Hoplodactylus tohu, Scarsbrook & Walton & Rawlence & Hitchmough, 2023
|
publication ID |
https://doi.org/ 10.11646/zootaxa.5228.3.3 |
|
publication LSID |
lsid:zoobank.org:pub:42773A43-8AC3-43AF-A110-DFA5E7EC43B0 |
|
DOI |
https://doi.org/10.5281/zenodo.7542115 |
|
persistent identifier |
https://treatment.plazi.org/id/1BA7530E-52D7-44F7-9FC1-61C9A4A4B7AA |
|
taxon LSID |
lsid:zoobank.org:act:1BA7530E-52D7-44F7-9FC1-61C9A4A4B7AA |
|
treatment provided by |
Plazi |
|
scientific name |
Hoplodactylus tohu |
| status |
sp. nov. |
Hoplodactylus tohu n. sp.
Figures 2A View FIGURE 2 , 3A–D View FIGURE 3 , 4A–C View FIGURE 4 ; Supplementary Figures 5A–C.
ZooBank registration of Hoplodactylus tohu n. sp.: urn:lsid:zoobank.org:pub:BC2A430C-D97C-4F45-9AFB-228379864926 .
Naultinus pacificus .– Gray 1843: 203 (in part, not Gray, 1842).
Hoplodactylus duvaucelii [sic] McCann 1955: 39, fig. 3, pl. 4; McCann 1956a: 46; McCann 1956b: 15; Bustard 1963: 218; Sharell 1966: 49, pls. 28–31; Thoresen 1967: 197; Whitaker 1973: 122; Domrow et al. 1980: 295; Barwick 1982: 377; Bauer 1985: 90; Halliday & Verrell 1988: 260; Wilson & Freeman 1993: 1 – all in part (not Duméril & Bibron, 1836).
Hoplodactylus duvaucelii .– Holder 1960: 302; Kluge 1967a: 25; Kluge 1967b: 1013; Werner et al. 1978: 378;7 Kennedy 1979: 1; Bauer 1986: 9; Worthy 1987b: 416; Bauer 1990: 108; Thompson et al. 1992: 123; Daugherty et al. 1993: 439; Bauer & Henle 1994: 139; Cree 1994: 352 Daugherty et al. 1994: 318; Towns & Daugherty 1994: 327; Worthy & Holdaway 1994: 326; East et al. 1995: 256; Worthy & Holdaway 1995: 350; Worthy & Holdaway 1996: 314; Hitchmough 1997: 1; Worthy 1997: 93; Bauer 1998: 43; Girling et al. 1998: 139, fig. 4; Worthy 1998: 448; Bannock et al. 1999: 102; Lukis 1999: 12; Flannagan 2000: 4; Jones 2000: 1, fig. 2.9; Towns & Ferreira 2001: 219; Towns et al. 2001: 4; Seligmann 2002: 277; Whitaker et al. 2002: 1; Hay et al. 2003: 16; Todd 2003: 17; Seligmann et al. 2003: 130; Holmes 2004: 4; Naish 2004: 18; Hare & Cree 2005: 137; Armstrong & Davidson 2006: 74; Hare et al. 2007: 89; Nielsen 2008: 5; Kelly & Sullivan 2010: 208; Miskelly 2010: 3; Wilson 2010: 6; Frank & Wilson 2011: 16; Nielsen et al. 2011: 17; Bell & Herbert 2012: 8; Str̂ckens et al. 2012: 542; Garcia-Porta & Ord 2013: 2667; Hitchmough et al. 2013: 10; Bell 2014: 8; Romijn et al. 2014: 111; Heath & Whitaker 2015: 751; Mockett 2015: 73; Chapple 2016b: 4; Chapple & Hitchmough 2016: 116; Cree & Hare 2016: 174; Hare et al. 2016: 140; Hare & Cree 2016: 246; Hitchmough et al. 2016a: 9; Hitchmough et al. 2016b: 89; Morgan-Richards et al. 2016: 77, fig. 2; Romijn and Hartley 2016: 196; Towns et al. 2016b: 308; Worthy 2016: 71; Bell and Herbert 2017: 38; Bowers 2017: 64; Knox et al. 2017: 490; Lozito & Tuan 2017: 148, fig. 2 Sion 2017: 131; Stancher et al. 2018: 36; van Winkel et al. 2018: 114, pls. 31, 40, 46, 128; Skipwith et al. 2019: 10; Florence-Bennett 2020: 13; Glynne et al. 2020: 804; Herbert 2020: 12; Price et al. 2020: 231; Scarsbrook 2021: 19, figs. 1.5, 2.1, 3.1, 3.3, 4.1; Scarsbrook et al. 2021: 2; Scarsbrook et al. 2022: 3, fig. 1 – all in part (not Duméril & Bibron, 1836).
Naultinus duvaucelii .– Chrapliwy et al. 1961: 6 (in part, not Duméril & Bibron, 1836).
Woodworthia duvaucelii .– Jewell 2008: 50 (in part, not Duméril & Bibron, 1836).
Type material.— Holotype: New Zealand, Marlborough Sounds, Middle Trios Island , male, Y. M. McCann, February 1950, RE.000503 . Paratypes: New Zealand, Marlborough Sounds, Middle Trios Island , 40°50.53′ S, 174°0.00′ E, both male, C. H. Daugherty, 22 November 1988, RE.006685, RE.006686 (tissue clips: FT2047, FT2048 respectively) GoogleMaps .
Material examined.— The type material. Stephens Island ( OMVT949 ) . Trios Islands: Middle Trios Island (RE.000505,FT2046); South Trios Island (RE.006687). Sentinel Rock (RE.000948). Chetwode Island (RE.000949). Brothers Islands : North Brother Island (RE.002561, RE.005496, RE.006509, RE.006510, RE.007265, FT277, FT278). Northwest South Island (mainland): Gouland Downs , Holocene fossil (S.38813.2). Canterbury (mainland): Waikari , Holocene fossil (S.33501, S.33703.1, S.33703.7, S.33703.10, S.33703.11); Waitaki , Holocene fossil ( OMVT719 a, OMVT807 a, OMVT807 b, OMVT3331 , OMVT3332 , OMVT3333 ) .
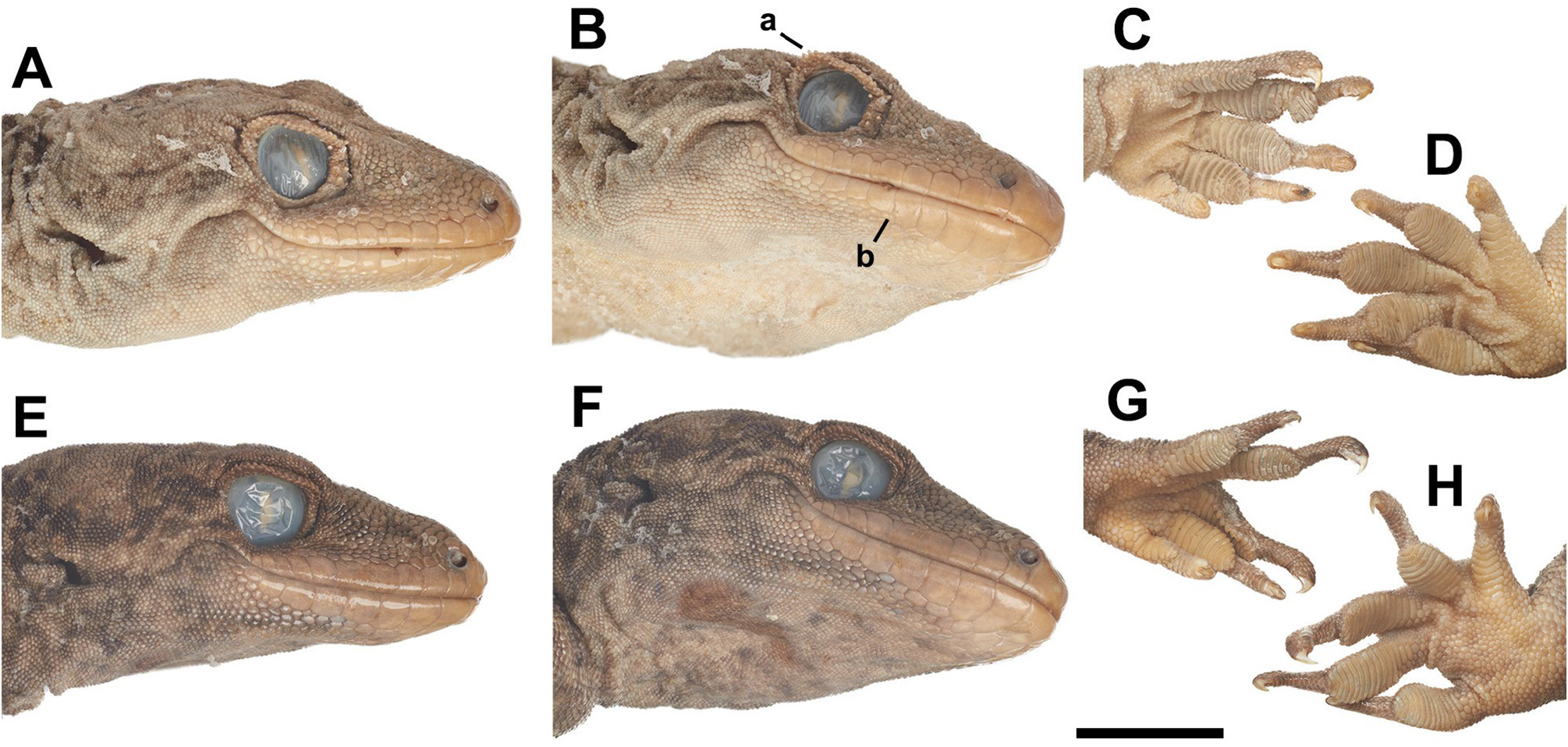
Diagnosis.— Hoplodactylus tohu may be distinguished from its only congener, H. duvaucelii , by several characters: H. tohu (generally) does not attain as great a size at maturity (Supp. Fig. 1 View FIGURE 1 ), has a more pronounced brillar fold ( Fig. 3A–B, E–F View FIGURE 3 , 4C, F View FIGURE 4 ), and less often bears a median cleft in the mental scale (Supp. Table 3). An abrupt size decrease at the 5 th infralabial scale characterizing H. tohu is far less common in H. duvaucelii , with most individuals of the latter examined exhibiting a gradual size decrease in infralabial scales ( Fig. 3B, F View FIGURE 3 ). H. tohu further differs from H. duvaucelii in generally having fewer subdigital lamellae on all digits of both the right manus and pes ( Fig. 3C–D, G–H View FIGURE 3 , Supp. Fig. 3 View FIGURE 3 ). The first digit of the right manus in H. duvaucelii differs in having a consistently less emergent claw and, usually, a comparatively bulbous distal end ( Fig. 3C, G View FIGURE 3 ). Previously reported differences ( Morgan-Richards et al. 2016) in the coloration and extent of patterning between these taxa ( Fig. 4 View FIGURE 4 ) are generalizations and can be misleading given fluid overlap between the two species. Dorsal body coloration and patterning in H. tohu often resembles those of young H. duvaucelii in being relatively more strongly contrasting ( Fig. 4A–B, D–E View FIGURE 4 ), with patterning generally becoming weaker at maturity. However, considerable variation in coloration and pattern (see Supp. Fig. 5) was observed throughout ontogeny in both species.
Description.— Coloration on dorsal surface grey to grey-brown, sometimes with olive and dark brown blotches; patterning of well-developed, roughly symmetrical transverse bands from nape to tail base centered along spine, resembling chevrons or paired diamonds, less defined on tail and generally less defined on older individuals; often bearing irregular series of longitudinal rows of pale grey or white spots dorsolaterally, extending onto limbs; ventral surface buff, with occasional light brown speckling, speckles less frequent on head. Mouth lining and tongue pink. Body moderately large (SVL: 81.2–115.7 mm), robust, stout (TrK: 25.4–50.3 mm). Head large (HL: 26.1–33.8 mm; HW: 18.3–27 mm; HH: 12.2–16 mm), robust, subtriangular; inflated laterally between posterior edge of orbit and ear opening (EE: 7.7–11.8 mm), narrowing towards craniovertebral junction; neck clearly demarcated. Snout oviform (EN: 7.4–11.1 mm); slight indentation in medial nasal region, often blunt along anteriormost margin between nares (IN: 4–5.8 mm). Dorsum of occiput/nape covered in small granular scales that abruptly increase in size at level of anterior edge of orbit towards anterior margin of snout (2–3 times diameter of occipital granules); one row of enlarged, oval scales posterior to internasal(s) and dorsal to supralabials, broader than high; twice the diameter of adjacent loreal scales. Eyes approximately one fifth head length; varying in shade from pale olive-buff to dark greens or browns; pupils lenticular with weakly crenulated margin. Supraciliary scales elongate, conical, directed increasingly posterior posteriorly; brillar fold very pronounced; frontal narrowing anteriorly (IO: 7.8–11.8 mm), supraocular portion deeply furrowed. Ear opening moderate (EL: 2–4.1 mm), ovoid, twice as high as wide; oriented obliquely (widest posterodorsally to anteroventrally), skin fold covering dorsal limit. Rostral subpentagonal, much broader than high; contacted dorsally by 2 enlarged, oval supranasals and 1–3 smaller (homogenous), round internasals; medial rostral crease extending ventrally from upper margin ½ to ¾ length of rostral; usually terminating diffusely, but sometimes as an ovoid crease. Nares rounded, situated anterolaterally; bordered by rostral, supranasal, 3–5 small postnasals and first supralabial. Supralabials rectangular, rounded dorsally; slightly higher than broad; numbering 13–16 per side; gradually decreasing in size posteriorly; final 3–4 more narrowly convex; supralabials terminating beneath the posterior orbital margin (with 11 or 12 beneath orbit midline); posteriormost twice the size of loreal scales. Mental trapezoidal to subtriangular, with longest face along jaw margin, narrowing posteriorly; mental crease usually absent, extending ½ length of scale where present; mental shorter than laterally adjacent first infralabials; contacted posteriorly by 1 large or 2 smaller hexagonal postmentals, which separate infralabials. Infralabials numbering 9–14 per side; from the snout, infralabials 1–4 on each side are quadrate, more rounded ventrally, higher than broad; infralabial 5 usually marks commencement of a significant and discrete infralabial size decrease, sometimes on one side only, with subsequent infralabials becoming progressively smaller and increasingly circular in shape, terminating in-line with the posterior orbital margin. Anterior infralabials and postmental(s) bordered posteriorly by series of enlarged irregular chin shields; rows indistinct; anteriormost approximately ¾ diameter of postmental, with gradual transition to very small, rounded throat granules posteriorly. Dorsal scales small, mostly homogenous in size, bead-like, apically flattened, partially overlain; indistinct from scales of nape. Ventral scales roughly twice diameter of dorsal scales, circular, flattened, subimbricate, slightly enlarged in precloacal region, extending in rows onto thighs where they form a subtriangular patch; transition to granules at throat abrupt. Skin folds extending ventrolaterally along trunk; anteroposterior folds above fore- and hindlimb insertion. Limbs relatively short and robust, hindlimbs longer; scales on forelimb dorsum larger than dorsal body scales, subimbricate distally; ventral forelimb scales smaller than those dorsally; clear transition to enlarged scales of palm which resemble ventral body scales. Scales on preaxial surface of thigh enlarged, up to three times diameter of dorsal body scales at knee; circular, subimbricate; gradually decreasing in size both posteriorly and distally (along shin) to smaller granules; transition to scales of soles indistinct (resembling ventral body scales). 5–6 rows of precloacal pores in males, anteriormost 2 rows extending distally just over halfway along hind thigh; absent in females. Digits broad, with dilated pads on digits II–V that rapidly transition into slender distal extension, mostly of constant width but becoming narrowly tapered near distal end; digit I smallest on all feet, digit IV longest on manus, digit V longest on pes; angle at rest between digits IV and V greatest, greater on pes than manus; digits II–V of pes and I–IV of manus very weakly webbed; dorsal scales on digits large, especially on first digit; all digits bear recurved, mostly exposed claws. Basalmost 1–2 subdigital lamellae sometimes fragmented; unfragmented subdigital lamellae curved outwards, usually smoothly but sometimes more sharply curved medially; lamellar counts of right manus: 6–7 (I), 9–11 (II), 12–14 (III), 12–15 (IV), 9–11 (V), and pes: 6–9 (I), 10–13 (II), 14–16 (III), 14–18 (IV), 11–16 (V) digits. Tail (original) stout, shorter than SVL, gradually tapered to end, roughly circular in cross-section; often lost through autotomy and then regenerated. Regenerated tail demarcated by abrupt decrease in scale size and anteroposterior striations from point of detachment distally. Base of tail distinctly swollen (TW: 8.7–14.7), most notable in males at cloacal spurs, with enlarged hexagonal scales on underside roughly twice size of more anterior ventral scales. Caudal scales usually arranged in discrete, irregular rows, generally decreasing in size distally from the septum; autotomy septa visibly marked by one, sometimes two rows of large or smaller scales, separated by 9–11 scale rows; dorsal caudal scales approximately 1.5 times size of dorsal body scales, demarcating tail base; highly variable in both size and shape, circular to rounded rectangular; ventral caudal scales enlarged medially, twice as large as dorsal; rectangular to hexagonal, higher than broad, subimbricate. Cloacal spurs consisting of a set of greatly enlarged, conical scales, with most acute point orientated posterodorsally, situated adjacent to cloaca (laterally); often asymmetric in number: 2–5 (L) and 1–4 (R), first (largest) roughly twice size of ventral scales, decreasing in size posteriorly.
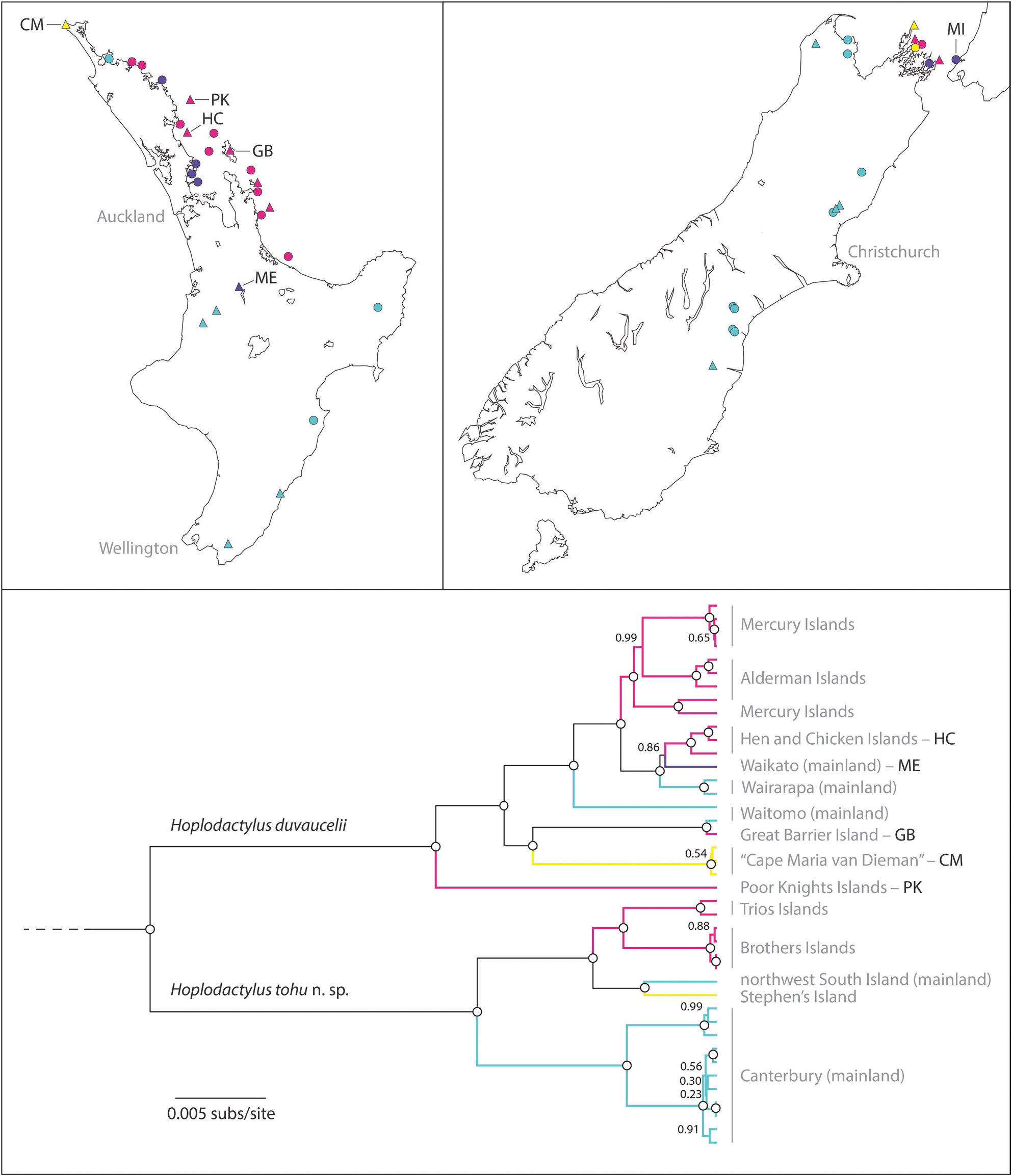
Distribution.— New Zealand: formerly throughout the South Island (Holocene); presently restricted to some islands in the outer Marlborough Sounds and Cook Strait ( Fig. 1 View FIGURE 1 ).
Remarks.— Hoplodactylus tohu has been recognized as distinct from H. duvaucelii for several years (MorganRichards et al. 2016; Hitchmough et al. 2021a). However, it has been unclear what rank to apply to this taxon. New Zealand diplodactylids frequently have highly conserved morphologies, and often greater intra- than inter-specific morphological variation ( Hitchmough et al. 2016b). With both Hoplodactylus species recognized here having undergone recent major range contractions resulting in significant declines in morphological and genetic diversity ( Scarsbrook et al. 2021; Scarsbrook et al. 2022), it is difficult to weight subtle character differences as these may have arisen, or increased in prevalence, recently, through chance, in relictual populations, and therefore not reflect deeper evolutionary divergences. That these taxa have been reported to produce viable cross-bred offspring in captive populations ( Morgan-Richards et al. 2016) fails to meet the criteria of the Biological Species Concept ( Mayr 1942). Reproductive isolation in the wild cannot be tested given their allopatric distributions. However, several pairings of lizard species that are widely recognized to be distinct ( Brennan et al. 2016; Leaché & Cole 2006; Olave et al. 2011), including some other New Zealand diplodactylids (e.g., Naultinus sp. ; Hitchmough et al. 2016b), have been shown to similarly produce viable hybrid offspring. Intergeneric hybrids have even been reported (RH pers. obs). Genetic distance (i.e., ~2.2% and ~4.0% across the mitochondrial genome and ND2 respectively) and estimated timing of lineage divergence of ~4.51 mya ( Scarsbrook et al. 2022) between the two Hoplodactylus species recognized here are comparable with those of other recognized diplodactylid species pairings (e.g., 3.8% ND2 divergence between Mokopirirakau kahutarae Whitaker, 1985 , and Mokopirirakau granulatus Gray, 1845 ; Knox et al. 2021). Their discrete (allopatric) distributions reflect commonly observed biogeographic patterns in other taxa ( Baker et al. 1995; Efford et al. 2002; Greaves et al. 2007; Liggins et al. 2008; Lloyd 2003), which further influences our treatment of species-level distinction.
Reports of interbreeding between H. tohu and H. duvaucelii in captivity makes careful management of both species essential to maintain species/genetic integrity. Individuals sourced for reintroduction or translocation purposes need to be of the correct species, and sourced from moderately large and stable populations (e.g. North Brother Island; Wilson 2010) to minimise impact on the source population. Further, source populations should be proximate (where practicable) to the site of translocation to ensure preservation of regional adaptations; a consideration more applicable to H. duvaucelii as extant populations are more numerous and occupy more ecologically varied habitats. It seems probable, for example, that H. duvaucelii rather than H. tohu , would have naturally occurred at Mana Island, off the southern North Island. However, the latter was translocated there from North Brother Island in 1998 through the release of 40 individuals ( Miskelly 2010), with a self-sustaining population reported 15-years later ( Bell & Herbert 2017).
H. tohu has been recognized as a distinct management unit by the Department of Conservation since 2021 ( Hitchmough et al. 2021a), listed as “nationally increasing” with the ‘Conservation Dependant’ and ‘Range Restricted’ qualifiers. H. tohu has a severely restricted distribution comprising a few islands ( Fig. 1 View FIGURE 1 ; Supp. Table 1 View TABLE 1 ) with highly anthropogenically modified habitats. The estimated total population size of the largest extant population, on the Brothers Islands, is between 583–677 ( Wilson 2010). Based on this, the suggested IUCN Red List status of H. tohu is ‘Critically Endangered A1 (a, b, c, e)’: population reduction of>90% observed, estimated, inferred, or suspected in the past where the causes of the reduction are clearly reversible AND understood AND have ceased. Occurrence of H. tohu on several managed predator-free islands that already have conservation management strategies in place does at least render moderate security to this species from localized disturbances such as fire or predator incursions. However, additional protection through translocations to fenced and more distant mainland sanctuaries (e.g. Orokonui Ecosanctuary near Dunedin, and the Mokomoko Dryland Sanctuary in Central Otago) could be beneficial, given the increased vulnerability of small and sparsely forested islands to the effects of climate change (e.g. sea-level rise, coastal erosion, storm intensity; Macinnis-Ng et al. 2021). Such translocation may also facilitate the re-establishment of ‘lost’ ecological interactions and morphological diversity (e.g. Scarsbrook et al. 2021) specific to mainland and densely forested ecosystems.
There is evidence of genetic sub-structuring ( Scarsbrook et al. 2022) within the distribution of H. tohu , although this has been greatly reduced with the extinction of mainland South Island populations. Careful management, and possible further research should consider if individual populations are experiencing inbreeding depression and might benefit from genetic rescue – establishment of ‘new’ populations through interbreeding of individuals from different populations to increase genetic ‘fitness’ (i.e., adaptability). Conversely, continued maintenance of discrete lineages may also be appropriate if those lineages reflect local habitat adaptations or if pooled populations would comprise too few individuals to maintain the resulting genetic diversity due to genetic drift.
Etymology.— The specific epithet was proposed by Dr Sharon Barcello-Gemmel, Rangatira of Te Ātiawa o Te Waka-a-Māui Trust, in recognition of the tupuna [ancestor] Tohu Kākahi. Tohu Kākahi was one of the two Parihaka prophets with whakapapa [genealogical] connections to Te Ātiawa – the iwi [tribe] with mana whenua [authority] over Ngāwhatu Kai Ponu [The Brothers], where the largest extant population occurs. Name used as noun in apposition.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Hoplodactylus tohu
| Scarsbrook, Lachie, Walton, Kerry, Rawlence, Nicolas J. & Hitchmough, Rodney A. 2023 |
Woodworthia duvaucelii
| Jewell, T. 2008: 50 |
Naultinus duvaucelii
| Chrapliwy, P. S. & Smith, H. M. & Grant, C. 1961: 6 |
Hoplodactylus duvaucelii
| Scarsbrook, L. & Verry, A. J. F. & Walton, K. & Hitchmough, R. A. & Rawlence, N. J. 2022: 3 |
| Scarsbrook, L. & Sherratt, E. & Hitchmough, R. A. & Rawlence, N. J. 2021: 2 |
| Florence-Bennett, B. J. 2020: 13 |
| Glynne, E. & Daza, J. D. & Bauer, A. M. 2020: 804 |
| Herbert, S. M. 2020: 12 |
| Price, S. J. & Grayson, K. L. & Gartrell, B. D. & Nelson, N. J. 2020: 231 |
| Skipwith, P. L. & Bi, K. & Oliver, P. M. 2019: 10 |
| Stancher, G. & Sovrano, V. A. & Vallortigara, G. 2018: 36 |
| van Winkel, D. & Baling, M. & Hitchmough, R. 2018: 114 |
| Bell, T. P. & Herbert, S. M. 2017: 38 |
| Bowers, L. 2017: 64 |
| Knox, C. D. & Jarvie, S. & Easton, L. J. & Monks, J. M. 2017: 490 |
| Lozito, T. P. & Tuan, R. S. 2017: 148 |
| Sion, G. 2017: 131 |
| Chapple, D. G. 2016: 4 |
| Hitchmough, R. A. & Patterson, G. B. & Chapple, D. G. 2016: 116 |
| Cree, A. & Hare, K. M. 2016: 246 |
| Hitchmough, R. A. & Barr, B. & Lettnik, M. & Monks, J. M. & Reardon, J. T. & Tocher, M. & van Winkel, D. & Rolfe, J. 2016: 9 |
| Hitchmough, R. A. & Patterson, G. B. & Chapple, D. G. 2016: 89 |
| Morgan-Richards, M. & Hinlo, A. R. & Smuts-Kennedy, C. & Innes, J. & Ji, W. & Barry, M. & Brunton, D. & Hitchmough, R. A. 2016: 77 |
| Romijn, R. L. & Hartley, S. 2016: 196 |
| Towns, D. R. & Hitchmough, R. A. & Perrott, J. 2016: 308 |
| Worthy, T. H. 2016: 71 |
| Heath, A. C. H. & Whitaker, A. H. 2015: 751 |
| Mockett, S. 2015: 73 |
| Bell, T. 2014: 8 |
| Romijn, R. L. & Nelson, N. J. & Monks, J. M. 2014: 111 |
| Garcia-Porta, J. & Ord, T. J. 2013: 2667 |
| Hitchmough, R. & Anderson, P. & Barr, B. & Monks, J. & Lettink, M. & Reardon, J. & Tocher, M. & Whitaker, T. 2013: 10 |
| Bell, T. P. & Herbert, S. M. 2012: 8 |
| Frank, H. & Wilson, D. 2011: 16 |
| Nielsen, S. V & Bauer, A. M. & Jackman, T. R. & Hitchmough, R. A. & Daugherty, C. H. 2011: 17 |
| Kelly, D. & Sullivan, J. J. 2010: 208 |
| Miskelly, C. 2010: 3 |
| Wilson, J. 2010: 6 |
| Nielsen, S. V. 2008: 5 |
| Hare, K. & Hoare, J. & Hitchmough, R. 2007: 89 |
| Armstrong, D. P. & Davidson, R. 2006: 74 |
| Hare, K. & Cree, A. 2005: 137 |
| Holmes, K. 2004: 4 |
| Naish, D. 2004: 18 |
| Hay, J. M. & Daugherty, C. H. & Cree, A. & Maxson, L. R. 2003: 16 |
| Todd, A. C. 2003: 17 |
| Seligmann, H. & Beiles, A. & Werner, Y. L. 2003: 130 |
| Seligmann, H. 2002: 277 |
| Whitaker, T. & Tocher, M. & Blair, T. 2002: 1 |
| Towns, D. R. & Ferreira, S. M. 2001: 219 |
| Flannagan, H. J. 2000: 4 |
| Jones, N. 2000: 1 |
| Bannock, C. & Whitaker, A. & Hickling, G. 1999: 102 |
| Lukis, K. 1999: 12 |
| Bauer, A. M. 1998: 43 |
| Girling, J. E. & Cree, A. & Guillette, L. J. 1998: 139 |
| Worthy, T. H. 1998: 448 |
| Hitchmough, R. A. 1997: 1 |
| Worthy, T. H. 1997: 93 |
| Worthy, T. H. & Holdaway, R. N. 1996: 314 |
| East, K. T. & East, M. R. & Daugherty, C. H. 1995: 256 |
| Worthy, T. H. & Holdaway, R. N. 1995: 350 |
| Bauer, A. M. & Henle, K. 1994: 139 |
| Cree, A. 1994: 352 |
| Towns, D. R. & Daugherty, C. H. 1994: 327 |
| Worthy, T. H. & Holdaway, R. N. 1994: 326 |
| Daugherty, C. H. & Gibbs, G. W. & Hitchmough, R. A. 1993: 439 |
| Thompson, M. B. & Daugherty, C. H. & Cree, A. & French, D. C. & Gillingham, J. C. & Barwick, R. E. 1992: 123 |
| Bauer, A. 1990: 108 |
| Worthy, T. H. 1987: 416 |
| Kennedy, C. M. 1979: 1 |
| Werner, Y. L. & Whitaker, A. H. & Whitaker, A. H. 1978: 378 |
| Kluge, A. 1967: 25 |
| Kluge, A. 1967: 1013 |
| Holder, L. A. 1960: 302 |
Hoplodactylus duvaucelii
| Wilson, K. J. & Freeman, A. 1993: 1 |
| Halliday, T. R. & Verrell, P. A. 1988: 260 |
| Bauer, A. M. 1985: 90 |
| Barwick, R. E. 1982: 377 |
| Domrow, R. & Heath, A. C. G. & Kennedy, C. 1980: 295 |
| Whitaker, A. H. 1973: 122 |
| Thoresen, A. C. 1967: 197 |
| Sharell, R. 1966: 49 |
| Bustard, H. 1963: 218 |
| McCann, C. 1956: 46 |
| McCann, C. 1956: 15 |
| McCann, C. 1955: 39 |
Naultinus pacificus
| Gray, J. E. 1843: 203 |