Eclipidrilus frigidus Eisen, 1881
|
publication ID |
https://doi.org/10.5281/zenodo.171279 |
|
DOI |
https://doi.org/10.5281/zenodo.6266042 |
|
persistent identifier |
https://treatment.plazi.org/id/039F8792-6D13-F146-FE8E-F91DA4EEFBB4 |
|
treatment provided by |
Plazi |
|
scientific name |
Eclipidrilus frigidus Eisen, 1881 |
| status |
|
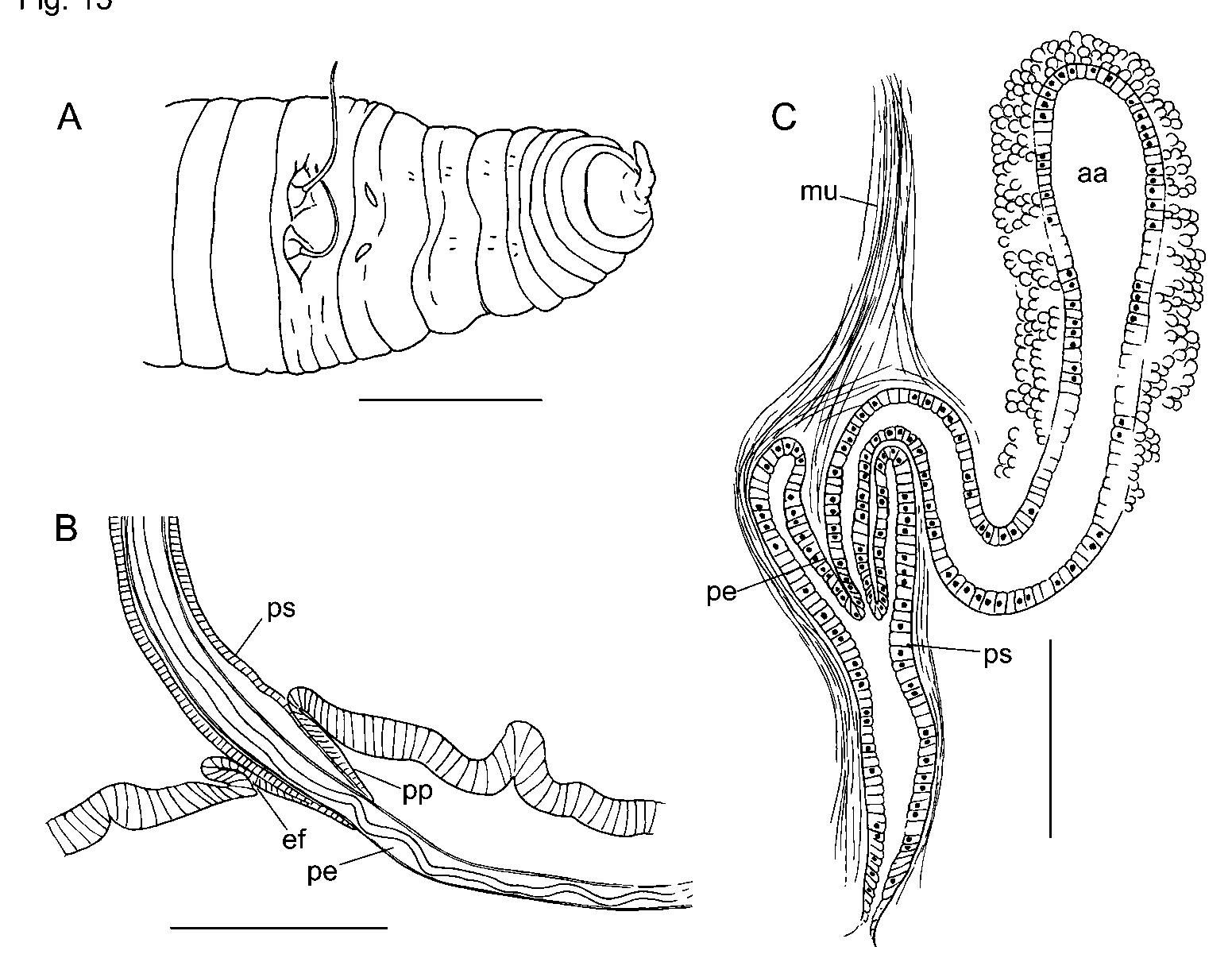
Eclipidrilus frigidus Eisen, 1881 ( Figures 11–12 View FIGURE 11 View FIGURE 12 )
MATERIAL EXAMINED: California: Amador Co.: Mokelumne River near Tiger Creek afterbay, collected by W. C. Fields, 8.VIII.1999. 1 dissected. Calaveras Co.: small spring near Calaveras Big Trees State Park, 10.XI.1996. 2 whole mounts. Plumas Co.: Upper Butt Valley Creek, collected by W. C. Fields. 1 whole mount, 6 dissected. Sierra Co.: Big Spring near Bassets, at North Yuba River, 39º35’47”N, 120º36’38”W, 9.VIII.1996. 1 dissected, 6 sagittally sectioned, 1 transversely sectioned. 4.XI.2002. 6 dissected. Trinity Co.: small creek, east side of Clair Engle Lake, 13.V.2001. 1 dissected. Tuolumne Co.: North Fork Stanislaus River, 4.XII.1996, collected by B. Quelvog. 2 dissected. Yuba Co.: Jackass Creek at Deadwood Creek, tributary to North Yuba River, 39º32’48”N, 121º05’34”W, 26.IX.2000. 10 dissected, 5 sagittally sectioned, all partially mature. Deadwood Creek, 5.X.1999, collected by W. C. Fields. 1 dissected, 1 whole mount. Idaho: Boise Co.: Little Gallagher Creek, tributary to South Fork Payette River, 30.V.1996. 1 dissected, 1 sagittally sectioned. Idaho Co.: Crooked River, 45º47’29”N, 115º32’47”W, 1.VII.2002, collected by D. L. Gustafson. 2 dissected. O'Hara Creek at Selway River, 46º05’06”N, 115º31’03”W, 3.IV.2001, collected by D. L. Gustafson. 2 dissected. Rainy Day Creek at mouth, 45º48’10”N, 115º41’38”W, 1.VII.2002, collected by D. L. Gustafson. 2 dissected. Snake Creek at mouth, 45º51’00”N, 114º44’24”W, 7.X.2001, collected by D. L. Gustafson. 1 dissected. Worms collected by S. Fend unless otherwise noted.
Supplementary description
Dorsal wall of pharynx much thicker than ventral wall anterior to IV; esophagus begins near 4/5. Some specimens with branched lateral blood vessels in posterior 1/3 of body, as described by Eisen (1885); other specimens with very short, blind lateral vessels that do not appear to be branched, and others with no apparent lateral vessels in posterior segments. Nephridial arrangement and morphology as described for E. pacificus (see above). Specimens from Idaho have small, external papillae posterolateral to the male pores ( Fig. 11 View FIGURE 11 A).
Penial structures very long (1000–1700 µm) and cylindrical, covered with a longitudinal muscle layer and a very thin, inner circular muscle layer. The muscle tube surrounds a thick layer of narrow epithelial cells, with basal nuclei. In the ectal 1/2 to 2/3 of the structure, these cells are very elongate and appear fibrous, similar to muscle tissue ( Fig. 12 View FIGURE 12 C), and the lumen is narrow and possibly cuticular. The lumen is variably coiled in most specimens ( Fig. 11 View FIGURE 11 C,F), but may straighten when the penis is extruded ( Fig. 11 View FIGURE 11 B). The lumen gradually widens and becomes ciliated in the ental portion of the penial structure.
Penial structures terminate on short papillae (or “collars” as termed by Eisen 1895), contained within small sacs ( Figs. 11 View FIGURE 11 F, 12A; see also Brinkhurst [1998, Fig. 1 View FIGURE 1 B]); ectally, the sacs terminate in small folds in the epidermis, which form the external male pores ( Fig. 11 View FIGURE 11 F). Small retractor muscles join the sacs to the body wall, dorsally at 10/11. In most mated specimens, fibrous extensions of lining cells are extruded through the terminal papillae to some extent, forming the actual penes. The extruded penes form a loose spiral, and may be contained within the penial sac ( Figs. 11 View FIGURE 11 F, 12A) or extruded beyond the body wall ( Figs. 11 View FIGURE 11 B–C). Male pores without distinct accessory glands.
Atrial ampullae 450–1450 µm long; nearly cylindrical but tapered ectally. Musculature of atrial ampulla with outer layer 7–24 (12) µm thick, of variably spiral muscle, with fibers in thin, radiating lamellae ( Fig. 12 View FIGURE 12 E) usually arranged at an angle about 20º to the long axis ( Fig. 12 View FIGURE 12 D), but ranging from parallel in some specimens to about 50º in one Idaho specimen. Inner, transversecircular muscle layer 1–5 µm thick, orthogonal to the long axis of the atrium ( Fig. 12 View FIGURE 12 E). Thickness of both muscle layers decreases with distension of atrium. Vasa deferentia narrow, diameter about 12 µm. Anterior male funnels with or without sperm, slightly smaller and thinner than posterior pair; testes in IX usually smaller than those in X. Numerous prostate glands are petiolate bundles of less than 10 cells each; length 25–50 µm ( Fig. 12 View FIGURE 12 E).
Spermathecal ducts complex. Ectal end of duct apparently an epidermal fold up to 200 µm deep; this section surrounded by a distinct transversecircular muscle layer under a thinner longitudinal layer ( Figs. 11 View FIGURE 11 G–I, 12F). In middle portion, each spermathecal duct widens into a globular expansion, 110–200 µm wide; elongate lining cells form irregular, transverse lamellae extending into the lumen ( Figs. 11 View FIGURE 11 G–I, 12F). Epithelium of spermathecal ampullae similar throughout or up to twice as thick entally versus ectally; cells are never highly vacuolated. Ampullae may be irregularly ovate ( Fig. 11 View FIGURE 11 H), or rounded ectally, with a narrowed ental “diverticulum” ( Fig. 11 View FIGURE 11 G). Sperm loosely packed throughout ampullae in all mated worms.
Remarks
Earlier descriptions of E. frigidus copulatory structures have been somewhat contradictory, due to limitations in the available material. Wassell (1984) stated that the penis was “formed from tissue lining anterior inside of atrium”, but also made the confusing statement that it was “continuous with the body wall muscles anteriorly”. Brinkhurst (1998) noted that in some lumbriculids the penis is composed of elongated lining cells, and Figure 1 View FIGURE 1 B of that paper showed a retracted E. frigidus penis with what appear to be elongate, fibrous lining cells, as in the new material. Nevertheless, he suggested that the penis of E. frigidus was eversible because the lining at the ectal end appeared detached from the muscle in a lectotype. The lining did not appear detached in the new material ( Fig. 12 View FIGURE 12 C), suggesting that the appearance of the lectotype specimen was an artifact of fixation.
Material examined by the above authors apparently did not include specimens with extended penes, but several of the new specimens have extended penes that appear to be formed of extruded (elongated) atrial lining cells. Gradual modification of the atrial duct lining was observed in partially mature worms ( Figs. 11 View FIGURE 11 D–F and 12A–B); this derivation of the penial structure contrasts with the development of pendant penes in E. palustris (see below, Fig. 15 View FIGURE 15 C). Coiling of the lumen in fully mature E. frigidus may be caused by contraction of a cuticular lining within the retracted penis, which further implies that the penis is extended by elongation rather than eversion. Other than the short papilla or collar within the ectal sac, there is no obvious pendant penis at any stage of development. Therefore, the primary mechanism appears to be extrusion of the duct lining. Although none of the new specimens had everted penial sacs, Brinkhurst (1998, Fig. 1 View FIGURE 1 B) indicated that the sac is eversible, and may also contribute to penial extension.
The musculature of the atrial ampulla was not discussed in detail by Brinkhurst (1998), although it was described as “spiral” in one specimen. Eisen (1895) described a thin circular layer and an exterior “spirally wound layer of longitudinal muscles” covering the penial structure, and implied that the same layers also covered the ampulla. The degree of spiraling in the new material, here indicated by divergence of the muscle fibers from the longitudinal axis, varies considerably within and among specimens, but is usually less than in the E. ( Premnodrilus ) or E. ( Leptodrilus ) species. Neither muscle layer has the crosshatched pattern seen in the inner muscle layer of E. lacustris (see below).
Somatic characters of the new material generally agree with the original description ( Eisen, 1895), except that the branching of lateral blood vessels in posterior segments is usually not pronounced. However, the lateral vessels were often difficult to see, especially in worms with gut contents. Eisen described nephridia as occurring in several preclitellar segments, as far forward as 3/4, but the most anterior nephridia in the new material were always on 6/7, and the next on 12/13.
Eclipidrilus frigidus has only been confirmed from California and Idaho. Mature Idaho specimens have small, external lobes, possibly functioning as claspers, posterolateral to the male pores on 10/11; these were not seen in the California material. Despite the apparent geographic range disjunction, there is little else to distinguish specimens from these two regions.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |