Cynopterus sphinx (Vahl, 1797)
|
publication ID |
https://doi.org/ 10.5281/zenodo.6448815 |
|
DOI |
https://doi.org/10.5281/zenodo.6794929 |
|
persistent identifier |
https://treatment.plazi.org/id/03AD87FA-FFC2-F62D-8CB5-374EFA8BF217 |
|
treatment provided by |
Conny |
|
scientific name |
Cynopterus sphinx |
| status |
|
Greater Short-nosed Fruit Bat
French: Cynoptére a nez court / German: GrofRer Kurznasenflughund / Spanish: Cynéptero de nariz corta
Other common names: Short-nosed Indian Fruit Bat, Sphinx Fruit Bat
Taxonomy. Vespertilio sphinx Vahl, 1797 View in CoL ,
Tranquebar, Madras, India.
Subspecies scherzeri might be a separate species, and subspecies babi might be conspecific with C. nusatenggara . Six subspecies recognized.
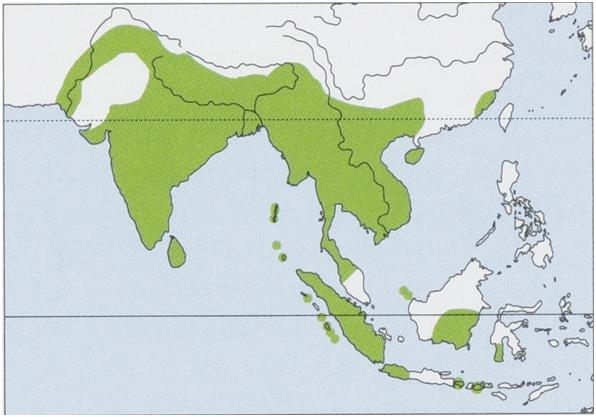
Subspecies and Distribution.
C.s.sphinxVahl,1797—IndiansubcontinentSoftheHimalayanfoothillsfromIndusRiverValleyinPakistanEtoMyanmarborder,includingNepal,Bhutan,allIndia (excludingTharDesert),Bangladesh,SriLanka,andtheAndamanandNicobarIs(exceptCarNicobarI).
C.s.babiLyon,1916—BabiI,offNWcoastofSumatra.
C.s.scherzeriZelebor,1869—CarNicobarI.
C. s. serasani Paradiso, 1971 — Natuna Is (Natuna Besar and Serasan). View Figure
Descriptive notes. Head—body 89-109 mm, tail 13-18 mm, ear 19-23 mm, hindfoot 14-5-20-5 mm; forearm 67-76 mm; weight 34-53 g (males) and 28-70 g (females). Sexual dimorphism varies, with males larger in northern India and females larger in southern India. The Greater Short-nosed Fruit Bat has white ear rims and wing digits. Muzzle is moderately short; nostrils are shortly tubular, with roughened rims. Philtrum is marked, ending in two joined upper lip pads; two large, triangular lower pads occur on lip. Skin of muzzle and ears and around eyes is darklilac gray. Forehead slopes,top of head is rather wide and flat; eyes are separated and mid-sized, and 1ris 1s chestnutbrown. Ears are moderately long and oval, and tips are slightly attenuated, with white rims on medial and lateral sides. Head is brown, slightly darker (russet-brown) on crown, and hairs are longer on nape and dorsum, cinnamon-fawn to olive-brown on females and typically deeper olive-brown on males, and lighter near tail. Tail is short and c.50% ofits length is free from uropatagium, which is narrow and naked dorsally; calcar is small. Ample brownish orange ruff entersside of head up to ear bases. Chest and belly are drab gray, merging to deep olive buff posteriorly; hairs are short. Index claw is present; patagial and feet are dark lilac gray; and wing membranes originate on sides of body and attach to first toe. Skull lacks basicranial deflection, rostrum is moderately short, forehead slopes gently, orbit is moderately large, braincaseis flattened anteriorly and more rounded posteriorly, zygomatic rootis nearly level with alveolar line, and zygoma is moderately thin, arched, and wide open. Dorsally, paranasal recesses are greatly inflated, reaching large postorbital foramen; postorbital processes are relatively thick and pointed posterolaterally; postorbital constriction is small; and temporal lines are joined medially in low sagittal crest. Palate is flat, post-dental palate is long and convergent, and palatine spine isjoined to mid-sphenoidal ridge. Ectotympanic is small and wide, more so anteriorly; entotympanic is along medial edge of ectotympanic. Mandible has sloping symphysis and straight body; coronoid is tall and sloping; condyle is slightly above lower alveolar line; and angle is round off. Dental formula for all species of Cynopterusis 12/2, C1/1,P 3/3, M 1/2 (x2) = 30. Upperincisors are small but with distinct crowns; C! is moderately long and straight, with convex anterior surface; P' is a spicule; and posterior cheekteeth are elongated, with cusps slanted forward and decreasing in height, and additional surface cusps are inconspicuous. Lower incisors and C, are small, Pis peg-like, next premolar (P,) is subequal in height to C,, and remaining cheekteeth decrease in size, with M, almost peg-like. Chromosomal complement has 2n = 34 and FN = 58, with 22 metacentric, five subtelocentric, and seven acrocentric chromosomes. X-chromosome is subtelocentric, and Y-chromosome acrocentric.
Habitat. Variety of habitats from relatively open, rural, and urban areas to primary and secondary forests from sea level up to elevations of ¢. 400 m. In undisturbed habitat, the Greater Short-nosed Fruit Bat is more common in subcanopies of old growth forests.
Food and Feeding. More than 90% of diet of the Greater Short-nosed Fruit Bat is fruit. It also eats flower products and leaves. It probably can exploit all bat-dispersed fruits in a given region and uses a greater proportion of larger fruits (less than 5 g) than smaller sympatric species of Cynopterus . It visits a fruiting plant within its home range, circling it and landing directly on the fruit, often also plucking fruit on the wing. Fruit is often taken to a night roost usually 20-100 m away from the plant, under protective foliage 10-30 m aboveground. Fruit is chewed, and large seeds are discarded; small seeds are swallowed with juicy pulp. Individuals shuttle continually between fruiting trees and night roosts, with two peaks of foraging activity; first peak is before midnight. Individuals forage alone and search for fruits offered regularly in small quantities such as Musa spp. Second foraging peak occursafter midnight and involves small groups of up to 13 individuals foraging on big-bang fruiting trees, such as kapok ( Ceiba pentandra, Malvaceae ) and particularly Ficus (Moraceae) that is heavily used throughout the distribution (e.g. k racemosa and F benghalensis in India). Other consumed fruit are Ebenaceae (Diospyros) , Anacardiaceae (Mangifera) , Annonaceae (Annona) , Rutaceae ( Acronychia , Atalantia ), Fabaceae (Pithecellobium) , Combretaceae (7 Terminalia ), Arecaceae (Phoenix) , Musaceae (Musa) , Oleaceae (Chionanthus) ; including introduced fruiting species (e.g. Psidium , Myrtaceae ). The Greater Short-nosed Fruit Bat also eats leaves of some species of Fabaceae (Cassia) , Sapotaceae (Mimusops) , Cucurbitaceae (Coccinia) , and Moringaceae (Moringa) , often later in the night after foraging for fruit. Leaves have higher concentrations of protein and calcium than fruit, which are protein poor but rich in carbohydrates (e.g. Musa ) or lipids (e.g. Psidium ). Gut passage can last c.12 hours or more, after which viable seeds are dispersed in excrement.
Breeding. Mating system of the Greater Short-nosed Fruit Bat is polygynous and seasonally polyestrous, with two distinct reproductive periods per year (winter and summer). Males struggle to maintain exclusive mating access to receptive females in a roost. Mating occurs in October-November, and pregnancy (winter) lasts 150 days due to postimplantation delayed embryonic development. Births occur in February— March. Females undergo postpartum estrus, so they can be pregnant and lactating, with a shorter pregnancy of 120 days (with no delayed development), giving birth again inJune-July. Males that hold territories shire 64-81% of young.Littersize is one, and females produce a maximum of two young per year. Males experience two peaks of spermatogenesis, but residual spermatozoa are retained in ducts of epididymides year-round. Females attain sexual maturity at 7-8 months old, giving birth to their first young in their first year. Males become sexually mature and are able to mate at 15-20 months old, depending partly on their birth season. Neonates weigh 11-13-5 g at birth (c.18% of postpartum female weight) and are altricial, remaining attached to nipples and carried in flight for 10-15 days, after which young are left in the roost when females forage. Young are born naked,eyes open at dayfive,ears unfold at about day seven, and hairs sprout at day nine. Females roost holding their young wrapped in their wings until ¢.30 days old when young start roosting on the side of their mother. At c.45 days, young start flying clumsily and trying to forage for fruit. Young are weaned after 55 days and are able to forage independently at 65 days old.
Activity patterns. Greater Short-nosed Fruit Bats are active from c.30 minutes after sunset throughout most of the night. Individuals significantly decreased activity in response to moonlight. Harem males spend c.50% of the night in a tent that they construct from leaves to defend their breeding spot; non-harem males spend less than 25% of the night in their tents. Greater Short-nosed Fruit Bats spend c.12 minutes or less in flight during the entire night, and the remainder of the time is spent in the tent (day roost) or the feeding (night) roost.
Movements, Home range and Social organization. Movements of Greater Short-nosed Fruit Bats are mainly from night roosts to feeding grounds and among fruiting trees and night roosts where fruit and leaves are eaten. Commuting flights from day roost to feeding grounds are up to 2-5 km but frequently 0-5-1 km. Flights to feeding areas were shorter for harem males (c.5 minutes) than non-harem males (8-12 minutes). Social organization is harem-based and centered on a male’s foliage tent that attracts reproductive females. Tents are made by chewing and severing vegetation parts, depending on tent type. Stem tents are built using branches of Polyalthia (Annonaceae) and Vernonia (Asteraceae) and fruit/flowerclusters of kital palm ( Caryota ; Arecaceae ), resulting in a spherical cavity open from below; umbrella tents are built using the palms ( Arecaceae ) Borassus , Corypha , Licuala , Livistona , Pritchardia , Sabal , and Roystonea , from which fronds are bitten in circles near their bases to collapse them distally. Greater Short-nosed Fruit Bats hang inside tents during the day; harem male roosts separately from his cluster of females, often displaying with spread wings. Roosting colonies can have 1-5 harems, each in a separate tent controlled bya single reproductive male, with occasional satellite males roosting outside but near a tent. Up to 19 females (mostly 1-6) roost in a given tent; females move among tents intermittently, exposing males to females outside his defended harem. Thus, harem organization is fluid temporally, with individuals shifting among roosts resulting in a social organization matching a fission-fusion model.
Status and Conservation. Classified as Least Concern on The IUCN Red List. The Greater Short-nosed Fruit Bat is generally common, except in more eastern locations such as Borneo where it is relatively rare. Population trend is increasing because it probably can take advantage of secondary habitats that become available with changes in land use and primary forest degradation. No major threats are known, as long as some forest cover remains. It is hunted for food and medicinal purposes in certain regions (e.g. southern China), but these practices do not seem to affect overall population. It is considered an orchard pest throughoutits distribution.
Bibliography. Andersen (1912b), Bates, Bumrungsri, Molur & Srinivasulu (2008), Benda (2010a), Bhat (1994), Bumrungsri (2002), Bumrungsri et al. (2007), Elangovan & Marimuthu (2001), Elangovan, Marimuthu & Kunz (1999, 2000, 2001), Elangovan, Satya Priya et al. (2003), Harada, Minezawa et al. (1982), Marimuthu et al. (1998), Meenakumari & Krishna (2005), Meenakumari et al. (2009), Nathan et al. (2005), Ruby et al. (2000), Shilton etal. (1999), Simmons (2005), Storz & Kunz (1999), Storz, Balasingh et al. (2001), Storz, Bhat & Kunz (2001a, 2001b).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Cynopterus sphinx
| Don E. Wilson & Russell A. Mittermeier 2019 |
Vespertilio sphinx
| Vahl 1797 |