Parosmodes morantii morantii Trimen, 1873
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3724.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:7D05BB2E-4373-4AFB-8DD3-ABE203D3BEC1 |
|
DOI |
https://doi.org/10.5281/zenodo.7044038 |
|
persistent identifier |
https://treatment.plazi.org/id/0385994A-FFAD-FFF8-9BFD-F8C9FCC1BB5D |
|
treatment provided by |
Felipe |
|
scientific name |
Parosmodes morantii morantii Trimen, 1873 |
| status |
|
Parosmodes morantii morantii Trimen, 1873 View in CoL
Nominate subspecies morantii was described from South Africa (Natal) and recorded from there north along the western mountains of Tanzania, to Uganda, Angola and Zaire. There is an apparently disjunct population in southeastern Kenya, where subspecies morantii is commonest at the coast and is recorded inland to the Teita Hills, Mt Sagalla ( Larsen 1991) and the Chyulu Hills, where van Someren (1939) recorded a few at 915–1370m (3,000 – 4,500 ft.). MJWC has also found it further inland at Kibwezi Forest and Kiboko. Both Clark (in Dickson & Kroon 1978) and Sevastopulo (unpublished) have documented the life history of this species; the former, based on material from Durban, South Africa, found that sometimes there are six caterpillar instars. Henning et al. (1997) repeat Clark’s observations and include photographs by R. Paré of caterpillars and pupa.
Adult behaviour
The single adult MJWC encountered at Kibwezi Forest was sunbathing (cf. Figure 22.1 View FIGURE 22 ). Caterpillars and their distinctive final shelters are much more easily and commonly found than adults.
Food plants
The first food plant recorded is Combretum molle (= C. gueinzii ) ( Combretaceae ) in South Africa ( Platt 1921), and this is most likely the source of Pinhey’s (1949) and Gifford’s (1965) records of Combretum sp. Subsequent reports from South Africa expand the range of food plants: Dickson & Kroon (1978) record C. molle and Bridelia micrantha ( Phyllanthaceae , formerly Euphorbiaceae ); Pringle et al. (1994) add Syzygium cordatum (Myrtaceae) . Henning et al. (1997) and Woodhall (2005) add Terminalia sp. and Quisqualis sp. to this list, but these are probably based on East African records.
Thus, in his list of the food plants of East African Lepidoptera, Sevastopulo (1975) includes Combretum , Quisqualis and Terminalia . Sevastopulo (unpublished) recorded Quisqualis indica and Terminalia catappa as food plants at the Kenya coast, but attributes a record of Combretum sp. to Pinhey (i.e. Pinhey 1949), which as noted above, is probably derived from the earlier South African record. The food plants given by Kielland (1990), Larsen (1991, 2005) and Heath et al. (2002) repeat those of earlier workers.
In Kenya, MJWC has repeated Sevastopulo’s observations on Quisqualis indica (Diani Beach, 89/99) and Terminalia catappa (Diani Beach, 89/103, 95/108), and also reared this species from Combretum pentagonum (Diani Beach, 90/107) and Syzygium guineense (Kiboko, 95/100). The four penultimate instar caterpillars collected on this last host plant were transferred to T. catappa when their original food plant was no longer available, and although they accepted this new food, only one reached the pupal stage (and it was parasitized).
Ovum
The ova are laid individually in the middle of the leaf upper surface. They measure 1.4mm (n=3) in diameter at the base by 0.8mm (n=2) high. An unhatched ovum associated with this species ( Figure 23 View FIGURE 23 ) had 15 strong ribs from the base to a broad, pale, mat area on top; the sides were straight rather than rounded (but see discussion below).
Leaf shelters
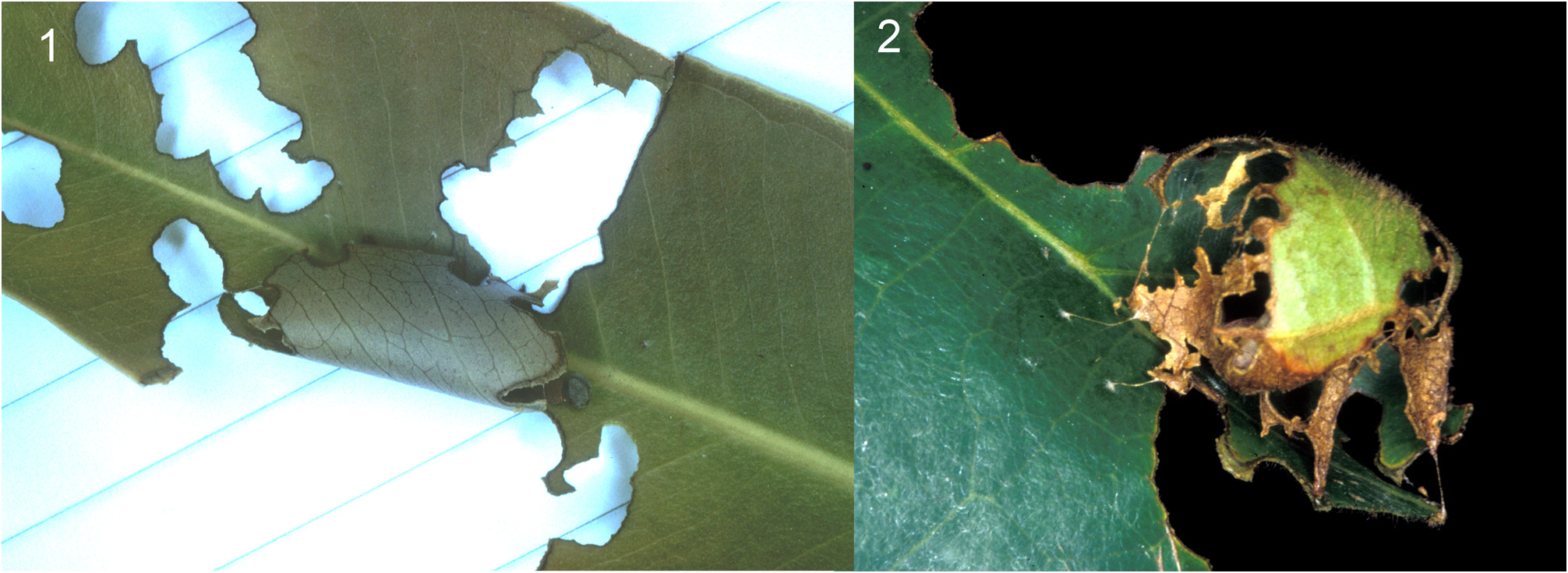
One form of the second shelter made on the large stiff mature leaf of Terminalia catappa is nearly globular, and is formed on a leaf main vein by rolling a flap up and over, leaving the veins and irregular flaps at the edge which are held in place with a scaffolding of silk strands; additional strands of silk anchor the structure to the leaf surface ( Figure 24.2 View FIGURE 24 ; 89/49B). The caterpillar rests across the main vein with its head at the end of the flap. Another form of stage 2 shelter was found on Syzygium guineense , which has smaller, less stiff leaves ( Figure 24.1 View FIGURE 24 ; 95/100A); one shelter was made on a flush leaf, and another three on mature leaves. It appears to have been formed as a two cut shelter, the lid folded over onto the leaf upper surface; the edges of the shelter and adjacent leaf have deep, irregular notches cut in them; the distal angles of the shelter have been eaten (or cut) to make the lid oval rather than a segment of a circle
The final instar caterpillar rests in the pupal shelter which it prepares. The shelter is made from a section of leaf containing a major vein; on Quisqualis indica all except the basal 10–25mm of leaf is used ( Figure 25.1 View FIGURE 25 ); on T. catappa an arc is cut out of the leaf apex ( Figure 25.2 View FIGURE 25 ). The whole shelter is about 40mm long; the leaf mid rib is bared for about 5mm at the base of the shelter, and strengthened with red silk (after partially biting it through in the case of T. catappa , so that it bent); the shelter hangs down by this bared section; the basal chamber in which the pupa is formed is about 20mm long, 9mm wide and 10mm high, and in cross section is nearly square; a deep notch is cut from the leaf, distal to the basal chamber, and the distal flaps (c. 12mm) are held together with silk. The shelters turn brown as the leaf portions desiccate, and the empty shelters may remain on the food plants long after the adult has emerged ( Figure 25.2 View FIGURE 25 ).
Caterpillar
The head capsule of the n-2 instar caterpillar measured 1.2 x 1.5mm (n=1) wide x high; the head was widest nearest the base with the sides relatively straight; brown; covered with short, pale setae, stalked with a palmate top. The penultimate instar caterpillar found in the shelter shown in Figure 24.1 View FIGURE 24 (95/100A) measured 15mm; head 1.8 x 2.1mm (n=4) wide x high; shape as previous instar; dark brown, covered with short pale setae, which have a very short stalk surmounted by an irregular, granular-surfaced, round plate; T1 concolorous with head; body dull browngreen with dark dorsal line, and covered with pale inconspicuous speckles; T2–T3 more yellow; A1–A8 a pinkishbrown, ventrolateral ridge; spiracles pale, those of T1 and A8 quite conspicuous; legs brown; prolegs concolorous.
The mature caterpillar is shown in Figure 27 View FIGURE 27 (89/49A). When collected, a similar caterpillar on Quisqualis indica (89/99A) measured 21mm; head 2.3 x 2.8mm (n=3); similar shape to previous instar; dark mat brown, with a dense covering of very light brown scales, of similar structure to those of previous instar; these scales are dark brown in a stripe down centre of face, and a broader one from each vertex down face, diverging slightly near mouth parts; T1 mat brown with very narrow dark dorsal plate; body dull green with yellow speckles A1–A8; dark green dorsal line from A2 to A8; legs T1 brown, other legs concolorous; spiracles inconspicuous. The mature caterpillar (95/100B) developed wax glands over the whole of the ventral surface A3–A8, apart from the prolegs and ventral line.
The photographs in Henning et al. (1997) show two forms of the final instar: a brown form which resembles the penultimate instar shown here ( Figure 24.1 View FIGURE 24 ), but with the same head as the final instar ( Figure 27.2 View FIGURE 27 ), and a green form, which resembles that shown here for the final instar ( Figure 27 View FIGURE 27 ). Their text does not discuss the two forms or state where they were found. Sevastopulo (unpublished) noted that at the beginning of the final instar the body colouring resembles that of the previous instar, but becomes increasingly green as the caterpillar grows; it could be that this is the case with the photographs in Henning et al. (1997).
Pupa
The pupa ( Figure 28 View FIGURE 28 ) is formed in the basal section of the pupal chamber which is lined with silk and covered with white waxy powder. It is 16mm long; brown, rounded, but flattened ventrally; the proboscis sheath almost reaches the cremaster; thorax and head with pale, erect setae, those of head with entrapped white waxy powder; abdomen with pale, recumbent setae; spiracle T1 oval, light brown, protruding. Pupation takes 11–14 days.
Natural enemies
An unidentified larval-pupal tachinid parasitoid was reared from a pupa formed by caterpillar collected in the penultimate instar on Syzygium guineense , 17 Aug 1995, Kiboko, Kenya (95/100C). The mature fly larva emerged from the pupa and formed its puparium within the pupal shelter. The adult fly emerged 21 days after the host pupated. A pupa collected in an old shelter on T. catappa at Diani Beach, (22 Aug 1995, 95/108) had two round holes laterally in the abdomen suggesting that it had been parasitized, perhaps by a Brachymeria sp. as there was no indication of puparia or parasitoid cocoons in the shelter.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
SubFamily |
Hesperiinae |
|
Genus |