Lithocolletinae Stainton, 1854
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3594.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:B00799F3-F397-438C-B1E1-A8440E636921 |
|
DOI |
https://doi.org/10.5281/zenodo.5259374 |
|
persistent identifier |
https://treatment.plazi.org/id/03ADE350-B11B-FF85-F1CF-FC408A7CC9D5 |
|
treatment provided by |
Felipe |
|
scientific name |
Lithocolletinae Stainton, 1854 |
| status |
|
Lithocolletinae Stainton, 1854 View in CoL
Diagnosis. This is based on adult characters of separate lithocolletine genera following prior work (e.g., Stainton 1857; Chapman 1902; Ely 1918; Meyrick 1927; Le Marchand 1936; Kumata 1961, 1963, 1993; 1995; Vári 1961; Bradley et al. 1969; Kuznetzov 1981; Watkinson 1985; Davis & Robinson 1998; Parenti 2000; Bengtsson & Johansson 2011):
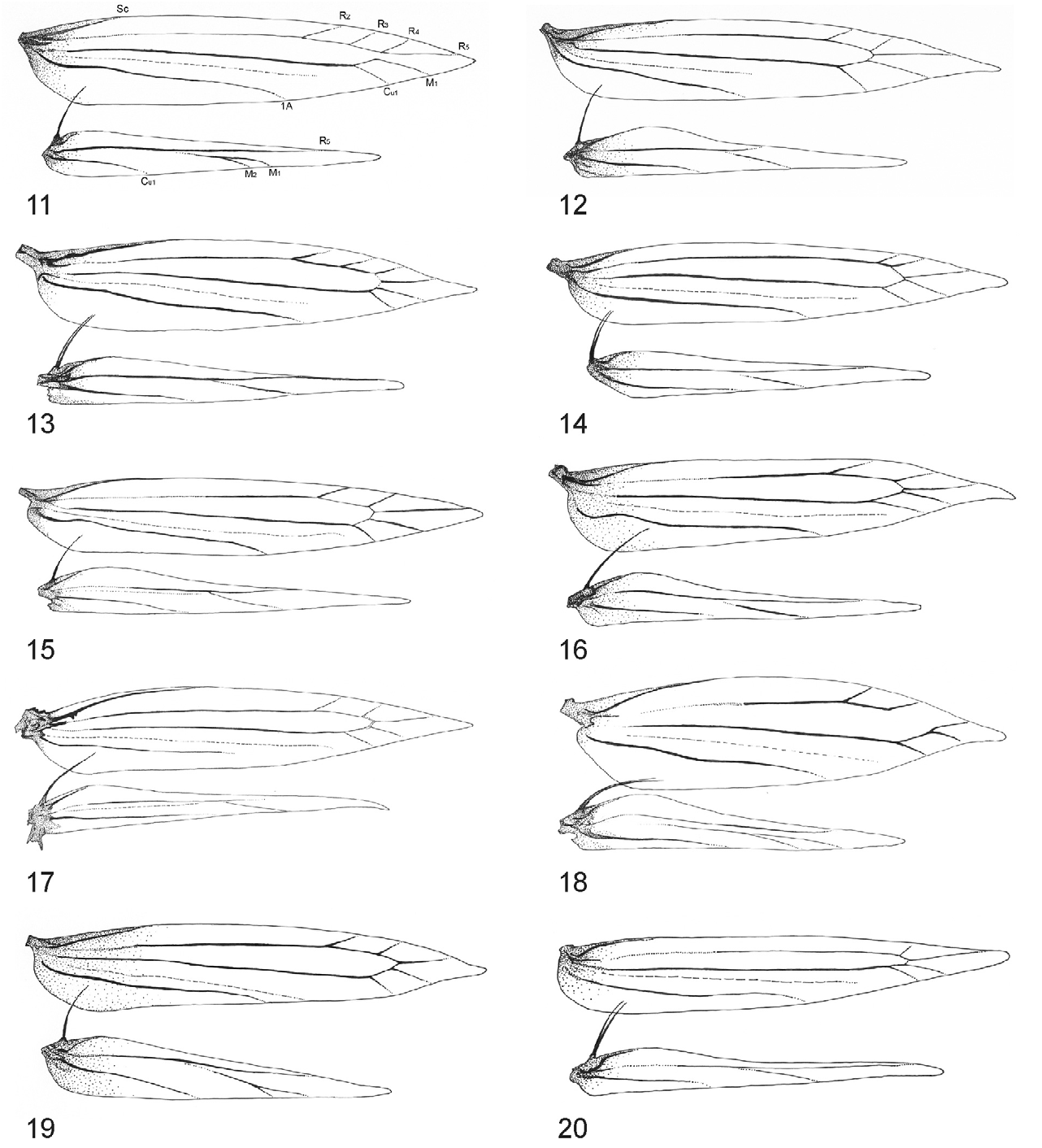
Hindwing with the vein Rs nearly parallel with vein M 1 or M 1+2 at the basal half of wing ( Kumata 1993; Davis & Robinson 1998).
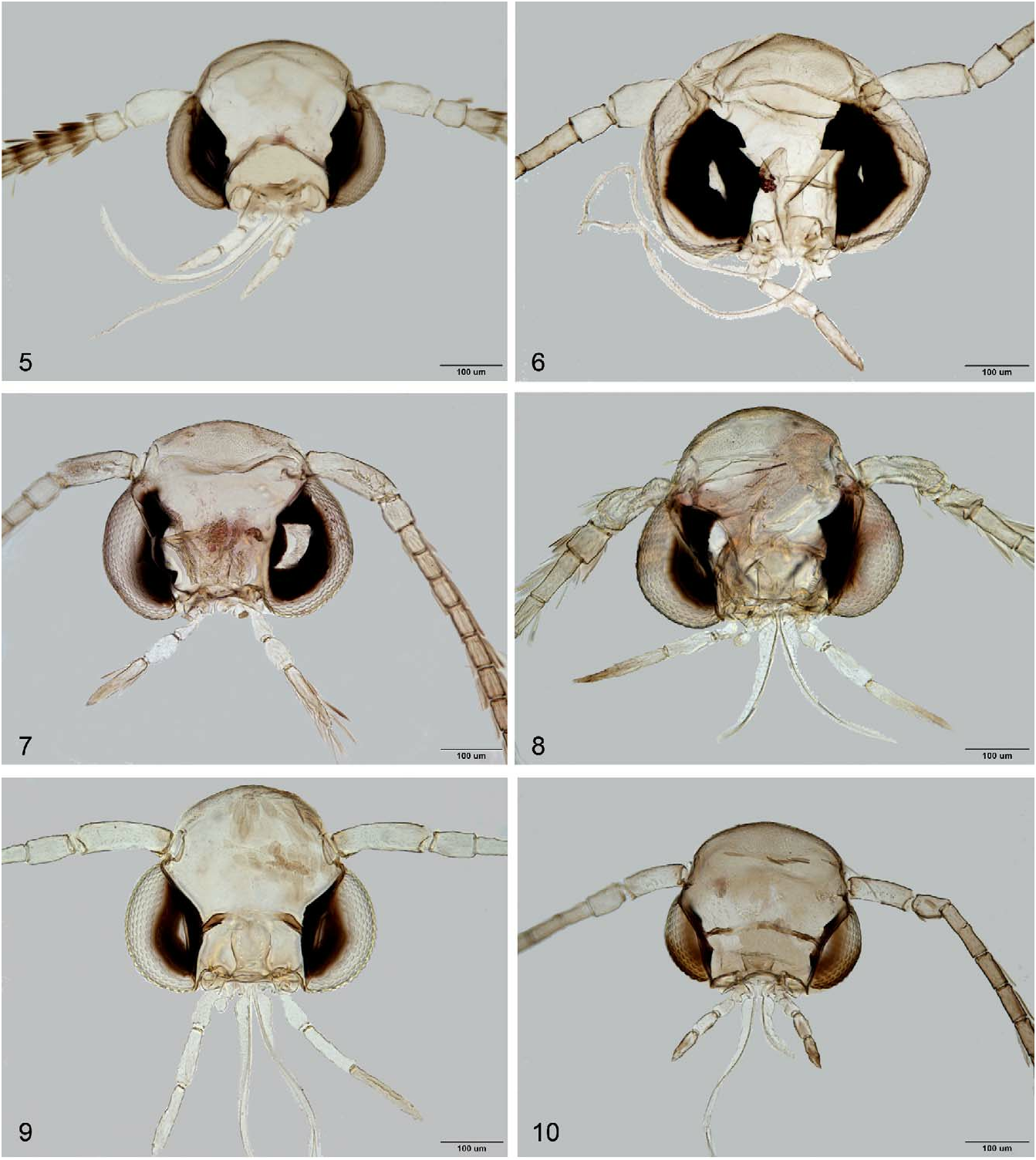
In addition to this hindwing venation character ( Table 2 View TABLE 2 ; Figs 11–20 View FIGURES 11–20 ), the following adult characters may also serve to separate the subfamily Lithocolletinae from the other subfamilies of Gracillariidae : i) tiny moths (less than 10 mm in wingspan), adults rest with body parallel to surface or with head end lowered; ii) background colour of forewing brilliantly ochreous, copper-golden, reddish-brownish, with white or silvery white striate, wedge-like markings, roundish spots, or brownish/black thin, lineal fascial and striate markings; iii) head tufted with longer or shorter occipital piliform scales; iv) labial palpus moderate, porrect or drooping straight, filiform, with approximate ratio of palpomeres from base 1:1:1.5 ( Figs 5–10 View FIGURES 5–10 ); v) forewing with seven to nine veins, CuP indistinct over entire length; vi) hindwing with five to six veins, M 1 indistinct at basal half; vii) eighth abdominal sternite of male modified, produced caudally, forming a flap laying under valvae except in Chrysaster , Leucanthiza , Macrosaccus , and Protolithocolletis .
Key to adults of lithocolletine genera
1. Eighth abdominal sternite of male modified, extended caudally, forming a flap laying under valvae..................... 5
– Eighth abdominal sternite of male unmodified, not extended................................................... 2
2. Hindwing with branched M 1 and M 2 ( Table 2 View TABLE 2 , line 1)............................................. Protolithocolletis View in CoL
– Hindwing with single M 1 ................................................................................ 3
3. Forewing with six or seven (R 2 rudimentary, indistinct, Cu 2 present) apical veins ( Table 2 View TABLE 2 , line 2)............. Leucanthiza View in CoL
– Forewing with five apical veins.......................................................................... 4
4. R 5 arising either from the base of R 4 or stalked with R 4; only apex of valva in male genitalia covered with elongate, stout setae; ductus bursae in female genitalia longer than seventh abdominal segment, signum on corpus bursae consisting of numerous microscopic spicules scattered or in a linear series on subcaudal part of corpus bursae ( Table 2 View TABLE 2 , line 3)........ Macrosaccus View in CoL
– R 5 stalked with M 1; setation of valva in male genitalia concentrated along costal margin owing to presence of hairy clasper; ductus bursae in female genitalia very short (ca. 1/5 as long as seventh abdominal segment), corpus bursae without signum ( Table 2 View TABLE 2 , line 4)............................................................................... Chrysaster View in CoL
