Simulium (Inaequalium) subnigrum Lutz
|
publication ID |
https://doi.org/ 10.11646/zootaxa.1506.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:9C4F12AF-DC25-4E84-92D0-9C5E4BCAD194 |
|
persistent identifier |
https://treatment.plazi.org/id/039087B2-E61C-A778-FF65-FC34FE63DE5D |
|
treatment provided by |
Felipe |
|
scientific name |
Simulium (Inaequalium) subnigrum Lutz |
| status |
|
Simulium (Inaequalium) subnigrum Lutz View in CoL
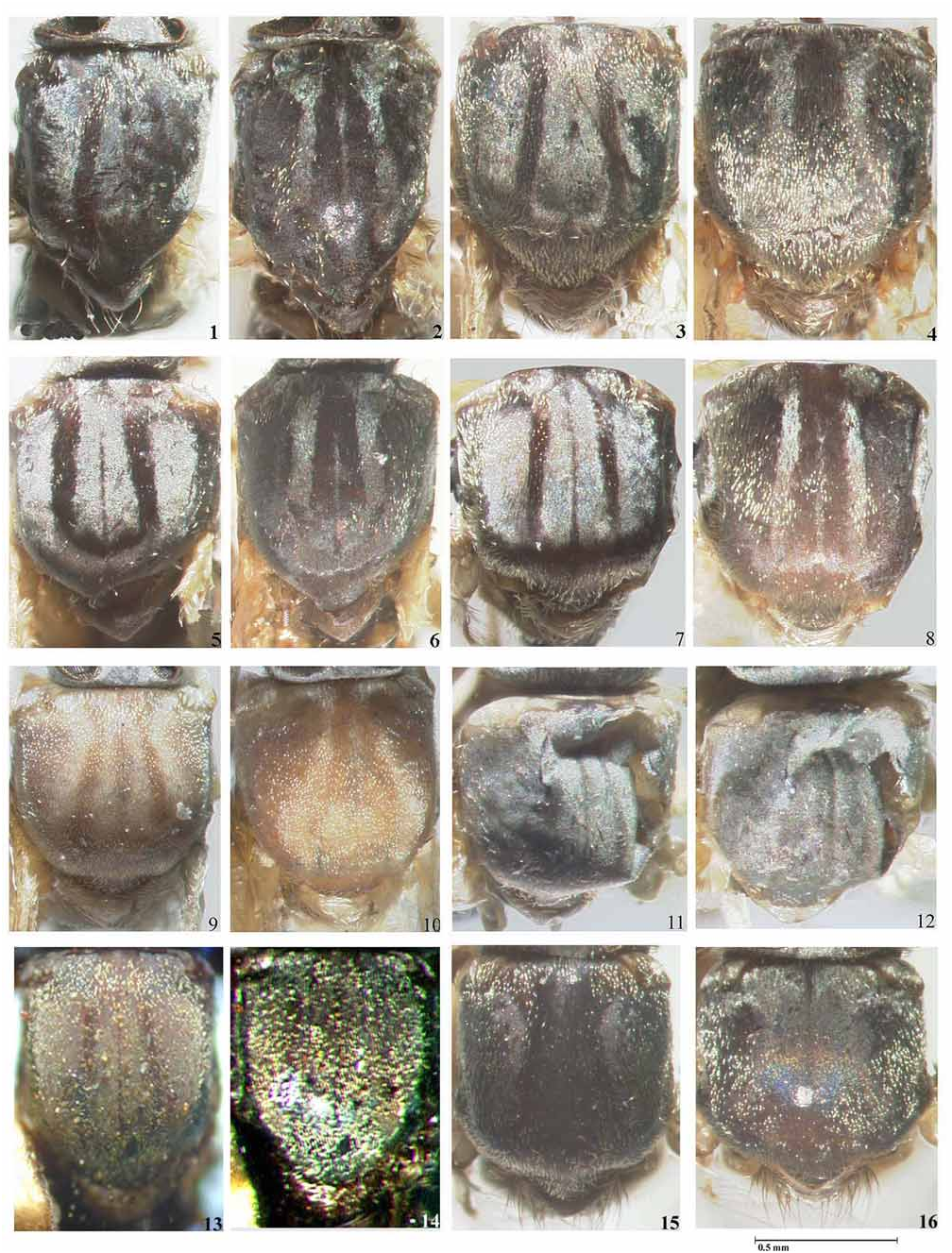
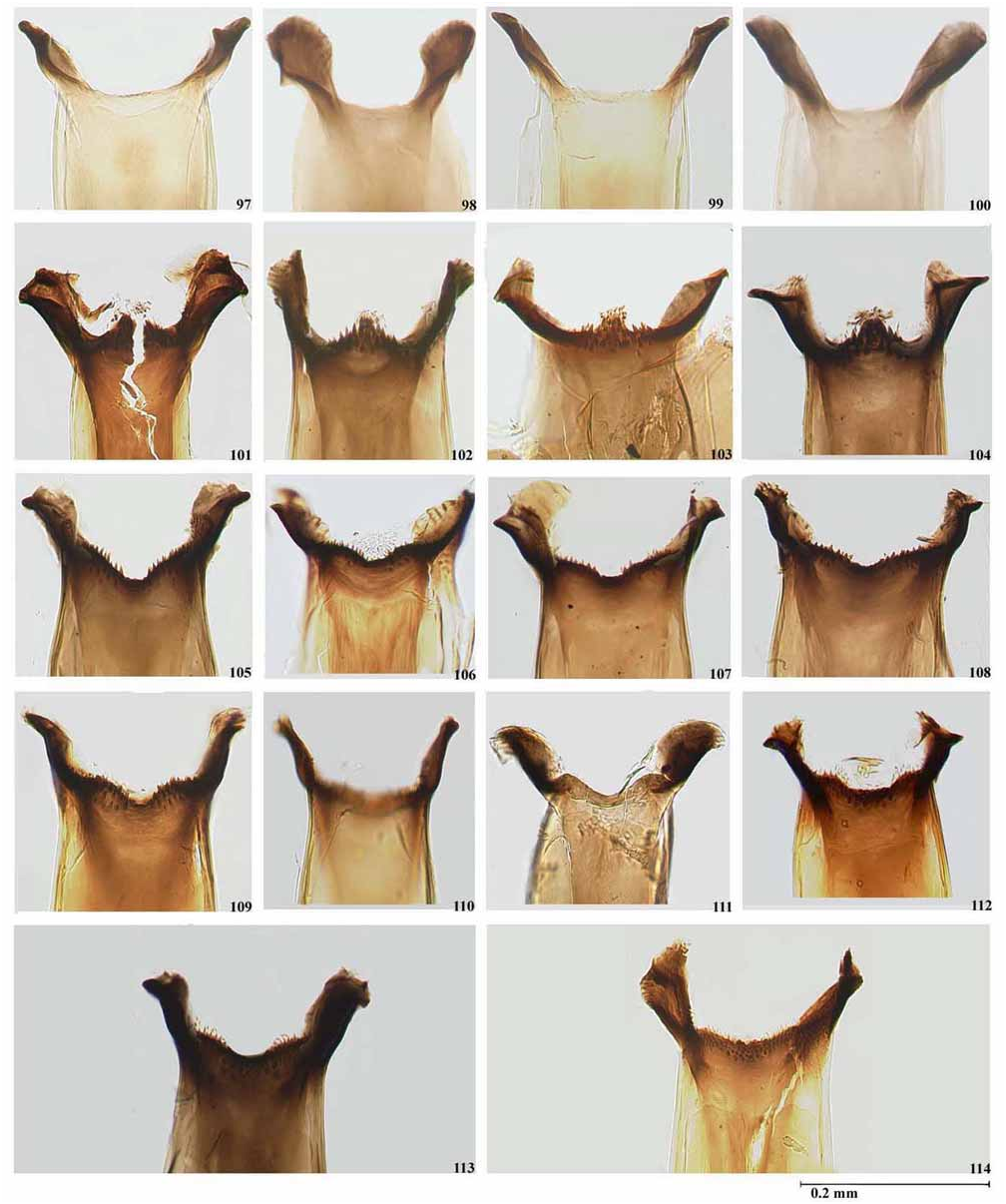
( Figs. 27–32 View PLATE 2 , 63–66 View PLATE 5 , 86 View PLATE 6 , 104 View PLATE 7 , 136–141 View PLATE 9 , 180–186 View PLATE 12 View PLATE 13 , 214–216 View PLATE 14 )
Simulium subnigrum Lutz, 1910: 239 View in CoL . SYNTYPES ♀♀ ♂♂ and pupal exuviae. BRAZIL: São Paulo State (Rivers Pacaembú and Anhangabahú is this correct?), Rio de Janeiro State (Mendes and Petrópolis); [Without date or collector’s name.] (IOC). [Examined.]
Simulium diversifurcatum Lutz, 1910: 258 View in CoL . SYNTYPES pupae, BRAZIL: São Paulo State (Serra de Bocaina, Capivary View in CoL ), Rio de Janeiro State (near Petrópolis); [Without date or collector’s name.] [Synonymy by Coscarón & Wygodzinsky, 1984: 90.]
Simulium subclavibranchium Lutz, 1910: 260 View in CoL . LECTOTYPE pupal exuviae, BRAZIL, Rondônia State, estrada de ferro Rio Madeira-Mamoré , [Without locality, date or collector’s name.] (IOC, 12.372, Bd. 120, no. 236) [Examined.] New type designation. New synonymy.
Simulium nogueirai D’Andretta & González, B., 1964: 103 View in CoL . HOLOTYPE ♀ (reared), BRAZIL: São Paulo State: Aldeinha , (E.F.S.); 7.vii.1957 (C.D’Andretta Jr. & M.V.Nogueira) (ICBUSP) [ Py-Daniel & Moreira (1989: 85–95) regarded the type series of S. nogueirai View in CoL as “not lost, but misplaced”.] New synonymy.
Simulium mbarigui Coscarón & Wygodzinsky, 1973: 142 View in CoL . HOLOTYPE ♂ (reared), ARGENTINA: Missiones, 20 km N. de Apósteles; 18.vii.1972 (Coscarón) (MLP) [Examined.] [Synonymy by Coscarón & Wygodzinsky, 1984: 90.]
Simulium (Simulium) beaupertuyi Ramírez Pérez, Rassi & Ramírez, 1977:165 View in CoL . HOLOTYPE ♀, VENEZUELA: Território Federal de Amazonas, Departamento de Atabapo, Sierra de Parima; [Without date or collector’s name.] [Holotype said to be deposited in Ramírez Pérez’ private collection in the original description, but Strieder & Py- Daniel (2000) stated it is deposited in IDVC - see “Note on Ramírez Pérez’ Simuliidae View in CoL collection” in Material and Methods.] New synonymy.
Simulium nahimi Py-Daniel, 1984: 125 View in CoL . HOLOTYPE ♀ (reared, pupa exuviae in ethanol). BRAZIL: Mato Grosso State, Dardanelos, Aripuanã ; 13.xi.1975, [Antonio Faustino Neto, Waldomiro Albuquerque, Francisco Moraes & Eduardo V. da Silva- Collectors name not on label, but in publication] (INPA, 5125-6) [Examined.] New synonymy.
Inaequalium leopoldense Strieder & Py-Daniel, 2000: 15 View in CoL . HOLOTYPE larva [The life stage designated as holotype was not stated in the original description, but is a larva- pers. comm. M.N.Strieder to L.M.Hernández v.2006.], BRAZIL: Rio Grande do Sul, São Leopoldo, 13.x.1983, (H.G. Konrad) (LEU). New synonymy.
Simulium subnigrum View in CoL was described by Lutz (1910) based on several females, males and pupae collected in the states of São Paulo (Rivers Pacaembú and Anhangabahú) and Rio de Janeiro (Mendes and Petrópolis). He also illustrated the pupal gill filaments and discussed the gill configuration to recognize this species. According to Amaral-Calvão & Maia-Herzog (2003) there are 12 pinned females identified as S. subnigrum View in CoL in the Lutz collection at the IOC [two are identified with “doubt”]. In the slide collection there are three females, one male and 43 pupae [1 vial and 1 adult were recorded are “missing”]. We have examined 13 pupae on slides of this species in the IOC, which agree with the concept of S. subnigrum View in CoL of Lutz (1910) and the morphological characters given in Coscarón & Wygodzinsky (1984). However, there is some confusion regarding the provenance of this material. Therefore, we have refrained at this stage from selecting a pupa as lectotype until this situation is clarified. We have also examined 10 pinned females in the Lutz collection collected in Formoso-Bocaina in 1915. These specimens are not syntypes and have been labelled with doubt as S. subnigrum View in CoL by L.M.Hernández (see Material Examined.).
Several species have been treated as synonyms of S. subnigrum and we have the following comments to make. Lutz (1910) described Simulium diversifurcatum from pupae collected in the states of São Paulo (Serra da Bocaina, Capivary ) and Rio de Janeiro (near Petrópolis). He stated that this species could be separated from S. clavibranchium by the pupal gill filaments that are not swollen apically. In his key he referred to the wrong figure number ( Fig. 8 View PLATE 1 ) for this species, while in the figure legend he referred to S. diversibranchium . He also thought that this species was a “variety of typical subnigrum , found in the same place”. Coscarón & Wygodzinsky (1984) in their revision of the subgenera Psaroniocompsa and Inaequalium regarded S. diversifurcatum as a synonym of S. subnigrum . The syntype pupae of S. diversifurcatum could not be located in the IOC holdings (see Amaral-Calvão & Maia-Herzog (2003) for species list in the Lutz collection at IOC) and we presume they are now lost. However, we have studied the original description and the pupal gill configuration in Lutz (1910: 263, Fig. 12 View PLATE 1 ) for S. diversifurcatum , and agree that it falls within the variation of S. subnigrum , hence confirming Coscarón & Wygodzinsky’s (1984) synonymy. We have also examined 14 pinned females of the 27 in the IOC identified as the latter species ( Amaral-Calvão & Maia-Herzog, 2003), but they are not syntypes. We cannot verify this identification because most Inaequalium species are morphologically similar and can only be accurately identified on pupal gill configuration. Therefore, they have been identified with doubt as S. subnigrum and have been labelled accordingly (see Material Examined).
Another species here regarded as a junior synonym of S. subnigrum is S. subclavibranchium . Lutz (1910) described S. subclavibranchium from pupae collected in a stream near the “estrada de ferro Madeira-Mamoré, Rondônia State ” [a rail line linking the rivers Madeira and Mamoré]. In the original description Lutz stated the following: “…Cocoon of common appearance with thick fibres, 3.5 mm long and pupal gill filaments 3.5 mm long. Anterior part of cocoon composed of many thick, dark granules. The primary branches are three in number with the apex weakly enlarged, cone-shaped, and all filaments equidistant from the common base. The trichomes have 5–10 branches anteriorly, but they are bifid or simple posteriorly. The branching of the gill filaments is similar to S. clavibranchium but the bifurcations are more basal. The pupae can also be distinguished by the shape of the enlargement at the apex of the gill filaments that in S. subclavibranchium are gradual and less pointed. The adults were not found, possibly because the female does not bite man...” Coscarón & Wygodzinsky (1984) stated that they had examined pupae from the type locality of S. subclavibranchium in the IOC collection that agree with the morphology of other material that they had collected from São Paulo and Rio de Janeiro states. In the same paper, these authors figured the gill filaments of S. subclavibranchium [based on their specimens from southern Brazil] and S. clavibranchium , and stated that S. clavibranchium can be distinguished by the different morphology and branching pattern of the pupal gill filaments. In S. clavibranchium the gill filaments are more abruptly enlarged and more narrowed towards the apex, and the branching of the secondary branches in S. clavibranchium were more apical than in S. subclavibranchium . They also pointed out that some specimens of S. subclavibranchium are difficult to distinguish from S. subnigrum because in the latter species sometimes the filaments can be slightly enlarged.
Amaral-Calvão & Maia-Herzog (2003) recorded two larvae and three pupae of S. subclavibranchium in the Lutz slide collection in the IOC. We have studied four slides from “Rio Madeira”, with identification labels “ S. subclavibranchium ” deposited in the IOC. Three of the slides contain pupal exuviae (with Lutz’s numbers “235”, “236” and “237”) and a fourth slide (with Lutz’s number “238”) contains two larvae (see Material Examined). The pupal gill configuration agrees with the figures of Lutz (1910) of S. subclavibranchium . We have selected the slide with Lutz’s number “236” and IOC number “12.372, Bd. 120” as a lectotype and have labelled it accordingly. The other pupae have been labelled as paralectotypes (Material Examined); we did not include the larvae as type material because Lutz did not mention these in his publication. None of the apices of the gill filaments was expanded in the four slides that we examined and the gill configuration matches that found in S. subnigrum . Consequently, we consider S. subclavibranchium as a junior synonym of S. subnigrum .
A question now arises as to the identity of the material identified by Coscarón & Wygodzinsky (1984) as S. clavibranchium and S. subclavibranchium from localities in southern Brazil. They recorded that branching of the gill occurs at some distance from the base, especially the ventral secondary branch [=lower secondary branch of dorsal primary branch] a third the way along its length. Two figures refer to this (19L, not 29K as cited, and 19M). Neither illustrates this description except for the claviform apices to the gill. In Figure 19L View PLATE 2 the gill bifurcates basally forming a dorsal and ventral branch; the dorsal branch again bifurcates some distance from the base, the upper secondary branch being at a point almost a third the length of the gill. In Figure 19M View PLATE 2 the lower secondary branch of the dorsal primary branch is more basal than in Fig. 19L View PLATE 2 . In S. subclavibranchium , based on material from southern Brazil and not the type locality in Amazonia, the gill apices are described as slightly enlarged and the secondary dorsal branches bifurcate more closely to the base than in S. clavibranchium , especially the dorsal secondary (=upper secondary) branch illustrated in Fig 21N View PLATE 2 and in Fig 21O a View PLATE 2 variation on this gill configuration also with basal branching. The figure ( Fig. 21M View PLATE 2 ) shows no enlargement of the apices of the gill (a pattern found in S. subnigrum ). Apart from the type material of both S. clavibranchium and S. subclavibranchium we have examined numerous link-reared specimens identified by us as S. clavibranchium in the MLP, BMNH, AMNH, IOC, LEU and MZUSP collections. Variation in the branching of the dorsal primary branch and the enlargement of the pupal gill filaments in S. clavibranchium have been seen. The primary branches can bifurcate more basally or more apically (typical of the syntype series of S. clavibranchium ) ( Fig. 212 View PLATE 14 ). The filaments can vary from distinctly enlarged apically (as is commonly found in S. clavibranchium ) to less enlarged. Some of the filaments are not enlarged at all even in the same specimen ( Fig. 213 View PLATE 14 ). In the lectotype of S. subclavibranchium the gill apices are not claviform. In conclusion, we consider that all material identified as S. subclavibranchium by Coscarón & Wygodzinsky (1984) and other authors (e.g. Coscarón & Coscarón-Arias, 2007, Strieder et al., 1992; Strieder & Py-Daniel 1999, 2000) that has not been collected from Madeira-Mamoré in Rondônia State is a variation of S. clavibranchium .
Another species here regarded as conspecific with S. subnigrum is S. nogueirai . This species was described by D’Andretta & González B. (1964) based on two reared females and one male collected at Aldeinha in the State of São Paulo, Brazil. The type material of this species is said to be deposited in the ICBUSP, but Py-Daniel & Moreira (1989) stated that they could not find it there [preferring to consider the type series of S. nogueirai not as “lost” but “located in an unknown place”]. Our attempts to locate the type material of this species in the MZUSP have been unsuccessful. Since this “unknown place” has still not been located the specimens are considered to be lost. From the original authors’ description it is not clear as to which species S. nogueirai is most closely related. Mention is made by these authors of the similarity of the male with that of S. baiense , and S. nogueirai is included in the subgenus Byssodon with differences recorded in the female and pupa of S. (B.) benjamini [currently unplaced to subgenus] and to the adults and pupae of S. (B.) ganalesense [currently within Psaroniocompsa ]. Later, in the same paper they referred to differences in female and pupal morphology with S. jundiaiense [as jundiaiensis ]. Py-Daniel & Moreira (1989) later redescribed all life stages of S. nogueirai based on material collected in Santa Catarina State. We have studied the descriptions and illustrations of S. nogueirai given in D’Andretta & González B. (1964) and Py-Daniel & Moreira (1989), and compared them with numerous link-reared specimens identified as S. subnigrum in the BMNH, IOC and MLP collections. Neither species can be separated using the female and male thoracic patterns, morphology of the female genitalia or pupal gill configuration. However, Py-Daniel & Moreira (1989) were only able to distinguish S. nogueirai from S. subnigrum by the presence of a “small apical incision” in the spine of the gonostyle of the former species, first noted by the original authors. No reference was made to variation in this character in S. nogueirai (one specimen examined by D’Andretta & González B. (1964) and three specimens by Py-Daniel & Moreira (1989) and no data are given for S. subnigrum by the latter authors). Consequently, we regard this variation in the morphology of the gonostyle as insufficient to erect a new species. Since the configuration of the pupal gill filaments also falls within the morphological variation found in S. subnigrum , especially in that the dorsal branch can bifurcate more apically, at the same level or more basally than the ventral branch we consider S. nogueirai conspecific with S. subnigrum . Coscarón & Wygodzinsky (1984) did not include S. nogueirai in their revision of the subgenus Inaequalium , nor did Coscarón (1991) in his key to adults and pupae. However, Coscarón & Coscaron-Arias (2007), Crosskey & Howard (1997, 2004) placed it in this subgenus and Strieder & Py-Daniel (1999, 2000, 2002) in the genus Inaequalium .
Simulium mbarigui View in CoL was described by Coscarón & Wygodzinsky (1973) from females, males, pupae and larvae collected in Argentina (Missiones and Corrientes Provinces) and Paraguay by Coscarón. In 1984 Coscarón & Wygodzinsky synonymized Simulium mbarigui View in CoL with S. subnigrum View in CoL . However, Coscarón (1987) listed S. mbarigui View in CoL as a synonym of S. clavibranchium View in CoL in his catalogue of the genus Simulium View in CoL in the Neotropical Region. In a subsequent paper ( Coscarón, 1991) S. mbarigui View in CoL was again listed as a synonym of S. subnigrum View in CoL , an action that has been followed by Coscarón & Coscaron-Arias (2007), Strieder & Py-Daniel (2000) and Crosskey & Howard (2004). We have examined the female holotype and four paratypes of S. mbarigui View in CoL , which are deposited in the MLP. The holotype is in relatively good condition and has been glued to a card point by its left side ( Figs. 27, 28 View PLATE 2 ) together with its pupal exuviae ( Fig. 214 View PLATE 14 ). The left side of the pupa has five broken filaments, while the right side of the gill is intact. Some of the paratypes appear to have been recovered from alcohol and their thoraxes have slightly shrunk. We have studied the original description, the thoracic pattern ( Figs. 27, 28 View PLATE 2 ) and the general morphology of S. mbarigui View in CoL , including the pupal gill configuration ( Fig. 214 View PLATE 14 ), and compared them with a large numbers of link-reared specimens of S. subnigrum View in CoL . We agree with Coscarón & Wygodzinsky (1984) that S. mbarigui View in CoL falls within the morphological variation found in S. subnigrum View in CoL and hence confirm their synonymy.
We also consider three other species as synonyms of S. subnigrum . Ramírez Pérez et al. (1977) described S. beaupertuyi from a pinned female holotype, and one female pupa and one male pupa mounted on two slides. The holotype was said to be deposited “in the private collection of Ramírez Pérez”. However, Strieder & Py-Daniel (2000) recorded it deposited in the IDVC. We have been unable to obtain the type material of S. beaupertuyi for study (see Note on Ramírez Pérez’ Simuliidae collection in Material and Methods). Coscarón suggested in 1991 that S. beaupertuyi might be a synonym of S. subnigrum . He also pointed out that Ramírez Pérez (1983) did not include this species for Venezuela, even though Briceño-Iragorry (1943) and Briceño-Iragorry & Ortiz (1957) had previously recorded its presence in the Federal District, Bolívar and Miranda States in this country. The identification of S. subnigrum from the state of Aragua was then correctly identified as S. clarki by Ramírez Pérez (1990). We agree that all morphological characters in the description of S. beaupertuyi by Ramírez Pérez et al. (1977) fall within the variation found in S. subnigrum and hence regard both species as conspecific. We have examined several pupae and larvae identified as S. beaupertuyi identified by M. N. Strieder and descriptions and illustrations of this species in Strieder & Py-Daniel (1999) and consider them to be conspecific with S. subnigrum .
Py-Daniel (1984) described S. nahimi from numerous females, males, pupae and larvae collected in Aripuanã, Mato Grosso State, Brazil. We have examined the female holotype, one male paratype (as allotype), and topotype larvae and pupae deposited in INPA. All specimens have a locality label “Dardanelos, Rio Aripuanã, 13.xi.1975 ” (see Material Examined). The pinned female holotype is in good condition and has been glued to a card point. Its pupal exuviae was said to be stored in ethanol, but we were unable to examine it. Py-Daniel (1984) stated that S. nahimi was readily separated from all other Inaequalium species by the large number of sub-apical spines (between 4–10) in the gonostyle of the males, while in the other species only one is present. However, the number of spines in Inaequalium species is variable. We have found specimens of S. rappae and, more recently, of S. inaequale with one, two or three sub-apical spines in the gonostyle. Apart from this character, the morphology of the adult thoracic pattern ( Figs. 29, 30 View PLATE 2 ), female head (nudiocular area and cibarium), pupal gill configuration ( Fig. 214 View PLATE 14 ) and larval hypostomium fall within the variation found in S. subnigrum . We have dissected various specimens of both male and female from localities from São Paulo State, Brazil that conform with the descriptions given by Py-Daniel (1984) for S. nahimi from the river Aripuanã in Mato Grosso State, Brazil. The only difference between S. nahimi from both states and S. subnigrum is that the paraproct in S. nahimi protrudes below the ventral surface of the cercus by about one third the depth of the cercus compared to about two thirds in S. subnigrum ( Fig. 136, 139 View PLATE 9 ). We have studied the gonostyle of a male topotype of S. nahimi deposited at INPA ( Fig. 181 View PLATE 12 ) and the ventral plate and paramere ( Figs. 180, 182 View PLATE 12 ) of material identified by L.M.Hernández as S. nahimi deposited in the BMNH. We have compared this material with a large number of linked-reared specimens collected mainly in Brazil and Paraguay and identified as S. subnigrum in the BMNH, IOC and MLP collections. We found that the number of apical spines is variable along the distribution range varying from one (Rio de Janeiro) ( Fig. 184 View PLATE 13 ) to three to nine often with differences in number in the same specimen ( Fig. 185 View PLATE 13 ) in populations from the Federal District, Goiás State, São Paulo in Brazil and in Paraguay. Little is known about the intraspecific variation in the number of spines on the male gonostyle in Neotropical black flies. However, at this juncture we prefer to treat S. subnigrum as a polymorphic species. The same approach was used by Shelley et al. (1997, 2006) in assessing morphological characters for their revision of the amazonicum -species group and in dealing with the variation found in the male gonostyle of S. nigrimanum ( Shelley et al., 2000) . Therefore, we regard S. nahimi as a junior synonym of S. subnigrum until studies linking morphology to DNA and chromosome techniques may be used to assess their taxonomic status.
Strieder & Py-Daniel (2000) reviewed the species of Inaequalium (as a genus) and described a new species, S. leopoldense . This species was based on several pupae and larvae at São Leopoldo in the state of Rio Grande do Sul State, Brazil by H.G. Konrad. They mentioned that the holotype (no. 59-1) is deposited in LEU, but did not state the life stage (see Strieder & Py-Daniel, 2000). In recent correspondence with Dr. M.N. Strieder the first author of this paper was informed that the holotype of S. leopoldense is a larva. We were unable to obtain type material for study, but have examined the original description and figures of S. leopoldense as well as several pupae and larvae identified by M. N. Strieder. Strieder & Py-Daniel (2000) used the pupal gill configuration and length of the dorsal and ventral primary and secondary branches to separate S. leopoldense from S. subnigrum , and referred to Figs. 7A, 8E, J and K View PLATE 1 (see page 18). However, Fig. 8E View PLATE 1 shows the head capsule of a larva in ventral view. We consider that the pupal gill configuration of S. leopoldense falls within the variation of S. subnigrum based on specimens housed in the BMNH, IOC, MLP and LEU collections. Strieder & Py-Daniel (2000) also considered that larval morphological characters such as the length of the antennal segments in relation to the length of fan stalks, number of cephalic fan rays, body colour, and number of anal gill lobes distinguish S. leopoldense from the closely related species S. subnigrum . They stated that in S. leopoldense the antennal segments are longer than the cephalic stalk [they only reach the apex of the fan stalks in S. subnigrum ], the number of cephalic fan rays varies from 42–45 [in S. subnigrum they vary between 35–40] and the anal gill lobes have between 10–14 lobules [ S. subnigrum has between 15– 16 lobules]. Based on our experience of Neotropical Simuliidae taxonomy, we consider the differences in the latter morphological characters as intraspecific and hence they cannot be used to separate closely related taxa. It is probable that if a larger sample had been used the range in variation would have increased for each species and their ranges would overlap. Although the type series of S. leopoldense has an adequate number of larvae and pupae to determine intraspecific variation it is not clear from the original description how many specimens the authors have dissected to produce the ranges for those morphological characters. Strieder & Py- Daniel (2000) also gave differences in body coloration to separate the two taxa in question. Charalambous et al. (1996) have shown that coloration in the larval stage cannot be used for species identification, as it is highly variable even within a population of the same cytotype. Based on these minor morphological differences, we are here considering S. leopoldense as a junior synonym of S. subnigrum until integrated studies linking morphology to DNA and chromosomes differences are carried out to assess their taxonomic status.
Simulium subnigrum View in CoL is very close to S. inaequale View in CoL , showing only slight differences in the female and male genitalia ( S. inaequale View in CoL - Figs. 127–135 View PLATE 9 , 176–179 View PLATE 12 and Figs. 1U, 2N View PLATE 1 of Coscarón and Wygodzinsky, 1972; S. subnigrum View in CoL - Figs. 136–144 View PLATE 9 View PLATE 10 , 180–186 View PLATE 12 View PLATE 13 ), and in the more basal branching of the gill ( S. inaequale View in CoL - Fig. 211 View PLATE 14 and Fig. 3M View PLATE 1 of Coscarón & Wygodzinsky, 1972; S. subnigrum View in CoL - Figs. 214–216 View PLATE 14 ). Further examination of link reared specimens is required to assess the degree of variation in these characters before any synonymy is established. Simulium subnigrum View in CoL can be recognized by the thoracic pattern of adults ( Figs. 31, 32 View PLATE 2 , 65, 66 View PLATE 5 ), female head (cibarium and nudiocular area) ( Figs. 86 View PLATE 6 , 104 View PLATE 7 ), morphology of the genitalia of the adults ( Figs. 136–141 View PLATE 9 , 180–182 View PLATE 12 ) and the pupal gill configuration ( Fig. 215 View PLATE 14 ). This species is placed within the inaequalespecies group of the subgenus Inaequalium by Coscarón (1987, 1991), Coscarón & Coscarón-Arias (2007) and Crosskey & Howard (2004) following Coscarón & Wygodzinsky (1984). Strieder & Py-Daniel (1999, 2000, 2002) include this species in the genus Inaequalium .
Based on the synonymies here proposed S. subnigrum has a wide distribution in the Neotropical Region. In Brazil it has been recorded in the states of Bahía, Ceará, Federal District, Goiás, Minas Gerais, Mato Grosso, Mato Grosso do Sul, Paraná, Rio de Janeiro, Rio Grande do Sul, Santa Catarina and São Paulo. Elsewhere, it is found in Argentina (Missiones), Colombia [see also Porto, 1940], Paraguay, Trinidad and Venezuela ( Crosskey & Howard 2004; Material Examined).
The immature stages of S. subnigrum are commonly attached to vegetation in small to medium (3–5m wide, 20 cms deep), fast flowing streams with sandy or rocky bottoms. Strieder & Py-Daniel (1999) recorded it breeding in small spillways on dams together with S. inaequale and S. perflavum . In studies on the diversity of Simuliidae in Rio Grande do Sul, Strieder et al. (2002) found S. subnigrum in streams between 220 and 400m, and above 850m altitude together with S. inaequale . Strieder (2002) and Strieder et al. (2002) recorded S. subnigrum and also S. subclavibranchium [= S. subnigrum ] as very common in the state of Rio Grande do Sul. Pepinelli et al. (2005) recorded S. subclavibranchium and S. nogueirai [now synonyms of S. subnigrum in this paper] as the most frequent species in São Paulo State. Little is known about the female feeding habits of this species. It has never been found biting man along its distribution range, but Pepinelli et al. (2005) recorded females of S. subnigrum as anthropophilic in their studies in the Parque Estadual Intervales in São Paulo State, Brazil. However, this record still needs verification. Lutz (1910) stated that he collected females of S. subnigrum biting horses.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Simulium (Inaequalium) subnigrum Lutz
| HERNÁNDEZ, LUIS MIGUEL, SHELLEY, ANTHONY JOHN, DE LUNA DIAS, ANTONIO PAULINO ANDRADE & MAIA-HERZOG, MARILZA 2007 |
Inaequalium leopoldense
| Strieder, M. N. & Py-Daniel, V. 2000: 15 |
Simulium nogueirai D’Andretta & González, B., 1964: 103
| Py-Daniel, V. & Moreira, G. R. P. 1989: 85 |
Simulium nahimi
| Py-Daniel, V. 1984: 125 |
Simulium (Simulium) beaupertuyi Ramírez Pérez, Rassi & Ramírez, 1977:165
| Ramirez Perez, J. & Rassi, E. & Ramirez, A. 1977: 165 |
Simulium mbarigui Coscarón & Wygodzinsky, 1973: 142
| Coscaron, S. & Wygodzinsky, P. 1984: 90 |
| Coscaron, S. & Wygodzinsky, P. 1973: 142 |
Simulium subnigrum
| Lutz, A. 1910: 239 |
Simulium diversifurcatum
| Coscaron, S. & Wygodzinsky, P. 1984: 90 |
| Lutz, A. 1910: 258 |
Simulium subclavibranchium
| Lutz, A. 1910: 260 |