Ceropegia strophanthiflora Heiduk & D.Styles, 2023
|
publication ID |
https://doi.org/ 10.11646/phytotaxa.632.1.2 |
|
DOI |
https://doi.org/10.5281/zenodo.10435146 |
|
persistent identifier |
https://treatment.plazi.org/id/039B87FC-FFC4-BA12-5099-FA5FFBA4412D |
|
treatment provided by |
Plazi |
|
scientific name |
Ceropegia strophanthiflora Heiduk & D.Styles |
| status |
sp. nov. |
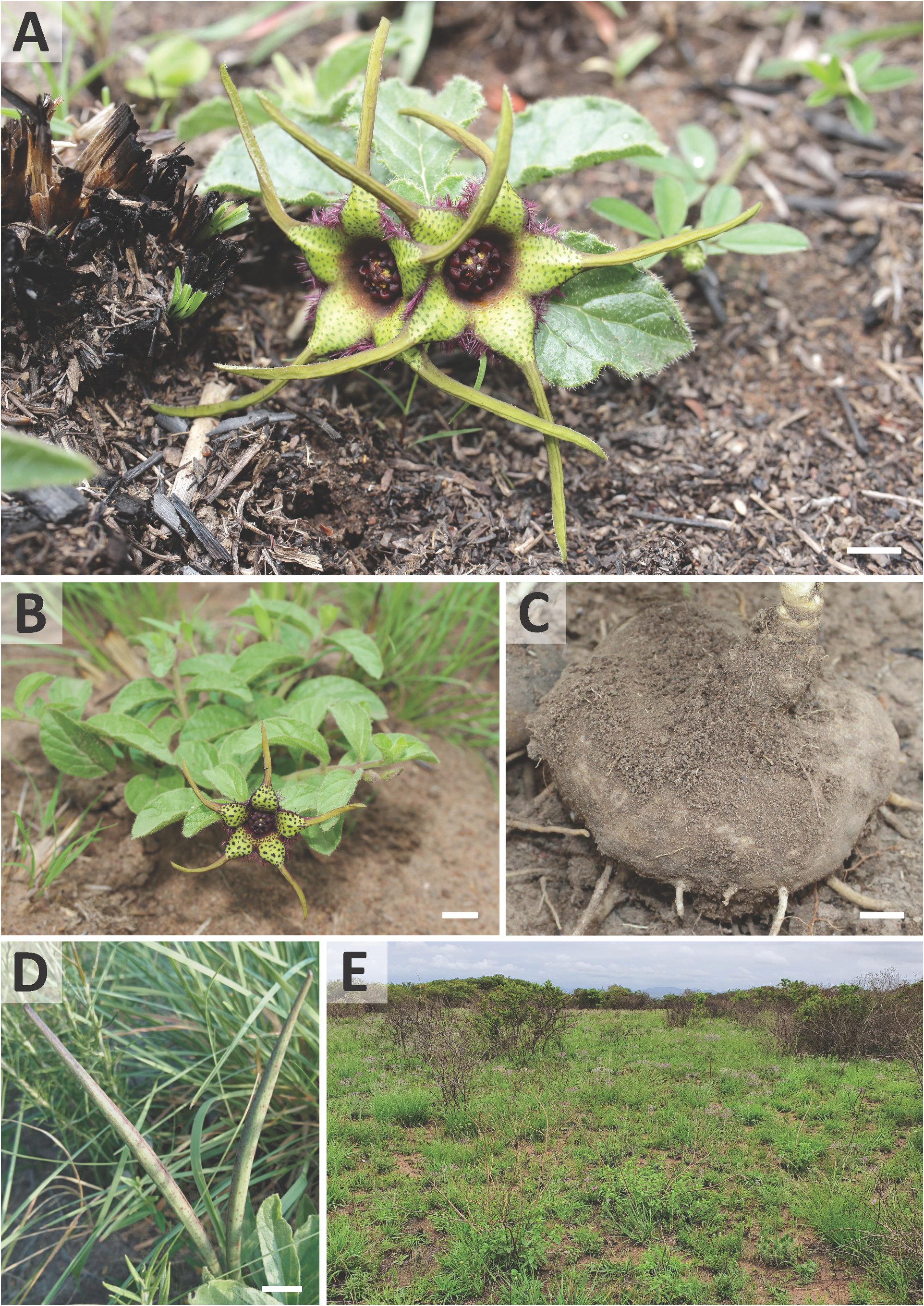
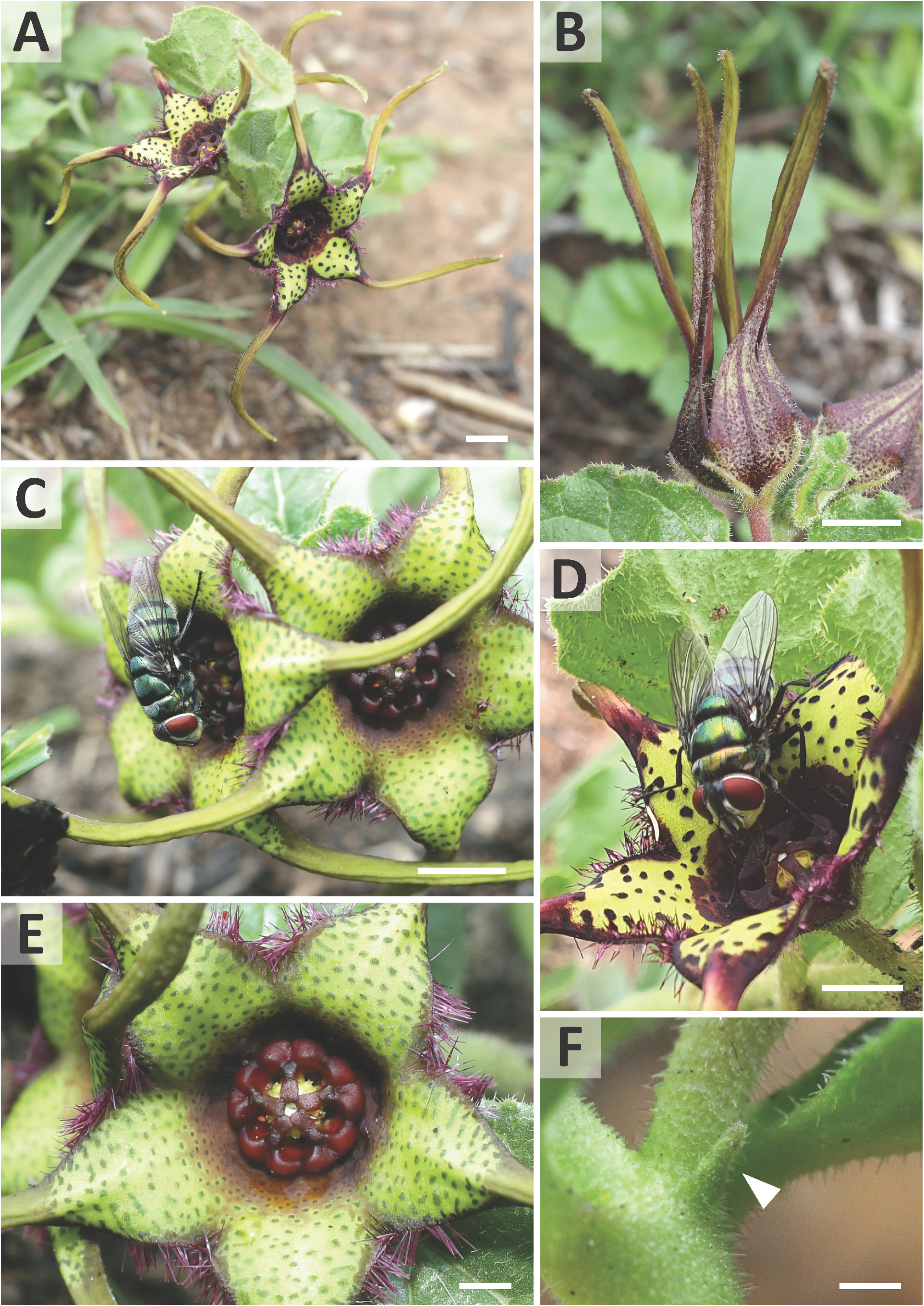
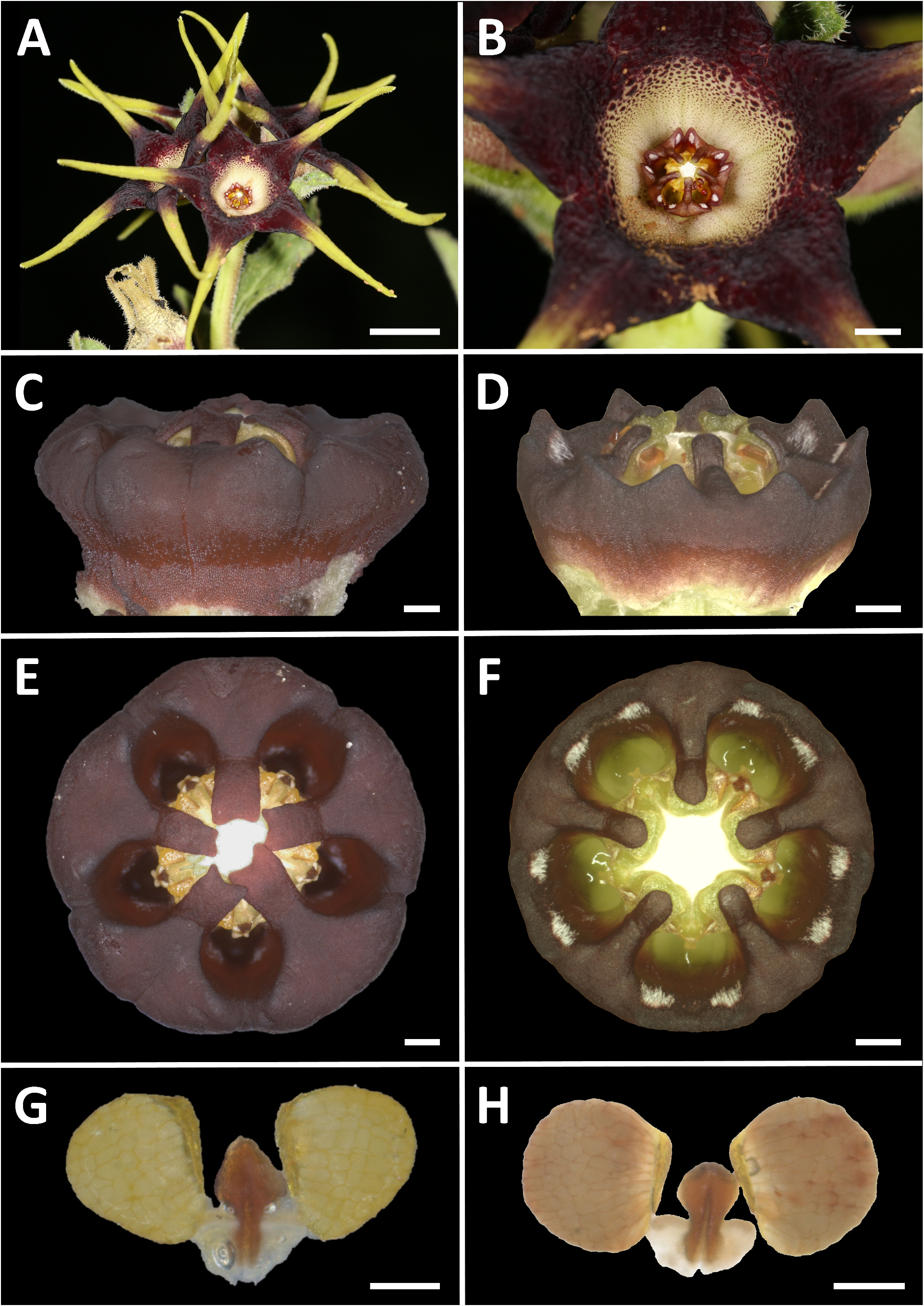
Ceropegia strophanthiflora Heiduk & D.Styles , sp. nov. ( Figs.1 View FIGURE 1 , 2 View FIGURE 2 , 3C,E,G View FIGURE 3 ).
Type: — SOUTH AFRICA. KwaZulu-Natal: inland of Mtubatuba, ca. 160 m, 08 November 2019, D.G.A. Styles & A. Heiduk 5773 (holotype NU! [ NU0094577 ]) .
Diagnosis: — Ceropegia strophanthiflora differs from C. rehmannii by longer petioles up to 4 mm long (vs. ± 1 mm in C. rehmannii ), usually 2-flowered inflorescences (vs. generally 4-flowered in C. rehmannii ), centrally purple flowers (vs. centrally creamish-yellow in C. rehmannii ) with greenish-yellow, purple speckled corolla lobe bases (vs. dark purplish-maroon in C. rehmannii ) and purple vibratile trichomes on margins of the corolla lobe bases (vs. glabrous in C. rehmannii ). Corolla lobe tips are more slender, much longer (15–27 mm vs. 10–25 mm in C. rehmannii ), reflexed and twisted (vs. usually straight and held upwards in C. rehmannii ). The gynostegial corona of C. strophanthiflora has thick, rectangular staminal lobes (vs. linear in C. rehmannii ) exceeding the anthers (vs. not exceeding anthers in C. rehmannii ), lacks distinct interstaminal corona lobes (vs. present in C. rehmannii ), and is glabrous throughout (vs. with tufts of white hairs on interstaminal lobes in C. rehmannii ).
Description: —Perennial herb. Tuber 60–80 mm in diam., ± 50 mm high. Stems 1–2, sub-erect or spreading, to 135 mm tall, ± 2 mm in diam., branched at first or second internode, annual, green, hirsute to puberulous, internodes 13–18 mm long. Leaves ovate to broadly lanceolate, 25.5–51.5 × 14.0– 22.5 mm, apex obtuse to acute, petiole 2–4 × 1.5 mm; lamina hirsute above and below; leave margins brownish, undulate, puberulous. Inflorescences extra-axillary, 1–2 flowered; bracts 1.7–2.0 mm long, narrowly triangular; flowers with foul scent reminiscent of dung, anthesis 1–2 days. Pedicel in ± 90° angle from stem, ± 5.5–8.0 × 1.3 mm, green often with brownish-purplish colourations, hirsute to puberulous. Sepals narrowly lanceolate, 5.5–6.5 × 1.5 mm, green often with brownish-purplish colourations, puberulous, ascending along the corolla base with often incurved tips. Corolla open-rotate, ± 52 mm in diameter. Corolla tube shallowly campanulate, ± 4.5–6.5 mm deep, ± 13 mm in diam., proportionally 1/5 or less of total corolla length, inside dark purple-brownish with darker spots, glabrous; outside brownish-purplish, scabrid. Corolla lobes spreading to reflexed, ± 4–5 times longer than tube, 15–27 mm in total length; corolla lobe bases 5.5–6.0 mm broad at base, 5.5–6.0 mm long, keeled from about half way, basally ovate-triangular, distally attenuate-tapering, merging into slender corolla lobe tips, lush green to greenish-yellow, dark-green or purple speckled, margins revolute, margin brownish-purple and densely fringed with filiform-subulate, purple vibratile trichomes of 1.5–2.0 mm length, otherwise glabrous above, scabrid below; corolla lobe tips confluent with lobe bases, caudate, slender, narrowly lanceolate, ± 15–17 mm long, revolute, slightly longitudinally furrowed, twisted, yellowish-green, glabrous above, puberulous below. Gynostegium sessile, dark purple throughout, robust and fleshy-sturdy. Gynostegial corona 4.7–5.5 mm in diam., ± 2.5 mm high, of complanate staminal and interstaminal parts, interstaminal corona lobes joined forming a cup with V-shaped thick margin, lobules reduced to obtuse bulges, confluent with inner lobes, glabrous; staminal corona lobes broadly linear-oblong, ± 2.5–3.0 × 0.6 mm, appressed to and arising along the anthers and stamen, with slight groove at their base, descending on style-head, not totally covering the latter, tips sometimes bilobed, partly overlapping irregularly, glabrous. Pollinarium with broadly ovoid pollinia tapering towards corpusculum, ± 450 × 380 µm, yellowish, with narrow insertion crest ± 250 µm long and 50 µm broad; caudicles ± 100 µm long; corpusculum sagittate, ± 390 × 215 µm, reddish brown. Follicles usually with both mericarps developed, erect, narrowly fusiform tapering at the tip, ± 80 mm long and 6 mm in diam., glabrous. Seeds linear-oblong, flattened, with broad margin, comose, coma white, ± 1 cm long.
Additional material examined
Ceropegia strophanthiflora :— SOUTH AFRICA. KwaZulu-Natal: inland of Mtubatuba, ca. 158 m, 28 November 2020, D. G. A. Styles & A. Heiduk 6142 (flowers in ethanol NU! [ NU 0094579]).
Ceropegia rehmannii :— SOUTH AFRICA. KwaZulu-Natal: Umgungundlovu District, north of Wartburg, ca. 735 m, 26 October 2020, D. G. A. Styles & A. Heiduk 6141 ( NU! [ NU 0094578]).
Distribution and habitat: — Ceropegia strophanthiflora occurs in an area of transition from Zululand Coastal Thornveld to Zululand Lowveld and falls within the south-western edge of the Maputaland Centre of Plant Endemism. These vegetation types are described in Mucina & Rutherford (2006), where assessed as Endangered and Vulnerable respectively ( Rutherford et al. 2006). South Africa’s 2018 National Biodiversity Assessment (NBA), which treated ecosystem types according to the International Union for Conservation of Nature (IUCN) Red List of Ecosystems (RLE) Framework ( Bland et al. 2017), reassessed them as Critically Endangered and Least Concern respectively ( Skowno et al. 2019). Subsequently, Zululand Coastal Thornveld was declared a Critically Endangered ecosystem in the Revised National List of Threatened Ecosystems ( South African Government 2022), where it is stated that it is “narrowly distributed with high rates of habitat loss in the past 28 years (1990–2018), placing the ecosystem type at risk of collapse”.
The population of Ceropegia strophanthiflora occurs within the grassland component of a thicket and grassland mosaic which appears to have been relatively protected from grazing and browsing by livestock. Review of aerial imagery and follow-up visits to the type locality indicate that the vegetation is infrequently burnt and the grassy growth may remain moribund for many years. Consequently, grassland habitat at the type locality is experiencing a significant degree of bush encroachment, with problem species including Dichrostachys cinerea ( Linnaeus 1753: 520 [no. 25]) Wight & Arnott (1834: 271), Lippia javanica Sprengel (1825: 752) and the weedy herb Helichrysum kraussii Schultz ‘Bipontinus’ (1844: 679). It is additionally invaded by the alien invasive species Chromolaena odorata ( Linnaeus 1759: 1205) R.M.King & H.Robinson (1970: 204) , Lantana camara Linnaeus (1753: 627) and a Eucalyptus L’Héritier (1788: 18) species. Interventions are urgently needed to protect the population and must include destroying woody and scrubby encroachers (requiring both cutting and use of herbicide), alien plant control and regular burning.
The most common constituents of bush clumps and thicket within the habitat are the following shrubs or small trees: Coddia rudis (E.Meyer ex Harvey 1859: i 22) Verdcourt (1981: 509), Diospyros dichrophylla ( Gandoger 1918: 56) De Winter (1963 : xxvi. 75), Euclea daphnoides Hiern (1873: 102) , Gymnosporia maranguensis Loesener (1908: 303) , G. senegalensis ( Lamarck 1785: 661) Loesener (1893: 541) and Scutia myrtina (Burman f. 1768: 60) Kurz (1876: 168) . Some are spiny and most are likely unpalatable or toxic to livestock. Aloe parvibracteata Schönland (1907: 139) is also quite common. Less often encountered are Acacia nilotica ( Linnaeus 1753: 521) Willdenow ex Delile (1813: 79) , Grewia occidentalis Linnaeus (1753: 294) , Hippobromus pauciflorus Radlkofer (1895 : iii, 5), and Trichilia emetica Vahl (1790: 31) . Herbaceous species co-occurring with C. strophanthiflora include Acrotome hispida Bentham (1848: 436) , Stylosanthes fruticosa (Retzius 1779–1791: Fasc. v. 26) Alston (1931: vi. suppl., 77), Crabbea hirsuta Harvey (1842: 27) , Gnidia capitata Linnaeus (1782: 224) , Justicia anagalloides T. Anderson (1863: 42) , Macledium zeyheri (Sonders) S.Ortiz subsp. argyrophullum ( Oliver 1884: t. 1461) S. Ortiz (2001: 743), Thunbergia atriplicifolia E. Meyer (1847: 226) , Vernonia natalensis Schulz ex Walpers (1843: 947) , and Waltheria indica Linnaeus (1753: 673) .
The type locality is situated within a larger natural area enclosed by a patchwork of informal settlement, next to which the heavy utilization of veld by cattle and goats has reduced much of the grass cover to near lawn-like consistency. While there is still some herbaceous grassland plant diversity, this tends to comprise unpalatable or very low-growing species. It is nonetheless possible that some plants could still occur here, but these conditions are not conducive to long-term survival.
A further issue highlighted is expansion of coal mining inland of Mtubatuba in future. Mining may displace existing settlement, and by providing improved access and temporary economic and employment opportunities, promote new and increased settlement with all of its accompanying impacts. This can transform potential habitat and unless it is well managed and mitigated cause further encroachment on the type locality.
Phenology: — Ceropegia strophanthiflora was seen in flower between November and January. Fruits were seen in January.
Etymology: —The specific epithet ‘ strophanthiflora ’ refers to the unusually long, caudate and twisted, reflexed corolla lobes reminiscent of flowers found in the genus Strophanthus .
Conservation status: — Ceropegia strophanthiflora is only known from the type locality which lies within a highly transformed area. Not more than 10 individuals were found to occur at this locality. The habitat is critically threatened by grazing, poor fire-management of grassland, bush encroachment and above all by continuing and expanding settlement and accompanying disturbance. We explicitly recommend that the conservation status of C. strophanthiflora is declared to be Critically Endangered (CR) under Criteria B 1(a)(b), C2(a)(i) and D (IUCN Standards and Petitions Subcommittee 2019). This species appears to be at the brink of extinction and there is a dire need to protect this exceptionally rare species, including from unscrupulous private collectors.
| NU |
Department of Microbiology, Faculty of Science |
| G |
Conservatoire et Jardin botaniques de la Ville de Genève |
| A |
Harvard University - Arnold Arboretum |
| B |
Botanischer Garten und Botanisches Museum Berlin-Dahlem, Zentraleinrichtung der Freien Universitaet |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.