5. Hindwing with branched M 1 and M 2 ....................................................................... 6
– Hindwing with single M 1 ................................................................................ 8
6. Four veins running to costa of forewing, six apical veins (R 2 present, M 1 not branched); tegumen in male genitalia with two or more pairs of apical setae; female genitalia with two pairs of apophyses ( Table 2 View TABLE 2 , line 5)...................... Hyloconis View in CoL
– Three or two veins running to costa of forewing (R 2 absent).................................................... 7
7. Forewing with six apical veins (R 2 absent, M 1 branched to M 1 and M 2); tegumen in male genitalia with one pair long and some short apical setae, valva not equal in width along its length, cucculus might be differently shaped; female genitalia with two pairs of apophyses (apophyses anteriores present) ( Table 2 View TABLE 2 , line 6)................................ Cremastobombycia View in CoL
– Forewing with four or five apical veins (R 2 absent, R 3 rudimentary, indistinct, M 1 not branched), hindwing with three pairs of sensillae on the dorsal margin which are associated with the veins Cu 1, M 1 and M 2; tegumen in male genitalia with one pair of apical setae, valva bar shaped, cucullus simple; female genitalia with one pair of apophyses (anterior apophyses absent) ( Table 2 View TABLE 2 , line 7).................................................................................. Porphyrosela View in CoL *
8. Forewing with six apical veins (R 2 present); tegumen in male genitalia with two pairs of apical setae ( Table 2 View TABLE 2 , line 8)............................................................................................... Neolithocolletis View in CoL
– Forewing with five apical veins (R 2 absent); apex of tegumen with one pair of apical setae or naked, without setae......... 9
9. Apex of tegumen in male genitalia naked without setae ( Table 2 View TABLE 2 , line 9)............................... Phyllonorycter View in CoL
– Apex of tegumen in male genitalia with a single pair of setae ( Table 2 View TABLE 2 , line 10)........................... Cameraria View in CoL **
* Vári (1961) did not illustrate sensillae in hindwing venation of the type species Porphyrosela desmodiella ( Clemens, 1859) . Sensillae on the dorsal margin of the hindwing in Porphyrosela might be almost invisible due to the very small size of the wing.
