Eucyclops Claus, 1893
|
publication ID |
https://doi.org/ 10.1080/00222933.2014.897766 |
|
persistent identifier |
https://treatment.plazi.org/id/039B7E0D-D404-FFC4-FD99-D07C670CFAA7 |
|
treatment provided by |
Felipe |
|
scientific name |
Eucyclops Claus, 1893 |
| status |
|
Genus Eucyclops Claus, 1893
Eucyclops elegans ( Herrick, 1884)
Material examined
Staatliches Museum für Naturkunde, Karlsruhe: one ♀ from Mammoth Cave National Park , Kentucky, USA (SMNK-01144) coll. Professor Jeannel. Muséum National d’ Histoire Naturelle, Paris : one ♀ (MNHN-Cp7225) and one ♂ (MNHN- Cp7226) from Argentina, coll. S.M. Frutos. National Museum of Natural History Smithsonian Institution, Washington DC: one ♀ (USNM-251652) from Elbow Lake Creek , Becker County, Minnesota, USA, coll. D.K. Shiozawa (date of collection not available) and one ♀ (USNM-242280) from Río Cai, Río Grande do Sul, Brazil, collected by P.T.C. Chaves on 24 April 1982 . Zooplankton Collection at El Colegio de la Frontera Sur, Chetumal: one ♀ (ECO-CH-Z-04948) from Arroyo en Sierra Fría , 21 mm of Norte de la Labor, Calvillo, Aguascalientes, Mexico, collected by M. Silva-Briano, 18 February 1989 .
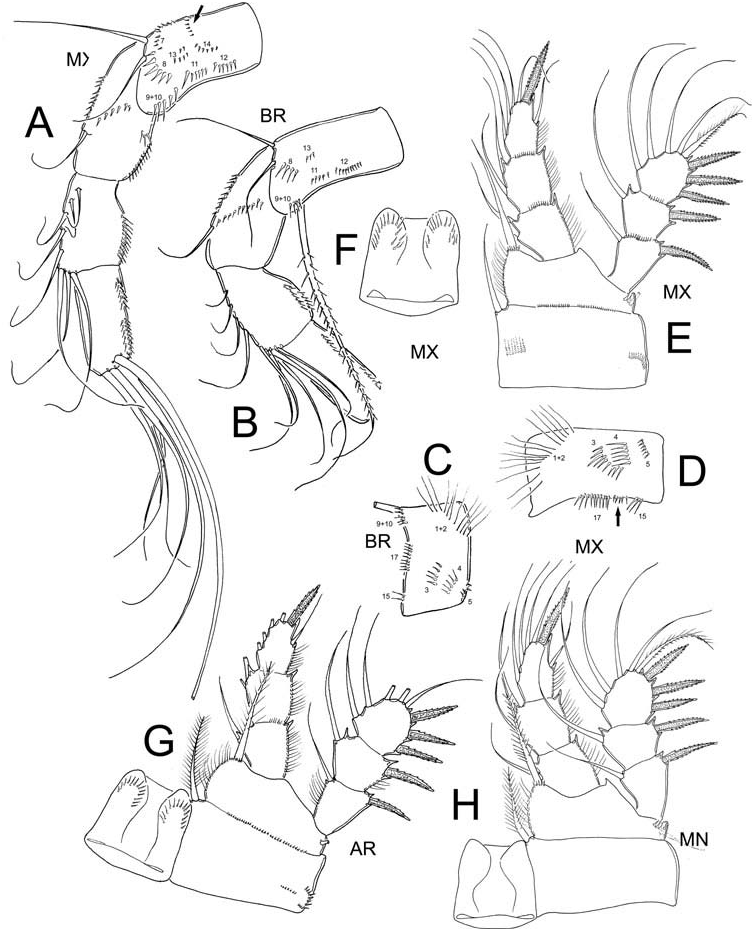
Among the main meristic and morphometric values used for the determination of species within the genus Eucyclops ( Reid 1985; Morton 1990; Dussart and Defaye 2001; Suárez-Morales 2004; Alekseev et al. 2006) only slight differences were found among the populations examined, although details of the ornamentation of the antennary basis are important to mention. The main measurements are summarized in Table 1.
Morphological remarks and comparisons of females
The adult females from MN and MX (1.2–1.4 mm) are slightly longer than the AR specimens (1.1 mm). In addition, the MX organisms have the entire body ornamented
Inner spine/length – 1.0 1.0 0.9 1.0 1.0 1.0 0.9 Enp3 P4
Outer spine/length – 0.7 0.8 0.7 0.8 0.8 0.8 0.7 Enp3 P4
Inner/outer spines – 1.4 1.2 1.3 1.3 1.2 1.3 1.3 Enp3 P4
Lateral inserted – 69 65 68 64 65 70 62
Length/width P5 – 1.7 1.2 – 2.3 1.2 – 1.9
Medial/outer – 1.6 2.1 – 2.3 2.4 – 1.7 setae P5
Medial seta/inner – 1.4 1.4 – 1.6 1.7 – 1.4 spine P5
Inner spine/length – 2.6 3.8 – 3 2.6 – 2.6 P5
with small pits forming cuticular patterns (see Figure 1E View Figure 1 ) which were not observed in the other groups of specimens. In all cases the prosome is expanded at the end of the cephalosome and following segment, representing 52–54% of the total body length. The prosomal fringes are finely serrated on dorsal surface (observed in MX specimens). The shape of the seminal receptaculum was among the main differences we observed in the material examined. In specimens from KN, MX and AR ( Figure 1B, E View Figure 1 ), this structure has the typical shape of the serrulatus group (see Alekseev et al. 2006), but in figures presented by Dussart and Frutos (1986) of material from AR, the posterior lobe is expanded and rounded (see Figure 25 in Dussart and Frutos 1986). The anal operculum in MX specimens is rounded and slightly serrated, while in AR specimens and also in those depicted by Dussart and Frutos (1986) from Argentina, the anal operculum is rounded but smooth. The length/width ratio of the caudal rami ranges between 6.7 and 8.0 in all specimens examined. Remarkably, the dorsal seta ( VII) is slightly shorter in the North American specimens (KN, MN, MX) than in the South American (AR) specimens, with a dorsal seta/caudal ramus length ratio = 0.4 in the former group and 0.5–0.6 in the latter ( Figure 1A–C, E View Figure 1 ). In addition, the dorsal seta ( VII)/outermost caudal seta ( III) ratio is variable in all specimens, the figure being 0.6 in the MX, 0.8 in MN, 1.0 in KN and 1.1 in AR. This ratio was not determined in the BR specimens. The innermost caudal seta ( VI)/outermost caudal seta ( III) ratio slightly differs among populations; in the MX and MN specimens the value is 1.0–1.1, whereas it is 1.2–1.3 in the other populations examined.
The antennules reach the posterior margin of the third suture of the prosome in most specimens groups, except for the MX material, in which antennules are slightly longer, reaching the posterior margin of the fourth suture of the prosome. The main differences we observed among the different specimens examined are in the ornamentation pattern of the antennary basis. In both MX and BR the frontal surface has the same pattern except for the MX specimens having all spinule rows with more elements than in the BR (see Table 2, Figure 2A, B View Figure 2 ). It is important to emphasize that in both populations the spinules of groups N1 and N2 are continuous; they lack a gap between them as in the typical serrulatus group ( Alekseev et al. 2006; Alekseev and Defaye 2011). The ornamentation pattern on the caudal surface also differs among populations; in the BR specimens we did not observe rows N7 and N14 which are present in the MX populations. Furthermore, the MX specimens have two extra rows of spinules (arrowed in Figure 2A, D View Figure 2 ) on the caudal surface, one between rows N15 and N17 and one on the frontal surface between N13 and N14. We had access to only one BR specimen so we could not confirm the presence or absence of these rows, but this character should be reviewed in South American populations. If such differences are consistent, they could represent a character strong enough to consider the BR specimens as representative of a separate species.
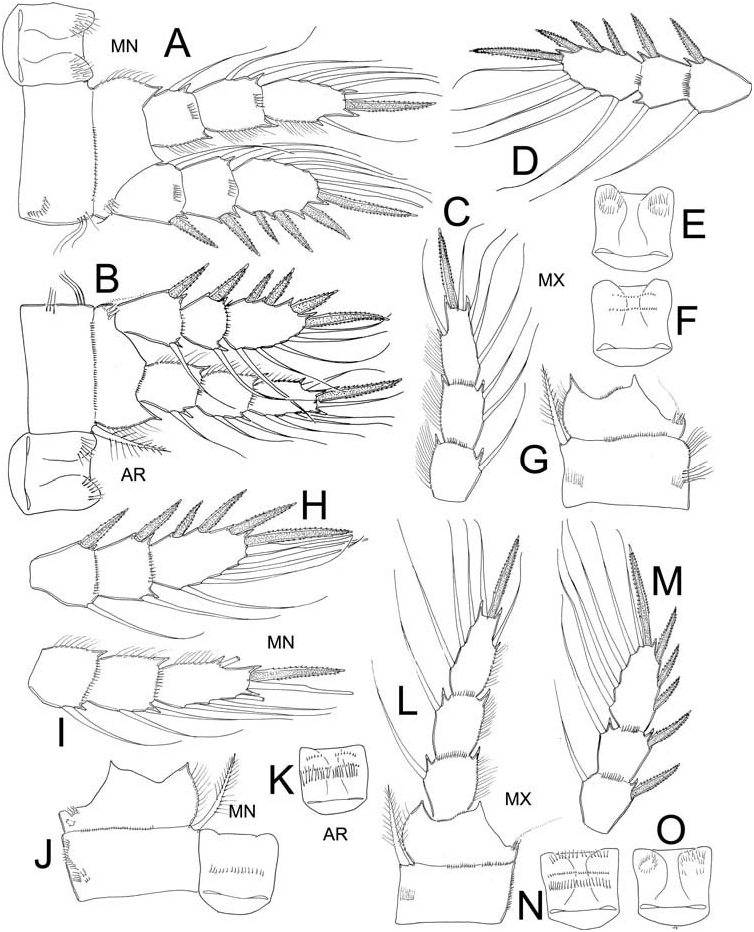
The slight differences of the ornamentation, armature and proportions of the Enps and Exps of the first three swimming legs are summarized in Table 1 and shown in Figures 2 View Figure 2 and 3 View Figure 3 . The coxal spinules formula of the fourth swimming leg was available only from MN (A-B-C + D-G-H), BR (A-C + D-E-G-H), and MX (A-C + D-E-F-G-H-J). The ornamentation of the intercoxal sclerite of leg 4 was also examined in these populations. In all specimens Row I of the intercoxal sclerite bears long hair-like spinules, the exception is the MX material which bears small, strong spinules (see Figure 4G, H View Figure 4 ). Row II and III are present in both BR and MX specimens. Row II bears long, strong spinules and is divided into three sections: two are close to the outer margins and the third is located on the middle surface (arrowed Figure 4E View Figure 4 ) in the BR specimens, while in MX this row is continuous and armed with short but strong spinules (arrowed Figure 4G View Figure 4 ). The inner coxal spine has a heteronomous ornamentation in all specimens; the inner margin bears long hairs basally and spinules distally, while the outer edge has 2–4 distal spinules and long hair-like elements basally.
The third endopodal segment of the fourth swimming leg is remarkably longer in the North American specimens, its length/width ratio ranging between 3.2 and 3.5, while in the South American forms this ratio is 2.4–2.7. The inner spine/length Enp3 P4, outer spine/length Enp3 P4, inner/outer spines Enp3 P4 ratios and the insertion point of the lateral caudal seta do not show differences among specimens (see Table 2).
In the populations of E. elegans examined the medial seta of the fifth leg is always the longest and the outer seta is shortest; the inner spine is long and strong, always longer than the outer seta. In the North American specimens, the inner spine/length
of segment P5 ratio (2.6–2.7) clearly differs from that in the South American populations (3.0–3.8).
Morphological remarks and comparison of males
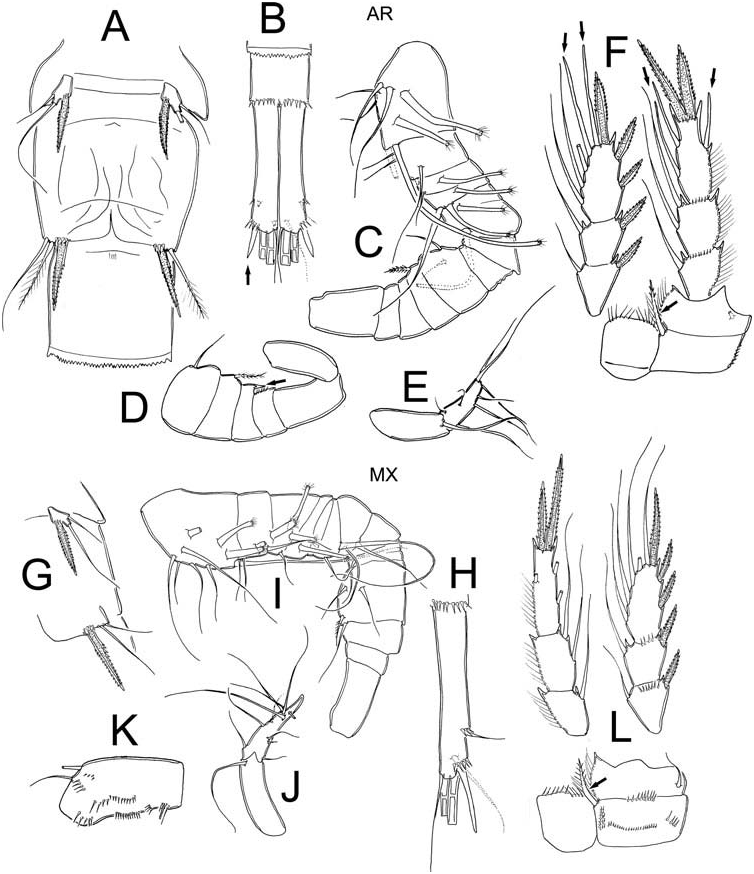
It was possible to examine the males from the AR and MX populations; figures of males were only available in Dussart and Frutos (1986) from Argentina. We provide a complementary description of the basic structures of male E. elegans of both populations (AR, MX); values between brackets correspond to those available from Dussart and Frutos (1986).
The urosome is six-segmented and slightly elongated; the urosomal fringes are strongly serrated in both populations examined. The caudal ramus is smooth along both the inner and outer margins, with strong spinules at the insertion of lateral seta (both AR and MX). The length/width ratio of caudal ramus was 6.1 in both populations (5). Dorsal seta ( VII) of both MX and AR were 0.5 (0.4) times as long as the caudal ramus and 1.8 (1.4) times as long as the outermost caudal seta ( III) in AR and 1.2 in MX; the outermost caudal seta were wider and blunt in AR specimens (arrowed Figure 5B View Figure 5 ). The innermost caudal seta ( VI)/outermost caudal seta ( III) ratio was 1.6 (1.6) in AR and 1.1 in MX. The lateral caudal seta ( II) is inserted at 75% in AR while it is at 71% (73%) of ramus length in MX. The armature of the antennules is as follows. In AR ( Figure 5C–D View Figure 5 ) it is 15-segmented: 1(5s + 3m), 2(4s), 3(1s + 1ms), 4(1s + 2ms), 5(1ms), 6(1s), 7(0), 8(0), 9 (2s), 10(1sp), 11 (cuticular protuberance arrowed Figure 5D View Figure 5 ), 11(0), 12(0), 13(0), 14(1), 15(9). MX ( Figure 5I–J View Figure 5 ) has 15 segmented antennule but with some differences: 1 (6s + 3ms), 2(4s + 1ms), 3(1 + 2ms), 4(1ms), 5(0), 6(2s), 7(3s), 8(0), 9(1s), 10(4s), 11(0), 12(0), 13(0), 14(1), 15(9s + 1sp). The intercoxal sclerite of leg 4 shows Row I bearing long hair-spinules in AR and with short spinules in MX (differences consistent with those found in females); the spinule formula on the caudal surface is only observed in MX: A- C + D-G-H ( Figure 5L View Figure 5 ). The inner coxal spine has a heteronomous ornamentation: the inner margin has long, hair like elements basally and spinules distally in both populations. The outer edge has two spinules on its apical surface and naked proximally (arrowed Figure 5 L, F View Figure 5 ). The length/width ratio Enp3 = 2.6 in AR and 3.3 in MX, the inner spine/Enp3 P4 length ratio = 1.1 in AR and 1.2 in MX, the outer spine/Enp3 P4 length ratio = 0.8 in both populations and the inner/outer spines Enp3 P4 ratio = 1.4 in both populations. Modified setae are present on Enp and Exp of AR specimen (arrowed Figure 5F View Figure 5 ), but all setae in MX normal. The fifth leg ( Figure 5 A, G View Figure 5 ) is represented by a free subrectangular segment, 1.9 times longer than wide in AR and 1.8 in MX; it bears one inner spine and two setae, the medial seta is longer than both the outer and the inner spines in both populations. The ratio of the medial seta/outer seta is 2.4 in AR and 1.8 in MX, while that for the medial seta/inner spine is 1.7 in AR and 1.3 in MX. The sixth leg ( Figure 5A, G View Figure 5 ) is represented by a small, low plate adjacent to the lateral margin of the genital somite with one strong but short inner spine and two unequal setae. In both populations the inner spine reaches the medial margin of the third urosomite, but differences in the relative size of setal elements are noteworthy. In the AR specimens the inner spine is about 0.9 times longer than the medial seta while in MX inner spine it is as long as the medial seta; in AR inner spine is 0.7 times longer than the outer seta while in MX inner spine is 1.6 times longer than outer seta. Small but strong spinules are present at the insertion of the inner spine.
Remarks
Eucyclops elegans is here assigned as a member of the serrulatus group because it has the diagnostic characters established by Alekseev and Defaye (2011) to distinguish the group: (1) longitudinal row of spinules along most of outer edge of each ramus and without hairlike setae or denticles on dorsal or ventral surface; (2) antennules 12-segmented, with smooth membrane along 3 distal segments (in the material examined the hyaline membrane was always finely but uniformly denticulated); (3) frontal side of antennary basipodite with groups N1 and N2 (both with long hairs in E. elegans ); (4) coxopodite of P4 with strong inner spine (in E. elegans with heteronomous ornamentation, gap present in all specimens examined); and (5) fifth leg with wide and strong inner spine.
With the inclusion of E. elegans in the serrulatus group we confirm that this species is not a synonym of E. speratus – a species that is not a member of the serrulatus group. Reid and Marten (1995) stated that American records of E. speratus should be assigned to E. elegans , but the evidence presented here supports the notion that these records should be considered separately as there are differences between populations. Also, we support the assumption of Kiefer (1929) and Dussart and Frutos (1986) that E. elegans is not a variation of E. serrulatus because of the several important differences between these two species. On the frontal surface of the antennary basis, rows N1 and N2 are continuous in E. elegans whereas these rows are clearly separated in E. serrulatus . Both species share rows N3, N4, N5, N15 and N17, but E. elegans bears an additional row between N15 and N17 that is not present in E. serrulatus . The caudal surface of the antennary basis has some additional differences between these two species: row N8 is absent in E. serrulatus and sometimes N16 is absent too, but in E. elegans both rows are present. In this species one extra row of spinules is present, between N13 and N14; these are absent in E. serrulatus . Slight differences in the ornamentation of the antennary basis were observed among the specimens groups of E. elegans ; these differences should be evaluated in all American populations to establish whether they represent separate species or are intraspecific variations. It is also important to emphasize that the male P6 of E. elegans of both populations examined (AR and MX) are remarkably different from that of E. serrulatus , E. speratus , E. neumani titicacae and most of the American species of the genus, bearing a small but strong inner spine which barely reaches the medial margin of the third urosomite. The proportions of setae and spine of P6 should be considered important in the separation of the populations examined; this character alone could be useful to distinguish species, together with the antennules ornamentations. These differences separate the South American from the North American populations, so sampling the type locality of E. solitarius ( Herbst 1959) (considered as a synonym of E. elegans ) would be useful to analyse the micro-patterns studied herein and establish if in fact the North and South America populations represent different species. The differences advanced herein suggest that that they belong to different taxa, but are members of the serrulatus group, whose diversity in the Americas appears to be underestimated.
Other American Eucyclops with long caudal rami include the South American forms E. neumani s.str., E. neumani titicacae and the North American (Alaska) species Eucyclops borealis ; the first two having a caudal ramus with spinules only in the area adjacent to the lateral caudal seta (II) and the last species with spinules covering the last third or half of caudal ramus. Among other characters, the former subspecies ( E. neumani neumani ) differs from E. elegans , E. serrulatus and E. neumani titicacae in details of the antennary ornamentation, with group N1 formed by spinules and not hair-like elements. Eucyclops borealis can be easily distinguished from its congeners by the absence of N2 and the presence of N6 on frontal surface of antennal basis. Eucyclops neumani titicacae also differs from E. elegans and E. serrulatus for its unique ornamentation pattern of the intercoxal plate of P4 (see Figure 13 in Kiefer 1957; Fuentes and Suárez-Morales 2013).
| VI |
Mykotektet, National Veterinary Institute |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |