Melitaea pseudornata, Munoz Sariot & Sanchez Mesa, 2019
|
publication ID |
https://doi.org/ 10.1093/isd/ixac004 |
|
publication LSID |
lsid:zoobank.org:pub:D1410808-7450-4190-BA1A-C57BA477AD46 |
|
DOI |
https://doi.org/10.5281/zenodo.7183787 |
|
persistent identifier |
https://treatment.plazi.org/id/03B287EC-FF80-A373-2EBE-B218C6BDBA7E |
|
treatment provided by |
Felipe |
|
scientific name |
Melitaea pseudornata |
| status |
|
M. pseudornata and M. phoebe ,Two
Interacting Species
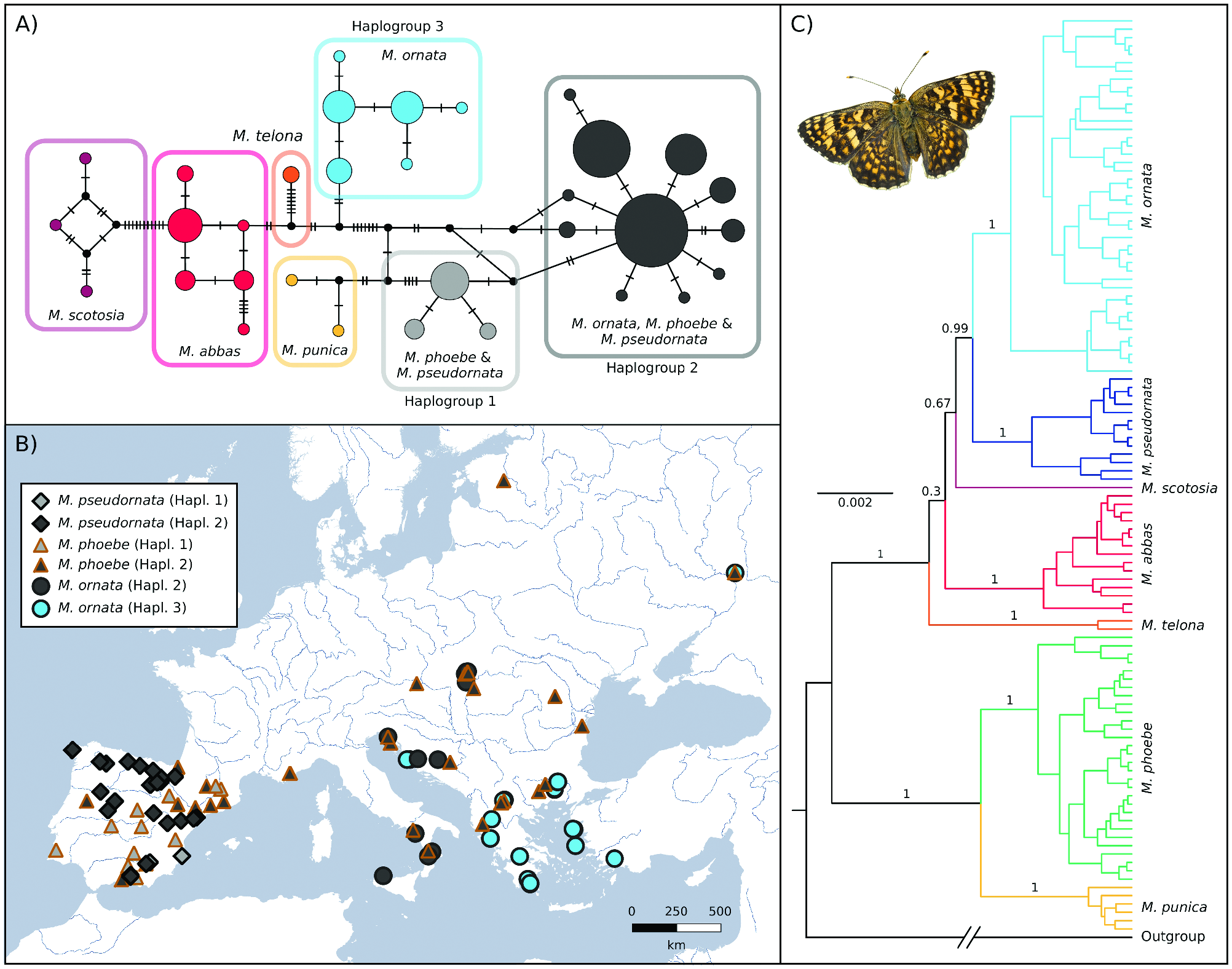
Mitochondrial DNA ( Figs. 2A,B View Fig ) showed that M. pseudornata shared two haplogroups (here, groups of haplotypes linked by two
or fewer mutations) with M. phoebe . One of the shared haplogroups, Haplogroup 1, is exclusive to Iberia. The second shared haplogroup, Haplogroup 2, was also found in M. phoebe from all Europe (including Iberia) and in M. ornata . Considering that M. ornata conserves a well diverged COI lineage in the Balkan Peninsula and eastwards (Haplogroup 3), the fact that in other parts of Europe this species is clustered in the same haplogroup with M. phoebe and M. pseudornata while nuclear markers differentiate them suggests mitochondrial introgression. Thus, M. ornata would have partially lost its original mtDNA in favor of an introgressed mtDNA presumably coming from M. phoebe , a scenario already proposed by Tóth et al. (2017). The same situation may apply to M. pseudornata , whose mtDNA could have been completely erased after the introgression events with M. phoebe —as occurred in other Iberian species such as Iphiclides feisthamelii ( Gaunet et al. 2019) . Overall, we cannot determine from these data how common hybridization between M. phoebe and M. pseudornata might be at present but, given that they share two well-differentiated haplogroups, introgressive hybridization seems to have occurred at least twice in the past.
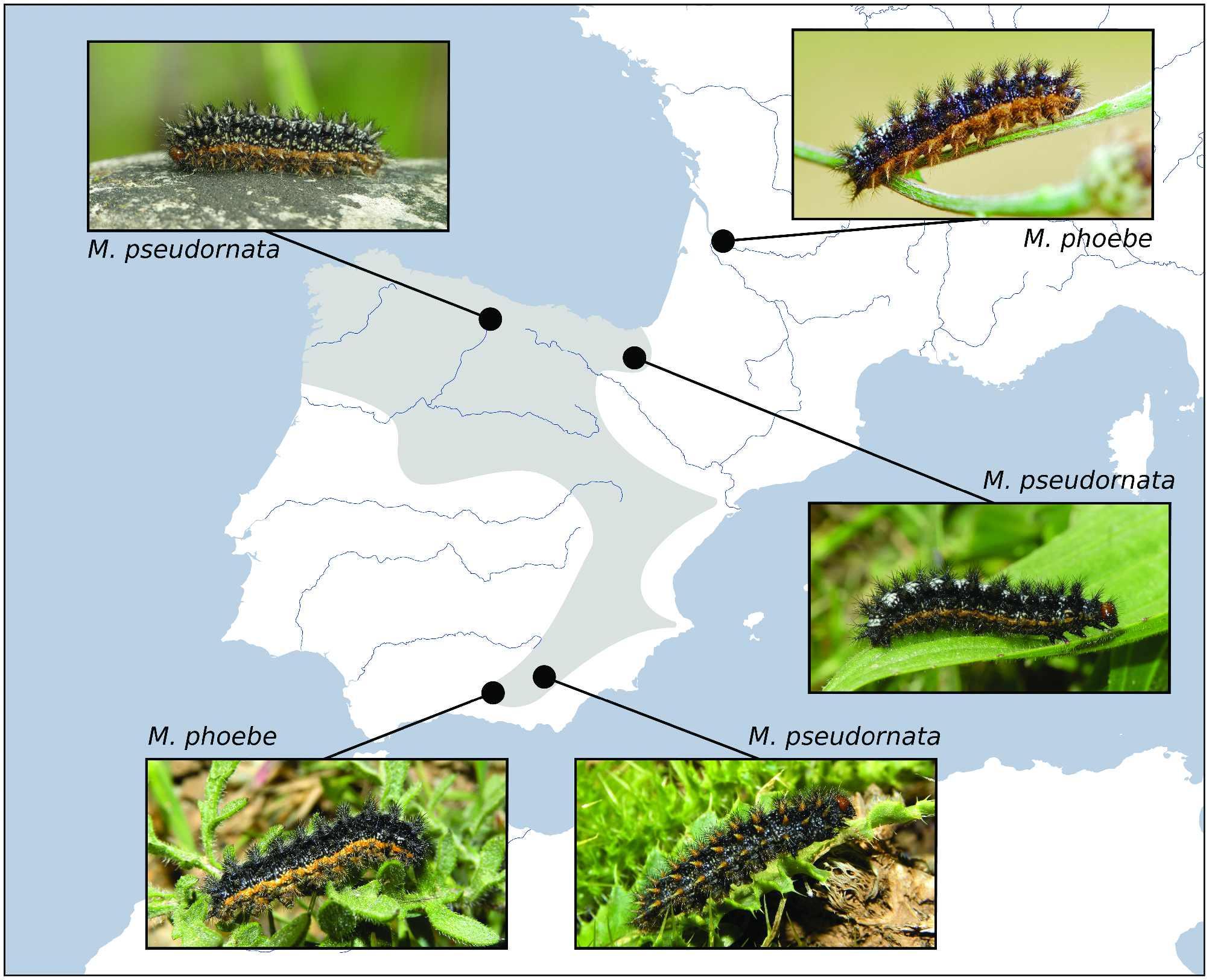
Past hybridization between M. phoebe and M. pseudornata could have had an impact on the morphology of the larvae and the adults. A sign of this can be the presence, in populations of northern Iberia, of the orange lateral stripe in the caterpillars, very similar to those present in the Iberian M. phoebe ( Fig. 4 View Fig ); these stripes are absent in M. ornata ( Russell and Tennent 2016) . Regarding the adults, a combination of traits of the wing underside such as the premarginal markings and color tone of the hindwings and are considered to be relatively useful to distinguish between M. ornata and M. phoebe ( Russell and Tennent 2016) . However, between the Iberian M. phoebe and M. pseudornata , these traits are regularly shared (Supp Fig. S4 View Fig [online only]). Furthermore, no other external traits seem to unambiguously differentiate the adults of M. pseudornata and M. phoebe .
M. pseudornata populations may also be affected by ecological character displacement regarding the larval host plant. In the Baetic System, M. pseudornata females are known to oviposit (or L 1 larvae were found) on Carduncellus Adans , Carduus L., and Cirsium Mill. , whereas M. phoebe oviposits on Centaurea L. Contrastingly , in central Navarre, an area where only M. pseudornata has been found, we only observed larvae (including L1) feeding on Centaurea jacea . This behavior could be influenced by a potential competitive pressure caused by the more generalist M. phoebe , a hypothesis already suggested by Tóth et al. (2015) for M. ornata .
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |