Sciaphobus (Neosciaphobus), Apfelbeck, 1922
|
publication ID |
https://doi.org/10.5281/zenodo.5302796 |
|
publication LSID |
lsid:zoobank.org:pub:8E2AF537-E612-4D8A-876D-015B61E5847F |
|
persistent identifier |
https://treatment.plazi.org/id/03AA879E-DE05-CD75-FED3-9E77FD5AFC61 |
|
treatment provided by |
Marcus |
|
scientific name |
Sciaphobus (Neosciaphobus) |
| status |
|
Subgenus Neosciaphobus Apfelbeck, 1922 View in CoL
Neosciaphobus Apfelbeck, 1922: 60 View in CoL (original description, subgenus of Sciaphobus View in CoL ).
Neosciaphobus View in CoL : WINKLER (1932): 1469 (catalogue); DALLA TORRE et al. (1937): 162 (catalogue); SMRECZYŃSKI (1966): 81 (fauna); DIECKMANN (1980): 251 (fauna); PODLUSSÁNY (1996): 200 (check-list); ALONSO- ZARAZAGA & LYAL (1999): 177 (catalogue); BENEDIKT et al. (2010): 107 (check-list); BOROVEC (2013): 385 (catalogue).
Type species. Curculio rubi Gyllenhal, 1813 View in CoL (= Thylacites ningnidus Germar, 1824 View in CoL ) by subsequent designation ( BOROVEC 2013: 85).
Diagnosis. Small to middle sized Sciaphilini ; body densely scaled; rostrum in basal half tapered, in apical distinctly enlarged, at apex wider than at base; frons large; epistome inconspicuous; epifrons separated from head by shallow transversal depression; antennal funicles distinctly longer than scapes; scapes not reaching hind borders of eyes when folded; elytra short- to long-oval.
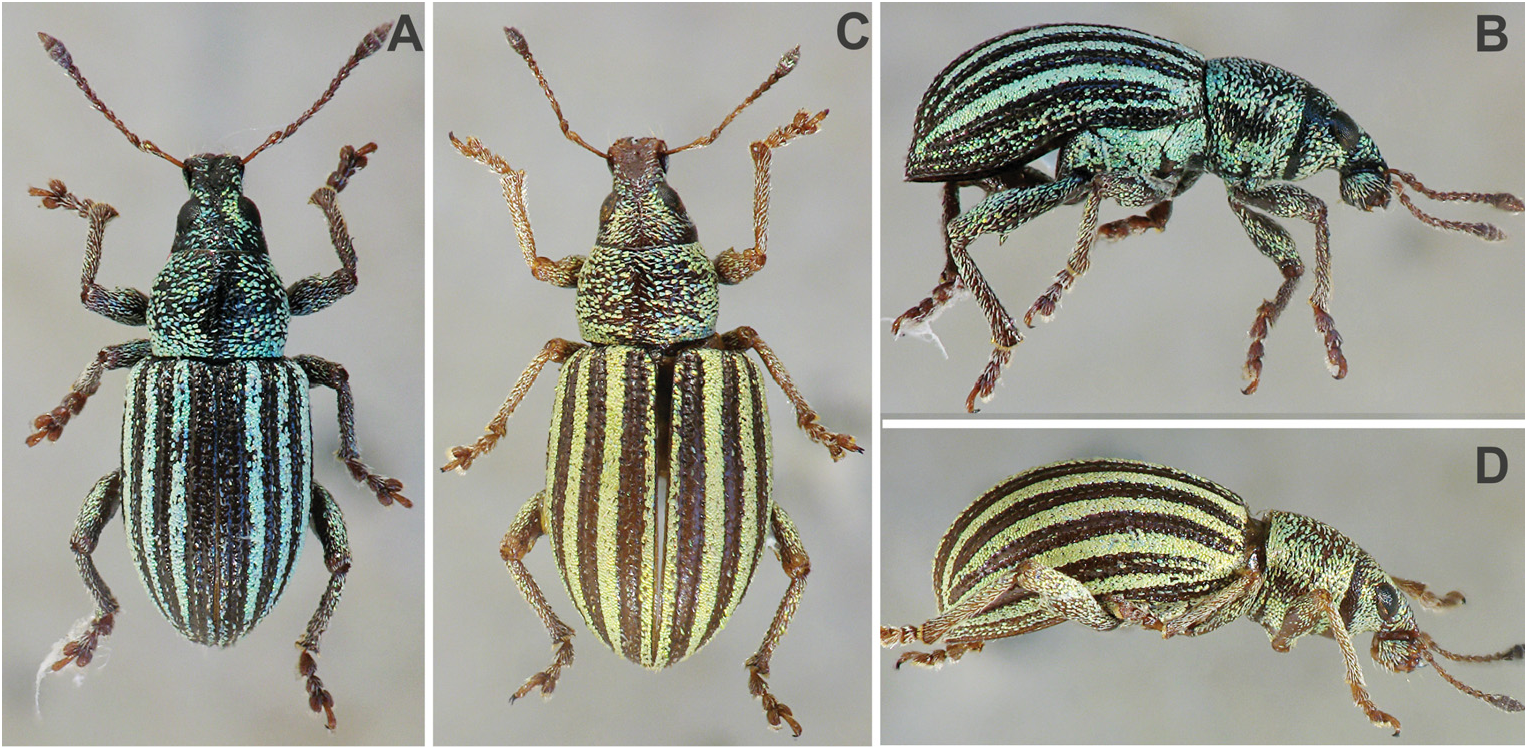
Redescription. Body length 3.1–7.3 mm. Body black; antennae and legs reddish or brownish, clubs and femora often darker. Elytra with rounded, short oval or long oval appressed scales, sparse or dense, brownish or greyish, mostly with intervals with one or two rows of very short and slender, indistinct, adherent setae, hardly visible in lateral view; elytral interval 7 and/or lateral intervals often lighter than colour of elytral intervals 1–6, only in S. vittatus with greenish scales on odd intervals. Pronotum with short or long oval scales directed transversally, with lateral longitudinal stripes made by lighter scales. Head and rostrum sparsely covered with slender scales. Antennal scapes and funicles with sparse, piliform setae, scapes with adherent, funicles with semierect setae; clubs with short adherent setae. Femora with long oval dense appressed setae; tibiae and tarsi with long, piliform, sparse, semiadherent setae. Abdominal ventrites densely or sparsely covered with long oval scales on ventrites 1 and 2, on ventrites 3–5 sometimes only in lateral parts, with semiadherent piliform setae.
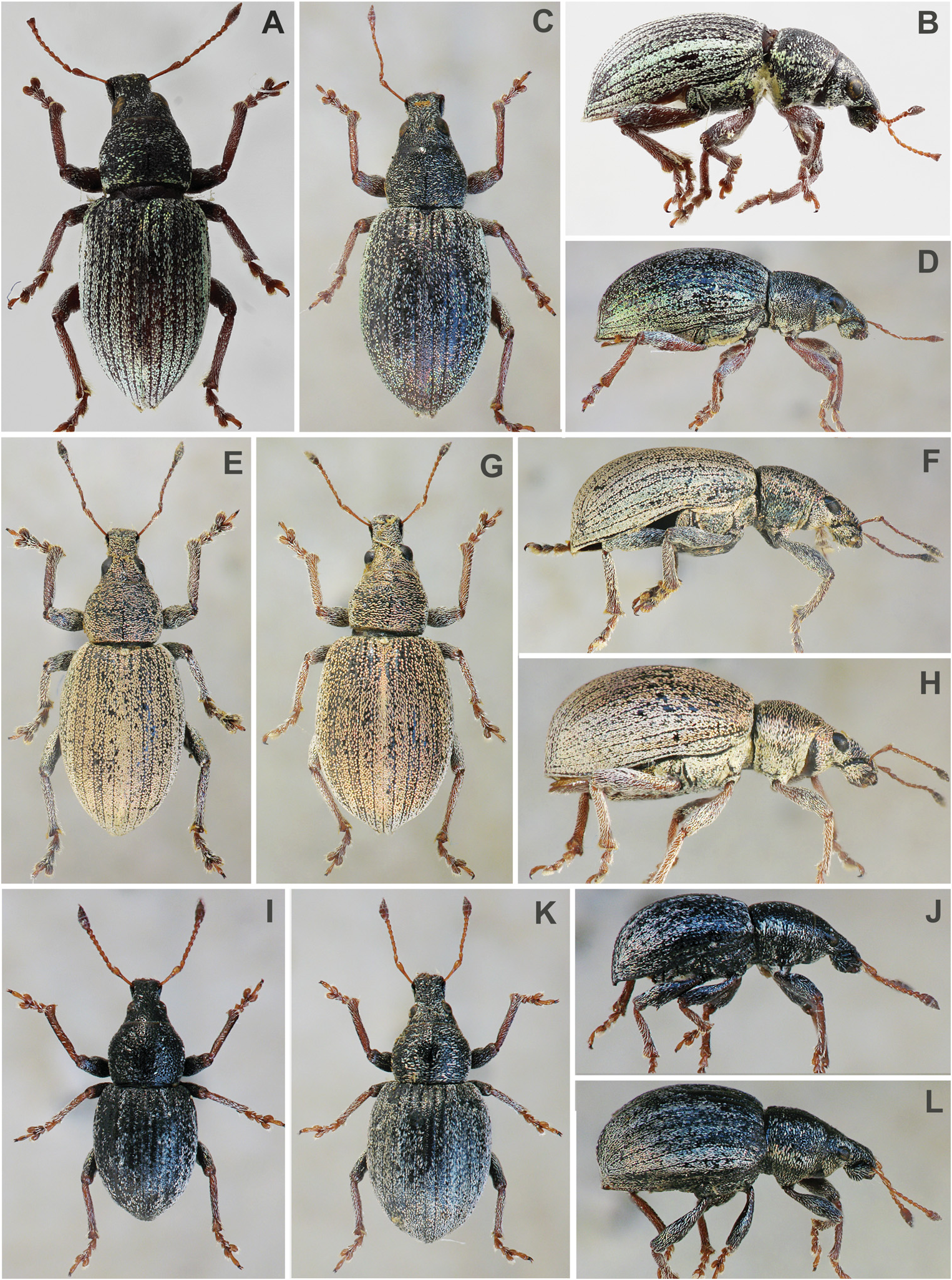
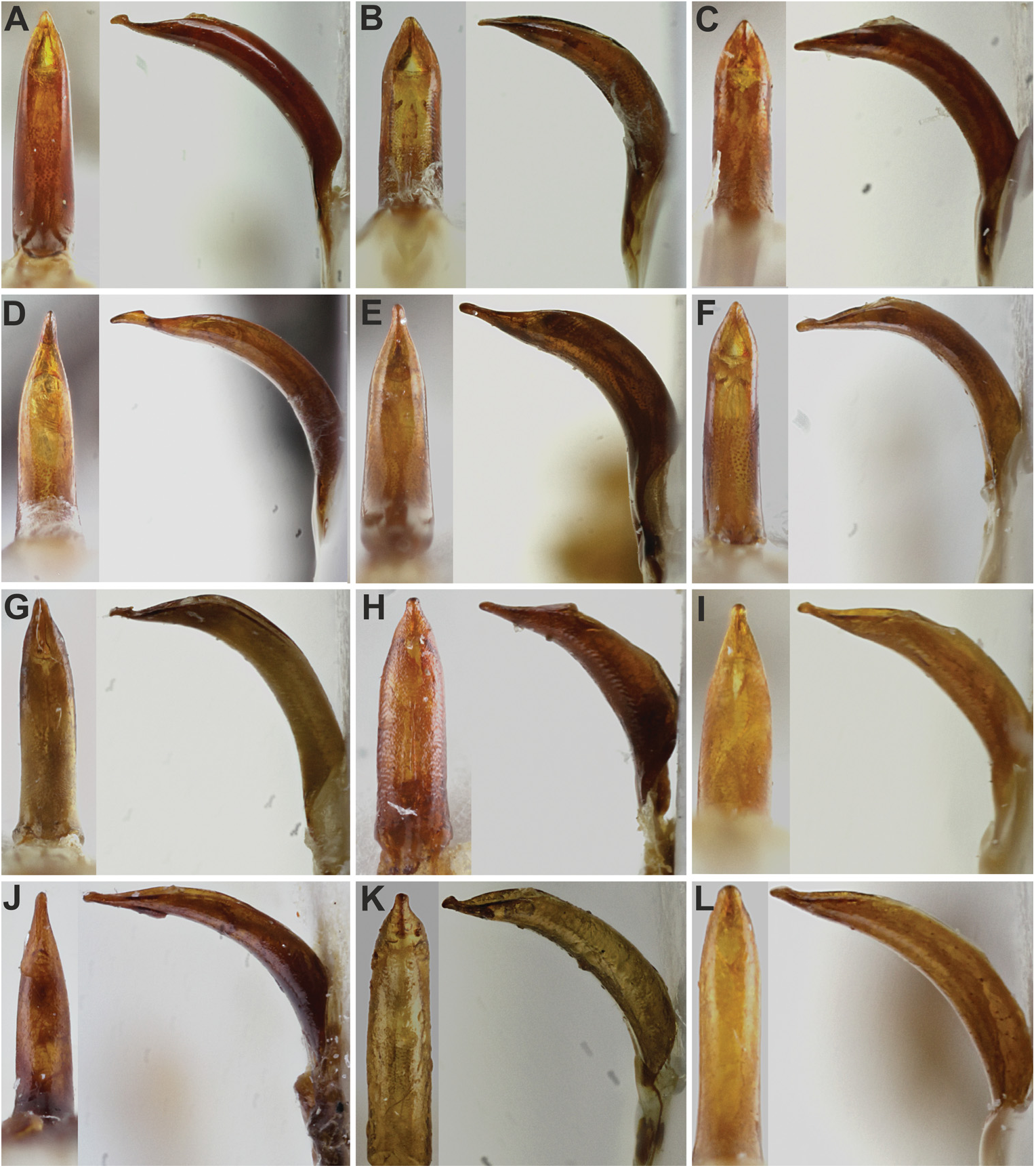
Head ( Figs 1A–L View Fig ; 2A–L View Fig ; 3A–D View Fig ; 4A–N View Fig ). Rostrum short to somewhat long with very similar shape in all species; basal half of rostrum tapered apicad with straight sides; apical half distinctly enlarged apicad with straight or rounded sides; rostrum at apex wider than at base. Frons large, flat or shallowly depressed; mostly shiny; on same level as epifrons or slightly angular in lateral view; with five pairs of slender, long setae. Epistome developed, inconspicuous, V-shaped, small, separated from frons by low carina. Epifrons weakly tapered basad, with distinct edges, at base somewhat narrower than space between eyes; flat, without carina or longitudinal furrow, separated from head by shallow, ill-defined transversal depression. Antennal scrobes in dorsal view visible in apical half as thin furrows, in lateral view furrow-shaped, well edged, glabrous in whole length; regularly curved; directed below eyes, reaching ventral border of rostrum. Eyes large, flat or convex, in lateral view placed near dorsal border of rostrum. Interocular space flat, in some species with narrow fovea. Head in dorsal view distinctly enlarged basad.
Antennae long and slender. Scapes very short, not reaching hind border of eyes when folded, 0.5–0.7× as long as funicles without clubs, slender in whole length, only in short apical part mallet-shaped enlargement. Funicle 7-segmented, segments long and slender, only in several species last two segments isodiametric or faintly transverse. Clubs long and slender, spindle-shaped.
Pronotum ( Figs 1A–L View Fig ; 2A–L View Fig ; 3A–D View Fig ) wide, with regularly rounded sides, without constriction behind anterior margin, with anterior margin slenderer than posterior one, regularly convex without longitudinal median carina or furrow, posterior margin straight; pronotum in lateral view with anterior margin straight, without setae.
Procoxal cavities contiguous, round, in middle of prosternum; procoxae subglobular.
Scutellum small, triangular, glabrous or big, subquadratic, densely squamose.
Elytra ( Figs 1A–L View Fig ; 2A–L View Fig ; 3A–D View Fig ) short to long oval, in some species globose, with strongly rounded sides, at apex narrowly rounded, convex, base straight, shoulders regularly rounded or weakly laterally projecting; striae punctate, glabrous; intervals almost flat, wide, in some species even intervals narrower than odd ones.
Mesocoxae semiglobular, narrowly separate, mesosternal process narrow, densely squamose, not reaching posterior margin of mesocoxae. Metacoxae transverse, separated by metasternal process narrower than transverse diameter of metacoxa.
Legs. Femora medially swollen, unarmed or with tooth, tooth usually smaller in pro- and mesotibia, in some species different also between sexes. Tibiae long, slender, straight; protibiae laterally straight, medially distinctly enlarged inwards, mucronate, apically rounded, with fringe of fine and moderately long yellowish setae; metatibiae with corbels oval to long oval, glabrous, shiny, fringed by dense yellowish setae. Tarsi moderately robust; tarsomere I longer than II or III; tarsomere II short, conical; tarsomere III wide and bilobed, wider than the others; onychium short or as long as tarsomere III, only exceptionally longer. Claws fused in basal part, parallel in whole length.
Abdomen. Abdominal ventrites subtriangular, ventrite 1 in middle longer than ventrite 2; ventrite 2 about as long as ventrites 3 and 4 combined, ventrites 3 and 4 equally long; first suture sinuose, fine; second suture weakly arched or straight, third and fourth sutures straight, second to fourth sutures wide and deep; metaventral process narrow, arrowhead-shaped or rounded.
Sexual dimorphism. Pronotum and elytra in males in the majority of species narrower than in females. Rostrum in males of several species also narrower than in females. Tooth on all femora in males of several species more distinct and larger than in females. Funicle segments in females in all species slenderer than in males. Ventrite 5 in males shorter, subtrapezoidal, in females longer, subtriangular and more pointed.
Male genitalia. Aedeagus ( Figs 5A–G View Fig ) long and slender, well sclerotised, temones about as long as body of aedeagus and 1.5–2.0× as long as tegminal manubrium. Tegmen ( Figs 6K, L View Fig ) with moderately wide ring, with diameter shorter than length of its manubrium, with long parameres, very near base or solidly fused in basal part. Sternite IX ( Fig. 6M View Fig ) with spiculum gastrale moderately long, anteriorly curved and enlarged to form wide apical plate, posteriorly with fused basal arms.
Female genitalia. Gonocoxites ( Fig. 6N View Fig ) moderately long and slender, weakly tapered anteriad, flat, subtrapezoidal with long and slender apical styli with 3–5 setae. Sternite VIII ( Fig. 6O View Fig ) with long and slender apodeme, terminated just inside plate, apically divergent, plate umbrella-shaped, short and wide, with basal margin ill-defined and apical margin thin but distinct, fringed with short numerous setae. Spermatheca ( Figs 6A–J View Fig ) large, long and slender, U-shaped, with long, slender, regularly pointed and at midlength distinctly curved cornu, corpus long and slender, curved at midlength, ramus and nodulus differing among species, usually developed but in all species very short and small.
Differential diagnosis. See Table 1.
Biology. The adults are polyphagous on different plants, shrubs and trees, mostly in xerothermic habitats. DIECKMANN (1980) listed Rubus idaeus L. and R. caesius L. ( Rosaceae ) as host plants of S. ningnidus and mentioned the occurrence of S. squalidus in fruit nurseries in Russia. The latter species was beaten from Salix L. in Romania by the first author. Jiří Krátký (pers. comm.) swept the same species in Hungary and Romania from different plants of herbal layer, with dominance of Aegopodium podagraria L. KOCH (1992) quoted S. scitulus as polyphagous on various species of Centaurea L. ( Asteraceae ), Salvia L. ( Lamiaceae ), Anthyllis L. ( Fabaceae ), and Fragaria L. ( Rosaceae ); and S. rubi on Rubus caesius L., R. ideaus L. RHEINHEIMER & HASSLER (2010) quoted previously published information and listed S. scitulus as polyphagous species on Centaurea scabiosa L., Salvia pratensis L., Anthyllis vulneraria L., and Fragaria vesca L. ( DIECKMANN 1980), and Medicago falcata L. (SPRICK & SCHIMDL 2004). Morphology of immature stages and biology are unknown. All species are amphigonic, except for S. ningnidus that is parthenogenetic through its range.
Distribution. The subgenus Neosciaphobus is mostly distributed in the Balkans. Six out of eight species live only there, each of them known only from a single country, and that would suggest that Neosciaphobus species have only a limited distribution. Sciaphobus ningnidus , due to its parthenogenetic reproduction, was able to spread across central Europe and western part of Russia. The only widespread amphigonic species is S. squalidus , distributed from the Balkans to southern part of central Europe and eastwards to Kazakhstan.
Remarks. The subgenus Neosciaphobus was proposed by APFELBECK (1922) for taxa without long erect elytral setae and without transversal carina between epifrons and frons on rostrum, and included the following species: S. balcanicus Apfelbeck, 1922 , S. globipennis Apfelbeck, 1922 , S. ningnidus ( Germar, 1824) , S. rasus ( Seidlitz, 1867) , S. reitteri ( Stierlin, 1884) , S. scheibeli ( Apfelbeck, 1922) , S. squalidus ( Gyllenhal, 1834) , and S. vittatus ( Gyllenhal, 1834) . Subsequent authors listed the same Neosciaphobus species in various catalogues (DALLA TO- RRE et al. 1937, WINKLER 1932, BOROVEC 2013). SMRECZYŃSKI (1966) and DIECKMANN (1980) defined Neosciaphobus not only by the elytra lacking erect setae, but also by an additional character: adherent scales grey or with cupreous sheen. DIECKMANN (1980) listed only two central European species of Neosciaphobus , S. ningnidus and S. squalidus .
APFELBECK (1922) omitted several species in his review of the genus, among them S. abbreviatus ( Desbrochers des Loges, 1871) and S. subnudus ( Desbrochers des Loges, 1892) . Both have the elytra without erect setae, and they were listed in the nominotypical subgenus in the subsequent catalogues ( WINKLER 1932, DALLA TORRE et al. 1937). As a matter of fact, the use of presence/absence of erect elytral setae as the main character at subgeneric level is misleading in Entiminae . For example, Phyllobius glaucus (Scopoli, 1763) and P. pomaceus Gyllenhal, 1834 are very similar and both belong to the subgenus Metaphyllobius Smirnov, 1913 , although one of them has long elytral setae, whereas the second is lacking them. The same inconsistency occurs in other genera of Entiminae , e.g. in Exomias Bedel, 1883 , Omias Germar, 1817 , Sitona Germar, 1817 . The colouration of adherent scales, green or greyish- -brown, could also be used only as an additional feature. For example, Sciaphobus dorsualis ( Gyllenhal, 1840) has green elytra with a large brownish spot covering the majority of elytral disc, therefore it is not possible to attribute it to any subgenus based only on the colour of elytral vestiture. The main character allowing separation of both subgenera of Sciaphobus is the presence or absence of 1) a narrow carina separating frons from epifrons and 2) a transversal sulcus between the rostrum and the remaining parts of the head. The diagnostic characters separating Neosciaphobus from Sciaphobus are summarized in Table 1.
Among the twenty-two presently known species of Sciaphobus only seven have rostrum separated from the head by a transverse sulcus and frons not separated from the epifrons by a narrow carina. In addition, all but alternately striped S. vittatus have elytra without erect elytral setae and with grey or brown adherent elytral scales. We include the following species in Neosciaphobus : S. globipennis , S. ningnidus , S. reitteri , S. scheibeli , S. squalidus , S. subnudus , S. vittatus , and S. angustus sp. nov. The remaining fourteen species that have rostrum on the same level as the head and frons separated from epifrons by a narrow carina belong to the nominotypical subgenus: S. abbreviatus ( Desbrochers des Loges, 1871) , S. barbatulus ( Germar, 1824) , S. caesius ( Hampe, 1870) , S. curvimanus Apfelbeck, 1922 , S. dorsualis ( Gyllenhal, 1840) , S. heteromorphus Apfelbeck, 1922 , S. megalopsis Apfelbeck, 1922 , S. paliuri Apfelbeck, 1908 , S. polydrosinus Apfelbeck, 1922 , S. rasus (Seidlitz, 1887) , S. scitulus ( Germar, 1824) , S. setosulus ( Germar, 1824) , and S. formaneki sp. nov. and S. pelikani sp. nov. described in this paper. All the above-mentioned species have: 1) elytral vestiture green, yellowish-green or brownish-green, except for S. dorsualis that has elytral vestiture green with a brown spot; 2) elytra with erect setae, except for S. abbreviatus , S. dorsualis , and S. rasus . The last is here newly transferred from the subgenus Neosciaphobus to the nominotypical subgenus.
The redescription of Neosciaphobus can also be used for Sciaphobus s. str., except for the characters on rostrum that separate both subgenera.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Sciaphobus (Neosciaphobus)
| Borovec, Roman & Skuhrovec, Jiří 2015 |
Neosciaphobus
| BOROVEC R. 2013: 385 |
| BENEDIKT S. & BOROVEC R. & FREMUTH J. & KRATKY J. & SCHON K. & SKUHROVEC J. & TRYZNA M. 2010: 107 |
| PODLUSSANY A. 1996: 200 |
| DIECKMANN L. 1980: 251 |
| SMRECZYNSKI S. 1966: 81 |
| DALLA TORRE K. W. VON & EMDEN M. VAN & EMDEN F. VAN 1937: 162 |
| WINKLER A. 1932: 1469 |
Neosciaphobus Apfelbeck, 1922: 60
| APFELBECK V. 1922: 60 |