Mixtoscutella ovata (Smitt, 1868)
|
publication ID |
https://doi.org/10.11646/zootaxa.5131.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:CF550031-D6A9-48A3-A953-A1BD40C72F5E |
|
DOI |
https://doi.org/10.5281/zenodo.7628955 |
|
persistent identifier |
https://treatment.plazi.org/id/03892374-0B65-3368-FF73-AB031D02FA9D |
|
treatment provided by |
Plazi |
|
scientific name |
Mixtoscutella ovata (Smitt, 1868) |
| status |
|
Mixtoscutella ovata (Smitt, 1868)
( Figs 27 View FIGURE 27 , 33G View FIGURE 33 )
Cellepora ovata Smitt, 1868a, p. 31 , pl. 28, fig. 197.
Cellepora ovata: Smitt 1868b, p. 485 ; Hincks 1877, p. 105, pl. 11, fig. 5.
Rhamphostomella ovata: Nordgaard 1906, p. 32 View in CoL , 41, pl. 4, fig. 56; Kluge 1962, p. 540, fig. 377; 1975, p. 657, fig. 377; Osburn 1912a, p. 245, pl. 26, fig. 63; 1933, p. 54, pl. 11, figs 5, 6; 1952, p. 432, pl. 50; Androsova 1958, p. 170, fig. 101; Gostilovskaya 1978, p. 227, fig. 143; Winston & Hayward 2012, p. 121, fig. 77; Grischenko 2013, p. 177, figs. 1q–r.
Discopora ovata: Nordgaard 1918, p. 78 .
Additional references. Rhamphostomella ovata: Kluge 1908a, p. 533 View in CoL ; 1928, p. 257; 1929, p. 22; 1961, p. 141; Osburn 1936, p. 542; 1955, p. 38; Gostilovskaya 1957, p. 455; 1968, p. 70; Hansen 1962, p. 40; Powell & Crowell 1967, p. 343; Powell 1968a, p. 2312; 1968b, p. 257; Gontar 1980, p. 18; 1990, p. 132; 1993b, p. 202; 1994a, p. 145; 2010, p. 153; Denisenko 1988, p. 13; 1990, p. 39; 2008, p. 187; 2011, p. 14; 2013, p. 184; Gontar & Denisenko 1989, p. 354; Grishankov 1995, p. 48; Kubanin 1997, p. 123; Gontar et al. 2001, p. 195; Shunatova & Ostrovsky 2001, p. 115, 118; Shunatova & Nielsen 2002, p. 263, fig. 1b; Grischenko 2002, p. 115; Kuklinski 2002b, p. 203; 2009, p. 228; Denisenko & Kuklinski 2008, p. 48; Ostrovsky 2009, p. 175, fig. 78a; 2013, p. 8, figs 1.11c, 2.41a; Denisenko et al. 2016, p. 366.
Material examined. Lectotype: SMNH-Type-9303, six fragments from one colony, Swedish Arctic Expedition , July 1861, Red Bay, west Spitsbergen, Svalbard and Jan Mayen, depth 64 m, mud . Paralectotype: SMHM-Type-9305, small colony growing on eroded fragment of another cheilostome, Swedish Arctic Expedition , September 1861, Advent Bay , Isfjord, west Spitsbergen, Svalbard and Jan Mayen, depth 35 m, mud with stones .
NHMUK 1911.10.1.1581A, one colony, ex Swedish Museum Natural History , from F.A. Smitt Collection, Spitsbergen . NHMW 44378 View Materials , one colony fragment, Kola Haven , collector H. Kluge [before 1907]. ZIRAS 36 /50120, three colony fragments detached from broken shells of the bivalve mollusc Chlamys sp. , MFRT Rodino, 12 September 1992, about 32 km from Cape Hayryuzova, western Kamchatka shelf, Sea of Okhotsk , 57°36.2ʹ N, 156°09.0ʹ E, depth 78–81 m, crab trap, collector A GoogleMaps . V. Grischenko. P. Kuklinski Collection, two colony fragments, RussianGerman Expedition Transdrift 1 , RV Ivan Kireev, Stn 48, 18 August 1993, Laptev Sea , 74°30.0ʹ N, 137°05.0ʹ E, depth 22 m, rock dredge, collectors M.K. Schmid and D. Piepenburg. GoogleMaps
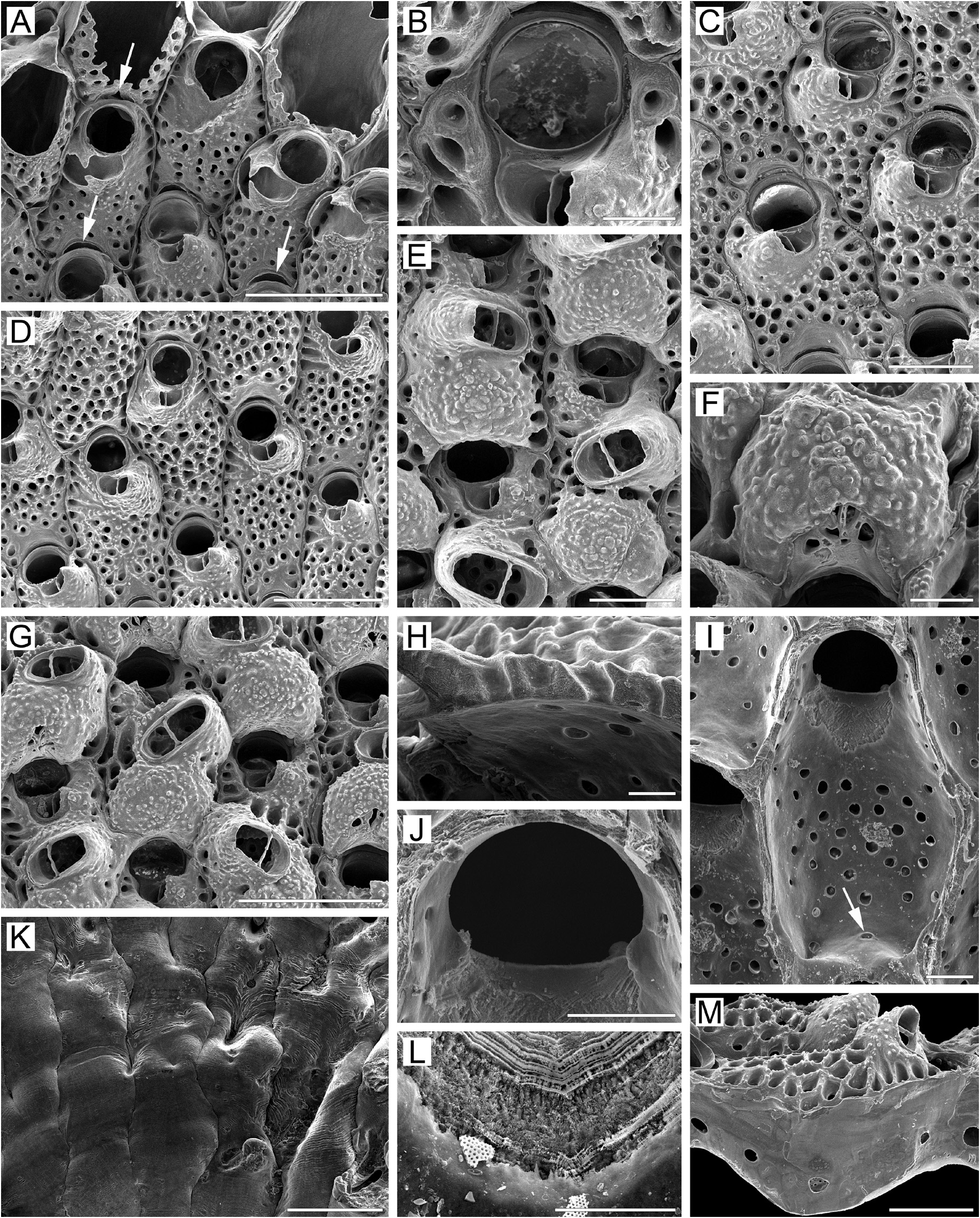
Measurements. ZIRAS 36/50120, western Kamchatka, Sea of Okhotsk ( Fig. 27A–M View FIGURE 27 ). ZL, 0.51–0.77 (0.63 ± 0.08). ZW, 0.32–0.53 (0.41 ± 0.05). ZD, 0.39–0.68 ( n = 2). OrL, 0.12–0.16 (0.14 ± 0.01). OrW, 0.15–0.20 (0.18 ± 0.02). OeL, 0.20–0.27 (0.23 ± 0.02). OeW, 0.28–0.38 (0.35 ± 0.03). Av(s)L, 0.12–0.17 (0.14 ± 0.01). Av(ad)L, 0.17–0.32 (0.23 ± 0.04). P(f)N, 29–40 (36) ( n = 10). P(oe)N, 2–7 (4) ( n = 10).
Description. Colonies encrusting, multiserial, unilaminar ( Fig. 27A View FIGURE 27 ), more or less circular or irregular in form, up to 22 mm in maximal dimension, yellow to pink-yellowish when alive, light brown to light yellow when dry. Zooids of medium size, oblong-hexagonal, oval to pyriform or rectangular, arranged in checkered pattern, demarcated by fine undulating sutures between lateral and transverse walls; sutures barely discernible in older parts of colony.
Frontal shield ( Fig. 27A, C, D View FIGURE 27 ) moderately convex, finely granulated, combining both umbonuloid and lepralioid portions. Marginal areolae lateral to orifice separated by short interareolar ridges, indicating umbonuloid area; rest of shield uniformly pseudoporous, with pits of various sizes and shapes (from circular and drop-like to oval and irregular), corresponding to lepralioid part. Pseudopores becoming more sunken and frontal shield coarsely reticulate with age, with 2–3 pseudopores sometimes lying in single depression ( Fig. 27G View FIGURE 27 ). Interior of frontal shield ( Fig. 27I View FIGURE 27 ) showing mixed nature. Ring scar irregular ( Fig. 27I, L View FIGURE 27 ), forming boundary between umbonuloid exteriorwall and extra-umbonuloid interior-wall microstructure. Umbonuloid component small, occupying about 20% of length of frontal shield (22% in one measured zooid).
Primary orifice ( Fig. 27A, B, J View FIGURE 27 ) broadly circular to transversely oval; distal and lateral margins formed by upper terminal part of distal transverse wall, incidentally with narrow rim and forming small, blunt condyles at proximolateral corners. Proximal margin shallow to moderately concave ( Fig. 27B, J View FIGURE 27 ), without lyrula. No oral spines.
Secondary orifice ( Fig. 27B View FIGURE 27 ) round to asymmetrically oval, cormidial; distolateral curvature formed by low, vertical thickening of proximal wall of daughter zooid and, occasionally, by lateral walls of adjacent distolateral zooids; proximally restricted by cystid of suboral avicularium on one side and very low thickening of frontal shield on opposite side, together forming inconspicuous peristome.
Cystid of suboral avicularium ( Fig. 27A–E View FIGURE 27 ) relatively small, broad, elevated, with coarsely granular surface and 1–4 minute communication pores, situated on left or right relative to orifice. Frontal surface (rostral/postmandibular areas) of avicularium slightly curved, concave, crossing zooidal midline, facing obliquely laterally. Rostrum ( Fig. 27B–D View FIGURE 27 ) elevated, short, lingulate, with weakly denticulate margin, directed laterally and frontally. Palatal foramen conforming to shape of rostrum, no cryptocystal shelf; opesia semioval. Crossbar complete.
Large adventitious avicularia developing in older areas of colony, occupying central to proximal part of frontal shield, frequently extending to marginal area of adjoining lateral zooid. Avicularian cystid broad, with coarsely granular surface ( Fig. 27E, G View FIGURE 27 ) and 2–5 minute communication pores. Frontal surface of avicularium facing obliquely distolaterally to proximolaterally, and frontally. Rostrum elevated, elongate oval to lingulate; palatal foramen conforming to shape of rostrum; opesia semioval. Crossbar complete.
Ovicells hyperstomial at all stages, cleithral ( Fig. 27E–G View FIGURE 27 ). Ooecium formed by distal autozooid around shallow crescentic concavity with communication pore at bottom, situated in proximalmost part of frontal shield adjacent to distal margin of maternal primary orifice ( Fig. 27A, C, D View FIGURE 27 ); pore leads to communication canal connecting ooecial and visceral coeloms, opening on inner side of frontal shield as oval communication pore near transverse wall ( Fig. 27I View FIGURE 27 ). Ooecium hemispherical, with weakly concave proximal margin and round to slit-like pseudopores, some occluded by secondary calcification, sometimes visible in limited subtriangular area of proximal surface of ectooecium ( Fig. 27F View FIGURE 27 ). Ectooecium initially smooth, rapidly overgrown by secondary calcification proceeding from frontal shields of daughter and adjacent zooids, frontal surface becoming granulated (except proximally), with 1–2 divergent sutures dividing calcification contributed by different surrounding zooids ( Fig. 27F View FIGURE 27 ).
Zooids interconnected by two mural pore chambers in each distolateral wall ( Fig. 27M View FIGURE 27 ). Communication pores form horizontal “band” or two multiporous septula in basal half of transverse walls.
Basal surface of zooids ( Fig. 27K View FIGURE 27 ) fully calcified, moderately convex, smooth or with coarse transverse lineation, lacking evident protuberances. Boundaries between zooids recognizable by fine, sinuous incisions.
Ancestrula and early astogeny not observed.
Remarks. Winston & Hayward (2012) mentioned neither condyles nor large adventitious avicularia in describing this species from the northwestern Atlantic. In contrast to our material, their colonies were loosely attached to substrata. Additionally, ooecia were almost free of secondary calcification, and the medial convex area had only a single pseudopore near the proximal margin, whereas ooecia in our specimens were heavily calcified, with several pseudopores of various sizes and shapes.
Ecology. Mixtoscutella ovata was recorded at depths of 3.5–582 m, mainly on mixed bottoms including silt, gravel and shells. Colonies encrusted brown and red algae, hydroid stolons, other cheilostome bryozoans ( Stomachetosella sp. ), mollusc shells, brachiopods and hydrocorals.
Distribution. This is a boreal-Arctic, circumpolar, sublittoral to upper bathyal species. Numerous Arctic records include the Barents Sea ( Smitt 1868a; Kluge 1915, 1929, 1962, 1975; Denisenko 1988, 1990), White Sea ( Gostilovskaya 1957, 1978; Grishankov 1995; Shunatova & Ostrovsky 2001; Shunatova & Nielsen 2002; Ostrovsky 2009, 2013), Kara Sea ( Nordgaard 1912; Kluge 1929, 1962, 1975; Denisenko 2021), Laptev Sea ( Kluge 1929, 1962, 1975; Gontar 1990), East Siberian Sea ( Kluge 1929, 1962, 1975; Gontar 1994a; Denisenko 2011), Chukchi Sea ( Kluge 1929, 1962, 1975; Denisenko 2008; Denisenko & Kuklinski 2008; Gontar 2010), Point Barrow, Beaufort Sea, Alaska ( Osburn 1952, 1955), Canadian Arctic Archipelago ( Nordgaard 1906; Osburn 1932, 1936), Baffin Bay ( Hansen 1962), Davis Strait ( Kluge 1962, 1975; Hansen 1962), Hudson Bay ( Gontar & Denisenko 1989), Labrador ( Hincks 1877; Gontar & Denisenko 1989), western Greenland ( Smitt 1868b; Kluge 1908b; Levinsen 1914; Osburn 1919; Denisenko & Blicher 2021), eastern Greenland ( Levinsen 1916; Denisenko & Blicher 2021) [ Smitt (1868b) just mentioned Greenland], Franz Josef Land ( Denisenko 1990), Iceland ( Hincks 1877); Spitsbergen ( Smitt 1868b, Gontar et al. 2001; Kuklinski 2002b, 2009) and northern Norway ( Nordgaard 1918). In the northwestern Atlantic it is recorded along the eastern coast of North America from the Gulf of St Lawrence ( Whiteaves 1901; Gontar & Denisenko 1989), Gulf of Maine, Woods Hole and Cape Cod ( Osburn 1912a, 1933; Powell 1968b; Winston & Hayward 2012). The only locality known from the northeastern Atlantic is the Faroe Islands ( Denisenko et al. 2016). In the northwestern Pacific it has been reported from Anadyrskiy Gulf in the Bering Sea ( Kluge 1961; Grischenko 2002) and from the waters along the eastern coast of the Kamchatka Peninsula including Kamchatskiy Gulf, Kronotsky Gulf ( Kubanin 1997; Grischenko 2002), Avacha Gulf ( Kluge 1961; Kubanin 1997; Grischenko 2002), Sea of Okhotsk in the waters along the southeast coast of Sakhalin Island ( Kluge et al. 1959; Kluge 1961), Shantar Archipelago ( Kluge 1961), western Kamchatka shelf (our data) and the Kuril Islands including Iturup, Zelenyy, Shikotan and South Kuril Strait ( Kluge et al. 1959; Kluge 1961; Gontar 1980, 1993b), Sea of Japan, in the waters of the southwestern coast of Sakhalin Island ( Androsova 1958; Kluge 1961) and continental slope of the northern part of the Sea of Japan ( Grischenko 2013). Northeastern Pacific records include Olga Bay and Leonard Harbor, Gulf of Alaska, and Punuk Island in the northeastern Bering Sea ( Osburn 1952).
| NHMUK |
Natural History Museum, London |
| V |
Royal British Columbia Museum - Herbarium |
| RV |
Collection of Leptospira Strains |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
SubOrder |
Flustrina |
|
SuperFamily |
Lepralielloidea |
|
Family |
|
|
Genus |
Mixtoscutella ovata (Smitt, 1868)
| Grischenko, Andrei V., Gordon, Dennis P., Taylor, Paul D., Kuklinski, Piotr, Denisenko, Nina V., Spencer-Jones, Mary E. & Ostrovsky, Andrew N. 2022 |
Rhamphostomella ovata :
| Kluge, G. A. 1962: 540 |
| Nordgaard, O. 1906: 32 |
Cellepora ovata Smitt, 1868a , p. 31
| Smitt, F. A. 1868: 31 |
Cellepora ovata : Smitt 1868b , p. 485
| Hincks, T. 1877: 105 |
| Smitt, F. A. 1868: 485 |