AEDINI
|
publication ID |
https://doi.org/10.1111/j.1096-3642.2004.00144.x |
|
persistent identifier |
https://treatment.plazi.org/id/03A57D7D-FF8C-FFE2-FE89-FA026A24FAB5 |
|
treatment provided by |
Diego |
|
scientific name |
AEDINI |
| status |
|
CLASSIFICATION OF AEDINI View in CoL View at ENA
The existing classification of Aedini generally follows Edwards’ (1932) concept of fewer genera but with numerous subgenera. Belkin (1962) stated ‘I am in full agreement with the practice, following Edwards, of recognizing few genera and many subgenera, since this appears to reflect the evolution of the family’. However, Belkin stated (p. 318) that the internal classification of Aedini was in need of thorough revision. He further noted (p. 326) that many of the subgenera might have to be subdivided into smaller natural groups because they appeared to be heterogeneous assemblages of superficially similar forms.
Despite more than a century of study, mosquito taxonomy is still largely at the descriptive level (alpha taxonomy) and relatively little attention has been given to the development of a natural classification (beta taxonomy). As Zavortink (1990) pointed out: ‘At the beta level of taxonomy,... the species are studied in greater detail and are reclassified into smaller and more numerous genera that indicate their genetic relationships more accurately.’ Using a mathematical equation that expresses the graphical distribution of species per genus in those groups of organisms that have achieved the beta level of taxonomy, Zavortink calculated that the total number of genera in Culicidae should be 225. Only 39 are currently recognized ( Reinert, 2001a). Likewise, the number of aedine genera falls far short of the number expected for a tribe the size of Aedini . In proportion to the family (about 3200 species), the tribe (about 1200 species) should theoretically include 87 genera.
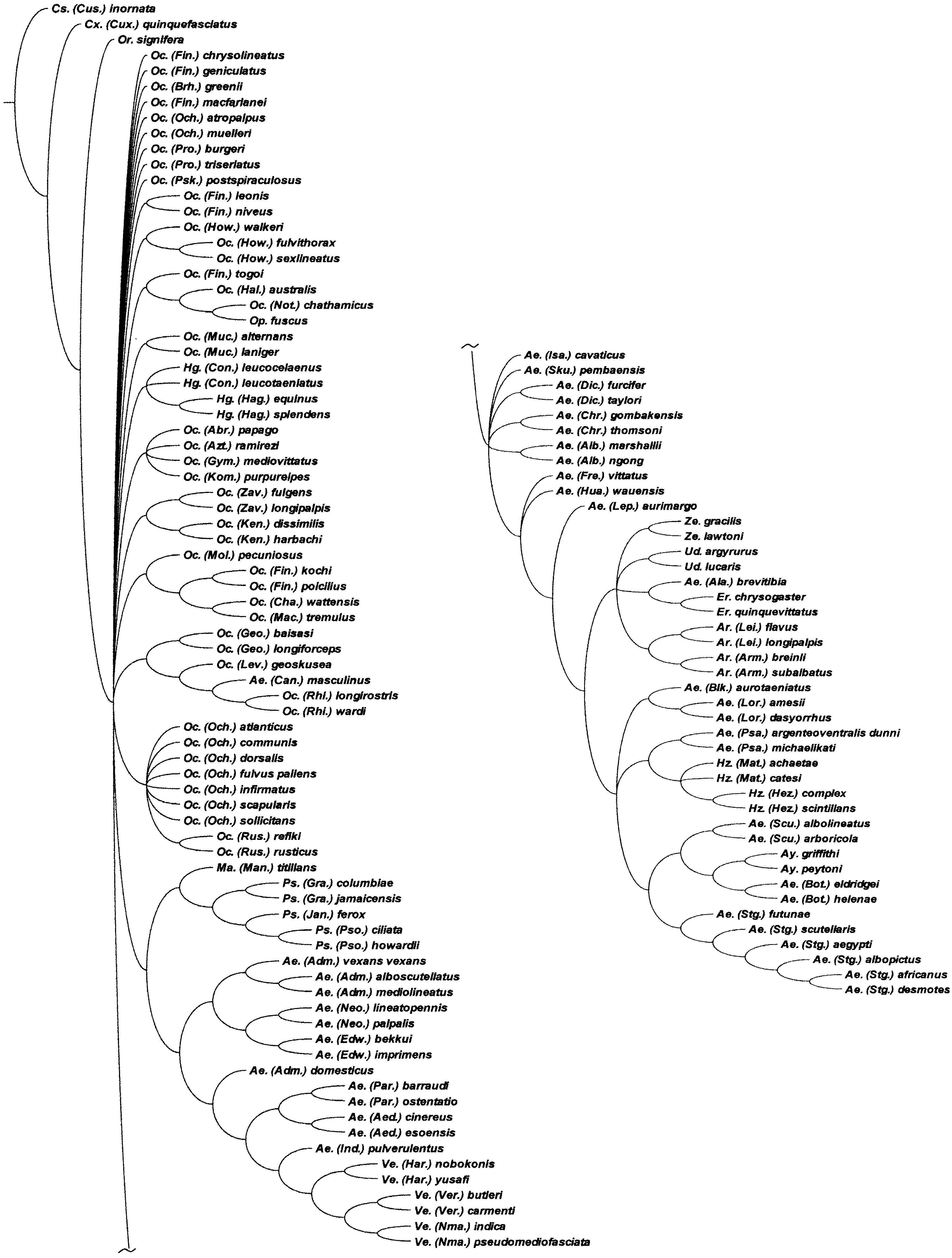
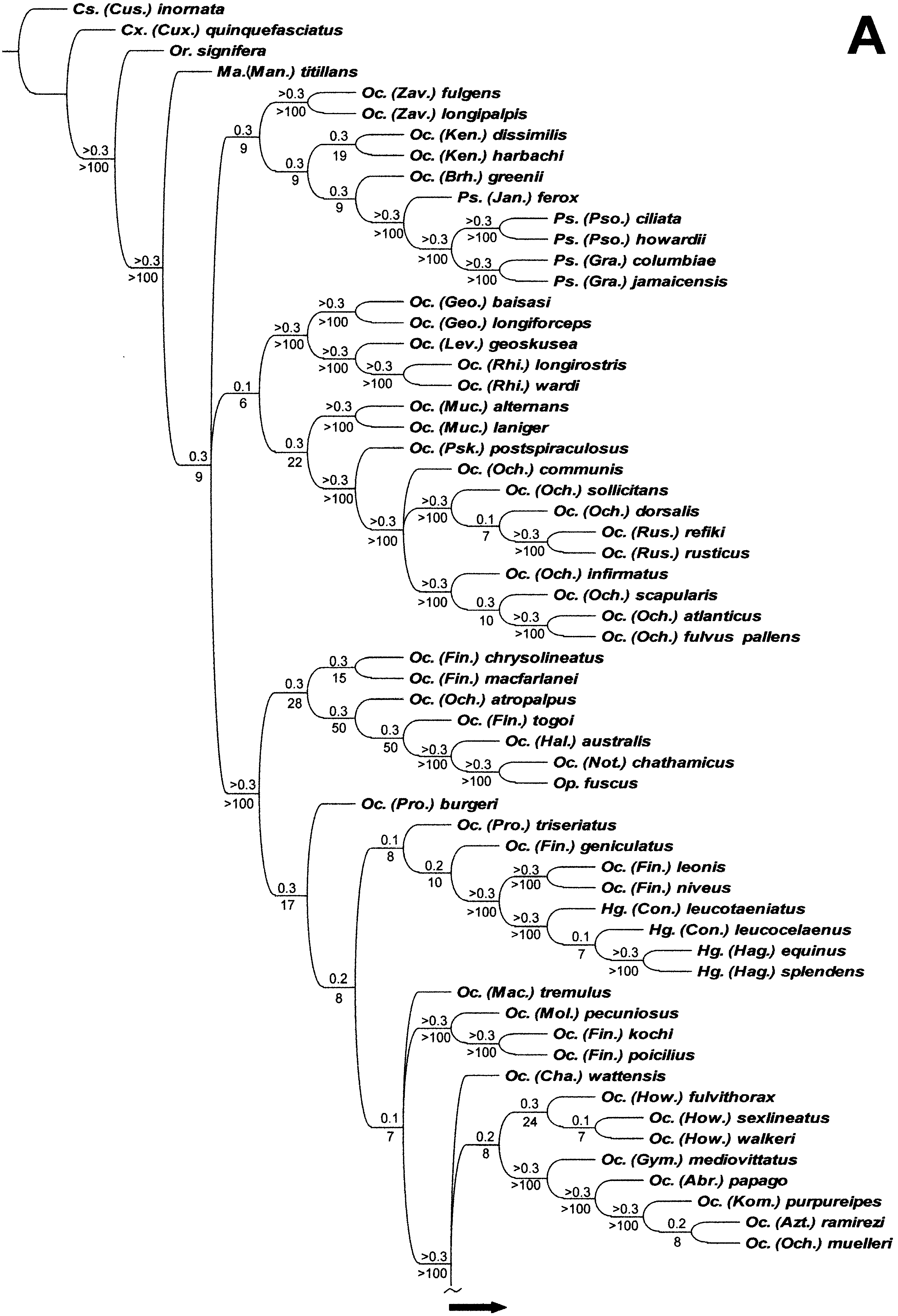
It is clear that the classification of Aedini is in need of revision and we consider that the results of the IW analysis of the combined adult and immature stages data would form the best present basis for doing so. However, we recognize that many of the included relationships are poorly supported ( Fig. 4 View Figure 4 ), with Bremer support values of only 0.1 or 0.2. The inclusion of new data or reinterpretations of our character coding could also markedly alter these groupings. A comparison with the results under EW ( Fig. 3 View Figure 3 ) shows also that many of the more inclusive groupings are dependent upon the weighting regime we employed and workers who reject differential weighting could feel justified in rejecting any resultant reclassification simply on that basis alone. We are therefore reluctant to make sweeping changes based on the patterns of relationship summarized in Figure 4 View Figure 4 .
However, we are equally reluctant to leave the generic classification of Aedini in its current parlous state, especially the two large and highly polyphyletic genera, Aedes and Ochlerotatus . Thus, we propose that a reasonable and conservative compromise would be to recognize as genera those groups that are ‘weighting independent’, i.e. those that are common to the results of both the EW ( Fig. 3 View Figure 3 ) and IW ( Fig. 4 View Figure 4 ) analyses. These groups are shown in Figure 7 View Figure 7 , which is the SCT formed from the 97 EW MPCs and eight IW MPCs.
There are 29 clades, each comprising between two and nine taxa. Another 30 taxa (including Mansonia ) fall into a basal unresolved polytomy. Nine of these are monobasic subgenera of Aedes and Ochlerotatus: Ae. ( Belkinius) , Ae. ( Fredwardsius ), Ae. ( Indusius ), Ae. ( Isoaedes ), Ae. ( Leptosomatomyia ), Oc. ( Abraedes ), Oc. ( Aztecaedes ), Oc. ( Gymnometopa ) and Oc. ( Kompia ). We propose to elevate all of these to genus level. In addition, we propose generic status for three small subgenera within the basal polytomy, i.e. Ae. ( Huaedes ) (three species), Ae. ( Skusea ) (four species) and Oc. ( Levua ) (three species), that we regard as monophyletic groups. Gymnometopa , Kompia , Leptosomatomyia and Skusea were originally recognized as genera and are thus restored to their original status. We treat the other 17 unresolved basal aedine taxa as incertae sedis (see Appendix 4). Further work is required to clarify their relationships and generic assignments. As two or more species of Ae. ( Aedimorphus ), Oc. ( Finlaya ), Oc. ( Ochlerotatus ) and Oc. ( Protomacleaya) are arrayed as singleton taxa within this group, it is most unlikely that these subgenera will be shown to be monophyletic.
Most of the clades with two or more included taxa have IW Bremer support values of at least 0.3. The exceptions are Eretmapodites + Ae. ( Alanstonea) (0.1), Ae. ( Neomelaniconion ) + Ae. ( Edwardsaedes ) (0.2) and Ayurakitia + Ae. ( Bothaella .) (0.1). Seven clades are existing genera ( Ayurakitia , Armigeres , Haemagogus , Psorophora , Udaya , Verrallina and Zeugnomyia ) and retain that status. Fifteen are presently treated as subgenera of Aedes and Ochlerotatus: Ae. ( Aedes) , Ae. ( Albuginosus), Ae. ( Bothaella ), Ae. ( Christophersiomyia ), Ae. ( Diceromyia), Ae. ( Lorrainea ), Ae. ( Paraedes ), Ae. ( Scutomyia ), Ae. ( Stegomyia ), Oc. ( Geoskusea ), Oc. ( Howardina ) Oc. ( Kenknightia ), Oc. ( Mucidus ), Oc. ( Rhinoskusea ) and Oc. ( Zavortinkius ). We propose to raise all of these to genus level. Thus seven of these taxa, Christophersiomyia , Diceromyia, Howardina , Mucidus, Paraedes , Scutomyia and Stegomyia , are reinstated to their original status.
The remaining seven clades require additional consideration. Ochlerotatus ( Finlaya) is divided into six lineages. We raise the clade consisting of Oc. ( Fin.) kochi, Oc. ( Fin.) poicilius and relatives (see Appendix 4) to generic rank as Finlaya ( type species = Culex kochi Dönitz ) and elevate Downsiomyia Vargas ( type species = Stegomyia nivea Ludlow ) from synonymy with Finlaya as the generic name for the clade comprising Oc. ( Fin.) leonis, Oc. ( Fin.) niveus and their relatives (see Appendix 4). Three of the remaining four species, Oc. ( Fin.) chrysolineatus, Oc. ( Fin.) geniculatus and Oc. ( Fin.) macfarlanei , fall within the basal polytomy of 30 taxa and are treated as ‘ Ochlerotatus ’ subgenus ‘ Finlaya ’ incertae sedis (see Appendix 4). The status of the fourth species, Oc. ( Fin.) togoi , is considered below.
The third clade comprises Ae. ( Alanstonea) and Eretmapodites . There are two options available, either raising Ae. ( Alanstonea) to genus rank or treating it as a subgenus of Eretmapodites . We choose the first, because support for the Ae. ( Alanstonea) + Eretmapodites clade is very weak (Bremer support = 0.1) and the supporting characters are all homoplastic. Further study may not corroborate a close relationship between these two taxa and so generic status for Ae. ( Alanstonea) is the more conservative course.
Fourth is a clade consisting of two Aedes subgenera, Ae. ( Neomelaniconion ) + Ae. ( Edwardsaedes ), the Bremer support for which, although not minimal, is still quite weak (0.2). We note that a unique character, 95: 1, does support this sister-group relationship (see Fig. 5C View Figure 5 ). However, it is part of a larger multistate character, the other states of which are optimized another 14 times on the cladogram, giving character 95 a total length of 15 steps (CI = 0.20, RI = 0.63). Thus, support for the Ae. ( Neomelaniconion ) + Ae. ( Edwardsaedes ) clade is not as strong as it might first appear and we again opt for the conservative line, i.e. we propose generic status for these two taxa.
The pairing of Heizmannia and Ae. ( Pseudarmigeres ) (fifth clade) has slightly higher Bremer support (0.3) but the six supporting characters ( Fig. 5 View Figure 5 ) are all homoplastic. Therefore, we choose to treat Ae. ( Pseudarmigeres ) as a distinct genus rather than reducing it to subgeneric rank within Heizmannia .
Ochlerotatus ( Ochlerotatus) (sixth clade) is divided into three lineages, two of which, Oc. ( Och.) atropalpus and Oc. ( Och.) muelleri , are part of the basal polytomy. The remaining seven taxa of Oc. ( Ochlerotatus ) analysed here, including the type species of Ochlerotatus , Oc. confirmatus Lynch Arribalzaga (currently treated as a synonym of Oc. scapularis ) form a reasonably well-supported group (Bremer support => 0.3), which can be regarded as Ochlerotatus s.s. However, nested within this group are the two species of Oc. ( Rusticoidus ). The support for this subgenus as the sister-group of Oc. ( Och.) dorsalis is minimal (Bremer support = 0.1) but that of the other branches within Oc. ( Ochlerotatus ) is much stronger (all 0.3 or higher) and the inclusion of Oc. ( Rusticoidus ) within this taxon appears reasonable. We therefore treat Rusticoidus as a subgenus within the restricted Ochlerotatus . The other species of Ochlerotatus s. s. are not assigned to subgenera as this is beyond the scope of the present study. Considerable work will be needed to resolve the relationships of these species in conjunction with those now treated as ‘ Ochlerotatus ’ subgenus ‘ Ochlerotatus ’ incertae sedis (see Appendix 4).
The final clade comprises Oc. ( Fin.) togoi, Oc. ( Halaedes) , Oc. ( Nothoskusea ) and Opifex . The support for all three branches within this clade is quite good (Bremer support = 0.3 or higher) but most supporting characters are homoplastic. The notable exceptions are two features relating to the male antennae, 93: 1 and 94: 0. These characters are part of the support for the sister-group relationship between Oc. ( Nothoskusea ) and Opifex , and are unique to these two taxa. We consider this close relationship between Oc. ( Nothoskusea ) and Opifex to be sufficiently strong to justify the transfer of Nothoskusea to Opifex as a subgenus. In contrast, the characters supporting the relationships of Opifex with Oc. ( Halaedes ) and Oc. ( Fin.) togoi are weaker and these taxa are better treated as separate genera. A new genus, Tanakaius gen. nov., is described in Appendix 3 to accommodate Oc. ( Fin.) togoi , the monotypic member of the Togoi Subgroup (subgroup VI) of Finlaya Group D ( Knight & Marks, 1952), and Oc. ( Fin.) savoryi . Halaedes (three species), Nothoskusea and Opifex (both monobasic) have distributions in the New Zealand Subregion of the Australasian Region. Halaedes also occur on the Australian continent. The distributional links between these three taxa, and especially between Nothoskusea and Opifex , provide additional evidence supporting a close relationship. The distribution of Tanakaius gen. nov. in the eastern Palaearctic is congruous with a more distant relationship.
A revised classification of Aedini based on the above proposals, which results in the formal recognition of 46 genera, is presented in Appendix 4. Those species not included in the analysis but whose classification is affected by the changes proposed here are also included. Proposed two-letter abbreviations for the genera recognized here, including applicable abbreviations recommended by Reinert (2001a), are listed in Appendix 5.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.