** Except the oriental species Cameraria fasciata Kumata, 1993 which has six apical veins in forewing (R 2 is present).
Biology
The immature stages of several lithocolletine species, including sap-feeding and tissue-feeding instars and their spectacular hypermetamorphosis are described in various publications (e.g., Watkinson 1985, Kumata 1961, 1963, 1993, 1995; Davis 1987; Bentancourt & Scatoni 2007; Davis & De Prins 2011). Most lithocolletine genera possess three sap-feeding and two tissue feeding instars. The first instar cuts through the epidermis of the mesophyllic cells and then feeds on the sap which is released by the feeding damage. The larva excavates a long narrow serpentine mine along a tougher leaf vein, the second instar widens the mine, and the third instar usually feeds laterally, creating a blotch mine that enlarges the gallery. In the fourth and fifth instars larvae feed on mesophyllic cells within the mined area without further enlargement of the mine. These cells are eaten out in small islets while the larvae move along the epidermis of the leaf without tearing it, keeping the larval ventrum adaxially to the epidermis of the leaf. The mine increases in depth by the fourth instar, and the spinning instar lays threads across the inner surface of the mine. At this stage the mine obtains a clearly defined shape which in most cases is one of four forms, and it is characteristic to a genus or species-group: tube, elongate wrinkled/smooth tent or roundish chamber. Some species of Lithocolletinae can significantly fold and distort the leaf. The species belonging to the Phyllonorycter loxozona group construct galls. The deposition of frass is an additional character which might assist in defining the generic or species group of Lithocolletinae in one or another way: i) it can be scattered around the mine; ii) neatly arranged along the vein of the leaf; iii) gathered neatly at one side of the mine; iv) spun with silken threads into a pile; or v) incorporated into the cocoon. However, the feeding habits and the deposition of frass also might differ between generations and climatic conditions. Pupation takes place within the mine, except in Chrysaster , without any opening or tear, within the protection of some form of cocoon or pupation chamber or pupa held in a fine net of silken threads; in more rare cases without any obvious protection of the pupa. The colour, size, shape and position of the cocoon within the mine is specific constant. Pupae are highly diagnostic for their presence of a cremaster on the caudal abdominal segment ( Phyllonorycter ), absence of a cremaster ( Cameraria ), or presence of the accessory cremaster on the seventh sternum ( Macrosaccus ), dorsal / ventral / lateral surfaces and position of spines and setae, proportial length of the appendages, etc. The number of generations differs greatly by species and climatic conditions. Although some species are septimavoltine, the majority tend to be bi- or trivoltine.
Host plants
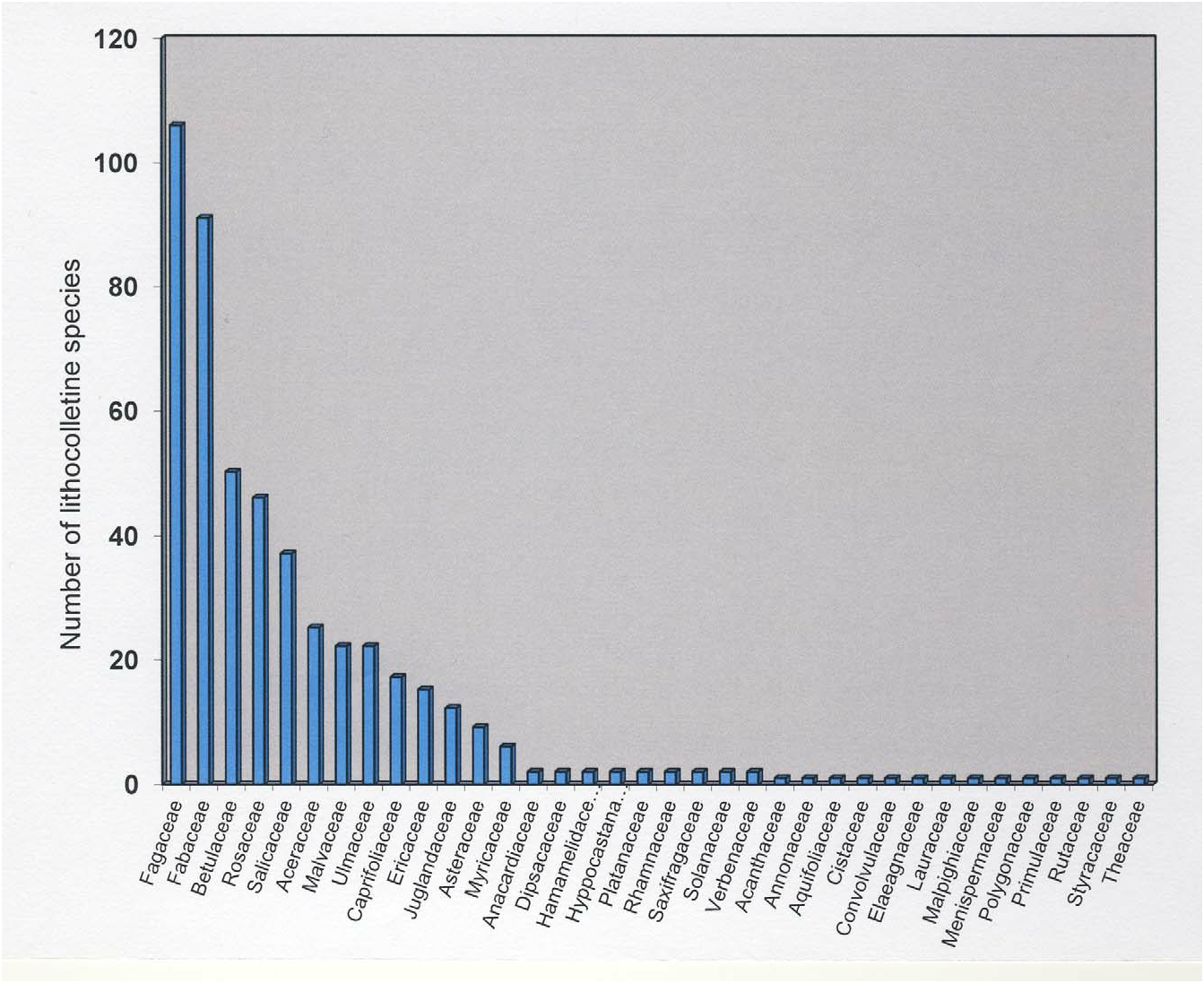
Currently, 870 different plant species are recorded as hosts for Lithocolletinae ( De Prins & De Prins 2012) . Of the 508 described lithocolletine species, at least one host plant is known for 461 (91%). Despite the predominance of oligophagy in Lithocolletinae confined within one host plant family, no less than 143 lithocolletine species are recorded from a single host plant only, indicating the often occurrence of the highly specialized monophagy within this subfamily. The 47 species that lack host plant data are predominantly tropical. Hosts of lithocolletine are represented across a broad spectrum of 36 plant families ( Fig. 1 View FIGURE 1 ). Most lithocolletine species utilize plants belonging to Fagaceae (106 species), Fabaceae (92 species), Betulaceae (51 species), Rosaceae (47 species) and Salicaceae (37 species). Each of the remaining 31 plant families serve as a host for a significantly lower number of lithocolletine moths ( Fig. 1 View FIGURE 1 , Table 3 View TABLE 3 ). However, species of most genera feed on only one or two host families, and only Cameraria and Phyllonorycter species appear to be truly polyphagous, feeding on multiple plant families. All lithocolletine genera, with the exception of Cremastobombycia , are associated with Fabaceae (species assigned to the genus Cremastobombycia feed on Asteraceae and Verbenaceae ). Moreover, all species belonging to Chrysaster , Hyloconis , Macrosaccus , Neolithocolletis , Porphyrosela and Protolithocolletis utilize exclusively Fabaceae plants. The correlation of lithocolletine moths with the plant family Fabaceae most probably is the ancestral condition of host plant preference within this subfamily. Leucanthiza feeds on Convolvulaceae , Fabaceae , and Theaceae . Cameraria and Phyllonorycter share very many hostplants, and the only host plant families that are not shared by Cameraria with Phyllonorycter are Annonaceae , Hippocastanaceae and Lauraceae . Host plants families utilized by Phyllonorycter belonging to Acanthaceae , Aquifoliaceae , Cistaceae , Dipsacaceae , Elaeagnaceae , Malpighiaceae , Malvaceae , Menispermaceae , Platanaceae , Polygonaceae , Primulaceae , Rhamnaceae , Rosaceae , Rutaceae , Saxifragaceae , Solanaceae , and Styracaceae are not shared with any other lithocolletine genus ( Table 3 View TABLE 3 ). In most cases, closely related moths feed on closely related host plants, and these usually belong to either the same plant genus or to closely related plant genera belonging to the same family ( Lopez-Vaamonde et al. 2003; De Prins & De Prins 2005, 2012). However, there are exceptions and some species show some plasticity in their host plant choice. In some rare cases, a species may feed on an unrelated host, if a female locates a host that is palatable for its larva. The best known example is Cameraria ohridella Deschka & Dimić, 1986 in Europe, where Acer pseudoplatanus L. ( Aceraceae ) may be chosen instead of the usual Aesculus spp. (Hippocastanaceae) ( Péré et al. 2010).
Distribution and diversity
Species diversity in Lithocolletinae is highly unequal, ranging from 401 species in Phyllonorycter Hübner, 1822 , to one in Protolithocolletis Braun, 1929 . Although lithocolletine species are known from all biogeographic regions, the vast majority is found in temperate regions, with 276 (54.3%) species in the Palaearctic, 145 (28.5%) in the Nearctic, 49 (9.6%) in the Oriental region, 26 (5.1%) in the Afrotropics, 18 (3.5%) in the Neotropics, and 8 (1.6%) species in the Australasian region ( Fig. 2 View FIGURE 2 , Table 4 View TABLE 4 ). Eight of ten genera are known to be restricted to the Nearctic region, seven to the Palaearctic and in the remaining regions three to five genera are known ( Table 4 View TABLE 4 ). Species are typically restricted to a single biogeographical region, rarely does their range span across more than one continent, however, some species are invasive where they were introduced either intentionally or unintentionally for different reasons ( Lopez-Vaamonde et al. 2010). For example, Cremastobombycia lantanella Busck, 1910 has an original Neotropical distribution (Mexico), but it was intentionally introduced into the Australasian (Hawaii) region in order to control Lantana sp. ( Busck 1910) , Neolithocolletis pentadesma Meyrick, 1919 is an Oriental species but it was unintentionally introduced to Seychelles ( Gerlach & Matyot 2006), Macrosaccus robiniella ( Clemens, 1859) has an original Nearctic distribution but it was accidentally imported into the Palaearctic with its larval foodplant, Robinia pseudoacacia L. ( Whitebread 1990).
The global species diversity of Lithocolletinae is clearly much greater than what is known ( Table 4 View TABLE 4 ). With the present treatment we aim to describe and illustrate a great number of species that were previously unreported.
In this study, we revise the Afrotropical Lithocolletinae utilizing morphology, life history, and molecular data. While it is ideal to conduct revisionary work for taxa at a global scale, we have chosen first to tackle the problem of Lithocolletinae within the Afrotropics, as the global diversity of Lithocolletinae is far too large and well beyond the scope of this study.
A brief history of Afrotropical Lithocolletinae studies
Francis Walker described the first Afrotropical Lithocolletinae species ( Lithocolletis aurifascia ) from St. Helena (British overseas territory) in 1875. According to Robinson (2009), the specimen was collected by Edith Wollaston. Walker (1875: 192) described Lithocolletis aurifascia as “an extremely minute and very beautiful moth”. However, this species is excluded from Lithocolletinae in the present study because of its head morphology, which resembles some Gracillariinae . Thus, it appears that the first Afrotropical lithocolletine was Phyllonorycter encaeria , described by Meyrick in 1911. This description was immediately followed by the description of Phyllonorycter melanosparta ( Meyrick, 1912) . Both species were discovered in South Africa, after which a few more species were described from other regions of the Afrotropics 25–30 years later ( Meyrick 1936; Ghesquière 1940). Lithocolletis urticicolella Ghesquière, 1940 , described from the Democratic Republic of the Congo, Kivu, valley of river Loso, was transferred to Tischeriidae ( De Prins & De Prins 2005) and its present generic combination is Tischeria urticicolella ( Ghesquière, 1940) ( Puplesis & Diškus 2005) . The middle of the 20th century was very fruitful to new species discoveries mainly due to the collecting trips by Pierre Viette in Madagascar (1949, 1951) and the detailed study by Lajos Vári (1961) in South Africa. In his monumental work, Vári (1961) treated most of the present genera of Gracillariidae , except Phyllocnistis , which was considered a different family ( Phyllocnistidae ) at that time. After describing 13 new lithocolletinae species, twenty years passed before one more Phyllonorycter species was described from Nigeria by Bland (1980), and again twenty five years before the descriptions of four more Phyllonorycter species discovered in Namibia, Kenya and Cameroon ( Triberti 2004; De Prins & Mozūraitis 2006; De Prins & De Prins 2007). Up to the present study 25 species of Afrotropical Lithocolletinae were described ( De Prins & De Prins 2012) and one species, Neolithocolletis pentadesma ( Meyrick, 1919) , was introduced to the Afrotropics from the Oriental region ( Gerlach & Matyot 2006). In the present paper we augment the list with 41 new species, totaling 66 Lithocolletinae species occurring in the Afrotropical region.
Key to the genera of Afrotropical Lithocolletinae
1. Forewing venation with three veins (R 3, R 4 and R 5) terminating along costa, hindwing venation with non-branched M 1 or with stalked M 1 and M 2 ..................................................................................... 3
– Forewing with four veins (R 2, R 3, R 4, R 5) terminating along costa, hindwing with non-branched M 1 ..................... 2
2. Hindwing with single, non-branched M 1; apex of tegumen with two pairs of setae ( Figs 12, 13 View FIGURES 11–20 )........... Neolithocolletis View in CoL *
– Hindwing with branched M 1 and M 2; apex of tegumen with more than 4 setae in male genitalia ( Fig. 11 View FIGURES 11–20 ).......... Hyloconis View in CoL
3. Hindwing with a unbranched M 1, apex of tegumen in male genitalia without setae or no more than one pair of setae....... 4
– Hindwing with stalked M 1 and M 2, apex of tegumen in male genitalia with two or more setae......................... 5
4. Apex of tegumen in male genitalia naked, without setae ( Figs 16, 17 View FIGURES 11–20 )................................. Phyllonorycter View in CoL
– Apex of tegumen in male genitalia with a single pair of setae ( Figs 14, 15 View FIGURES 11–20 )................................ Cameraria View in CoL
5. Apex of tegumen in male genitalia with a single pair of setae; anterior apophyses absent in female ( Fig. 20 View FIGURES 11–20 ).. Porphyrosela View in CoL **
– Apex of tegumen in male genitalia with more than one pair of setae, which may be of different length; anterior apophyses present in female ( Figs 18, 19 View FIGURES 11–20 ) Cremastobombycia View in CoL
* Except Neolithocolletis mayumbe which has three veins running to costa in forewing, but apex of tegumen with two pairs of setae in male genitalia.
** Anterior apophyses are absent in females of Cameraria varii also but all other morphological characters indicate the placement of this species into the genus Cameraria .
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.