Gorgocephalus yaaji, BRAY & CRIBB, 2005
|
publication ID |
https://doi.org/10.1093/zoolinnean/zlab002 |
|
publication LSID |
lsid:zoobank.org:pub:AAA956A8-14F7-49E4-888F-072FAC7D3826 |
|
DOI |
https://doi.org/10.5281/zenodo.5761788 |
|
persistent identifier |
https://treatment.plazi.org/id/03815953-FFA0-2E09-147F-7ACDFEF81AF3 |
|
treatment provided by |
Plazi |
|
scientific name |
Gorgocephalus yaaji |
| status |
|
GORGOCEPHALUS YAAJI BRAY & CRIBB, 2005 View in CoL
( FIGS 9D–F View Figure 9 , 10 View Figure 10 ; TABLES 3 View Table 3 , 6)
Synonym: ‘ Gorgocephalus sp. Aus’ of O’Dwyer et al. (2015).
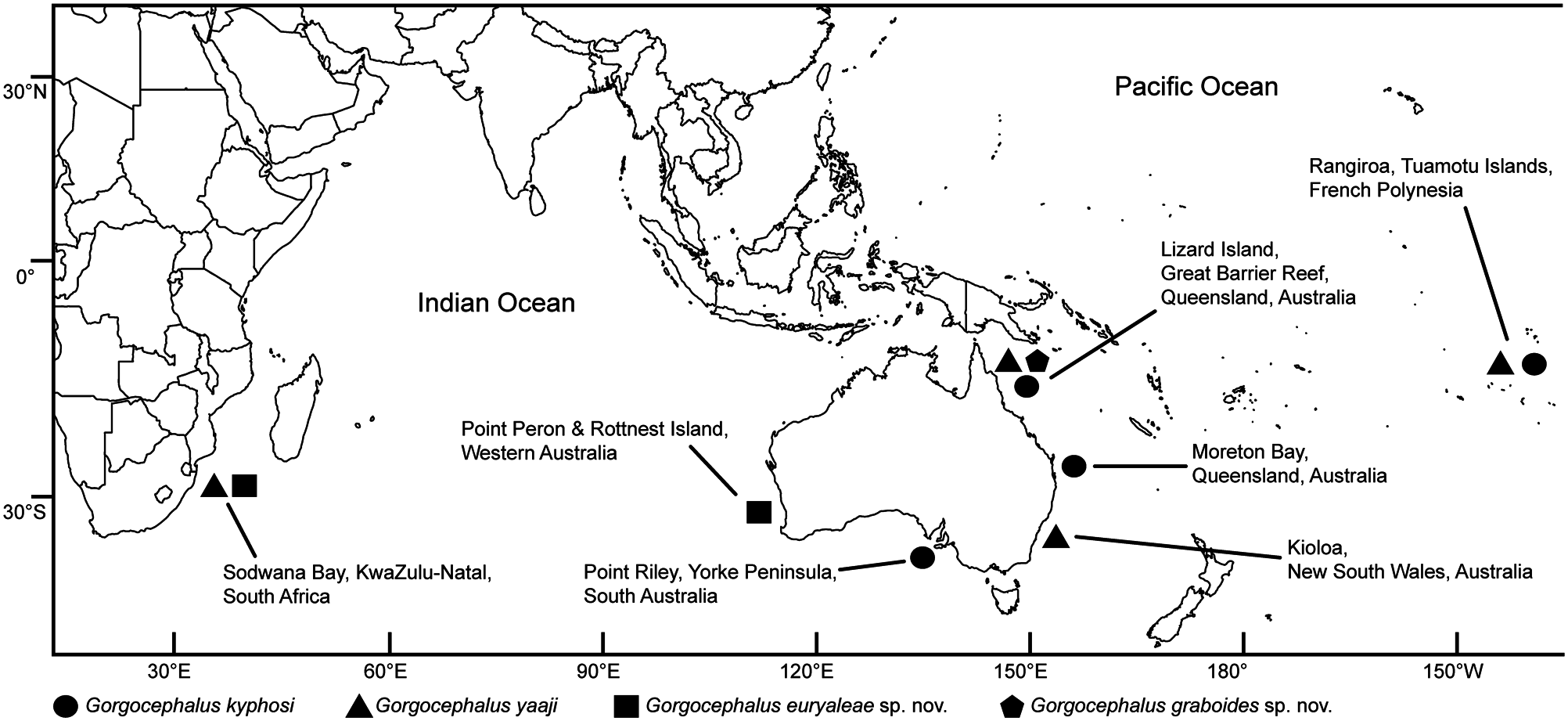
Type host and locality: Kyphosus vaigiensis (Quoy & Gaimard, 1825) ( Perciformes : Kyphosidae ), brassy chub from off Lizard Island, Great Barrier Reef, Queensland, Australia ( 14°41’10’’S, 145°28’15’’E).
Records: 1, Bray & Cribb (2005); 2, O’Dwyer et al. (2015); 3, Huston et al. (2016); 4, present study.
Definitive hosts: Perciformes , Kyphosidae . Kyphosus cinerascens (Forsskål, 1775) , highfin chub (3, 4); Kyphosus elegans (W. K. H. Peters, 1869) , Cortez sea chub (4); Kyphosus vaigiensis (Quoy & Gaimard, 1825) (1, 4).
Intermediate hosts: Gastropoda, Littorinimorpha , Littorinidae . Austrolittorina unifasciata (Gray) (2, 3); Echinolittorina austrotrochoides Reid, 2007 (3); Echinolittorina cinerea (Pease, 1869) (4).
Other localities: Gerringong , New South Wales, Australia ( 34°44’18’’S, 150°49’29’’E) (2) GoogleMaps ; Kioloa , New South Wales, Australia ( 35°33’38’’S, 150°22’27’’E) ( KI) (3) GoogleMaps ; Shellharbour , New South Wales, Australia ( 34°34’59’’S, 150°52’E) (2) GoogleMaps ; Sydney , New South Wales, Australia ( 33°52’42’’S, 151°12’34’’E) (2) GoogleMaps ; Ulladulla , New South Wales, Australia ( 35°21’17’’S, 150°27’43’’E) (2) GoogleMaps ; off Rangiroa , Tuamotu Islands, French Polynesia ( 15° 10’ 40’’ S, 147 °39’ 04’’ W) (4) GoogleMaps ; Sodwana Bay , KwaZulu-Natal, South Africa ( 27°32’24’’S, 32°40’41’’E) (SB) GoogleMaps (4).
Voucher material (adult): Ten whole-mount and three hologenophore specimens, ex K. vaigiensis from LI (QM G238592–G238604); six whole-mount and three hologenophore specimens ex K. cinerascens from RA (MNHN HEL1447–1455); one whole-mount specimen ex K. elegans from RA (MNHN HEL1456); three whole-mount and one hologenophore specimen ex K. cinerascens from SB (NMB 730); six whole-mount specimens ex K. vaigiensis from SB (NMB 731).
Voucher material (intramolluscan): Eight slides of rediae and cercariae ex E. cinerea from RA (MNHN HEL1457–1464).
Site in host: Upper intestine (definitive); gonad/ digestive gland (intermediate).
Representative DNA sequences: Nineteen sequences ( MW353650 View Materials – MW353668 View Materials ), using primers of Wee et al. (2017), and three sequences ( MW350141 View Materials – MW350143 View Materials ), following O’Dwyer et al. (2016) (see methods) deposited for COI mtDNA; 17 sequences deposited for 5.8S-ITS2- partial 28S rDNA ( MW353931 View Materials – MW353947 View Materials ); nine sequences deposited for partial 28S rDNA ( MW353889 View Materials – MW353897 View Materials ); see Supporting Information, Table S2. View Table 2
Description of adult ( Figs 9D –F View Figure 9 , 10 View Figure 1 A – C, F: Measurements in Table 3 View Table 3 ). Description based upon all adult voucher material and SEM images of three adults. Body elongate-oval, somewhat dorsoventrally flattened, broadest in region of ventral sucker, tapering slightly posteriorly. Tegument armed with alternating rows of partially overlapping comb-like scales; distal portion of scales forming up to 18 distinct tendrils. Eyespot pigment sparsely scattered in forebody. Oral sucker terminal, partially retractable, infundibuliform, broadest in anterior region with distinct reduction in diameter about mid-length continuing through to posterior margin; margin of anterior portion bearing crown of 14 bifid tentacles; outer branch of tentacles broad, conoid; inner branch of tentacles approximately equal in length to outer, tendril-like, tapering distally. Ventral sucker in anterior third of body, round, smaller than oral sucker. Prepharynx short, distinct, sigmoid or looped. Pharynx ellipsoidal to dolioform, in line with oral sucker or rotated up to 45°. Oesophagus short, bifurcates just posterior to pharynx with proximal section reaching to ventral surface and opening as ‘ventral anus’, and distal portion expanding to form caecum. Caecum single, broadest in anterior region, passes from mid-forebody to close to posterior extremity, terminates blindly; gastrodermis well developed.
Testes two, ellipsoidal, tandem, contiguous, in midhindbody. Vasa deferentia narrow, passing relatively direct from testes to cirrus-sac. Cirrus-sac elongate, cylindrical, winding, reaching from anterior testis to level of ventral sucker; anterior portion curves back posteriorly from ventral sucker to genital atrium. Internal seminal vesicle tubular, bends slightly about mid-length, occupies about half length of cirrus-sac. Pars prostatica distinct, vesicular, approximately equal in length to internal seminal vesicle, lined with anuclear cell-like bodies. Ejaculatory duct long, occupies recurved portion of cirrus-sac. Genital atrium broad, dorsal and approximately equal in size to ventral sucker. Genital pore large, round to irregular, opening dorsodextrally at level of ventral sucker.
Ovary pre-testicular, ellipsoidal to subglobular. Mehlis’ gland between ovary and anterior testis. Laurer’s canal opens dorsally posterior to ovary. Canalicular seminal vesicle saccular, contiguous with and dorsal to ovary. Uterus narrow, passes posteriorly from oötype to anterior testis, loops back, gently winding anteriorly, forming muscular metraterm about mid-level of pars prostatica, opening into genital atrium adjacent to ejaculatory duct. Eggs few, oval, operculate, large; length of eggs often exceeding that of ovary. Vitellarium follicular, in fore- and hindbody, fields reaching from pharyngoesophageal region to near posterior extremity; dorsal, lateral and ventral fields confluent, wrap around body from dorsal midline to ventrosinistral and ventrodextral regions anterior to testes, wrap entire body posterior to testes. Vitelline reservoir between ovary and anterior testis; collecting ducts indistinct. Excretory pore terminal; excretory vesicle Y-shaped, passes anteriorly, bifurcating in testicular region, ducts passing anteriorly sinistrally and dextrally, terminating as enlarged pyriform sacs on either side of cirrus-sac.
Description of redia ( Fig. 10D View Figure 10 ): Measurements in Table 6. Description based on voucher material. Body ellipsoidal, broadest posteriorly; distinctive protuberance arising from posterior extremity in most specimens. Cercarial embryos numerous, poorly developed. Mouth subterminal. Pharynx ellipsoidal. Intestine short, globular, immediately posterior and subequal in size to pharynx.
Description of cercaria ( Fig. 10E View Figure 10 ): Measurements in Table 6. Description based on voucher material. Oculate gymnocephalous cercariae. Body elongate, ellipsoidal. Eyespots two, in anterior forebody; eyespot lenses present in live naturally emerged specimens, not present in immature specimens. Oral sucker subterminal, infundibuliform. Ventral sucker post-equatorial, round. Prepharynx short, passes between eyespots. Pharynx ellipsoidal. Caecum single, terminating near anterior margin of ventral sucker. Tail bipartite; proximal portion bearing series of lateral projections; distal portion scaled, lacking lateral projections. Excretory vesicle Y-shaped; arms extending to ventral sucker, stem extending to near posterior extremity; posterior collecting duct visible to first few scales of distal portion of tail; anterior collecting ducts not visible beyond ventral sucker. Genital primordia darkly stained, dorsal to ventral sucker.
Remarks: Gorgocephalus yaaji occurs across a wider geographic range than any other species of the family. Indeed, to our knowledge the finding of G. yaaji in French Polynesia, South Africa, and in between, is the first report, supported by molecular data, of naturally occurring populations of a single marine digenean species occurring across the whole breadth of the IWP. Although it has fewer definitive host records than G. kyphosi , three gastropod intermediate hosts are known for G. yaaji , the most for any species of the family. O’Dwyer et al. (2015) described the first gorgocephalid cercariae from the littorinid A. unifasciata , but did not connect these infections to an adult gorgocephalid, because their sequences did not match those of G. kyphosi from the study of Olson et al. (2003), the only sequences available for the family at that time. Huston et al. (2016) described the cercariae of G. yaaji from Echinolittorina austrotrochoides from Lizard Island, GBR, and determined that, based on the difference in host, molecular differences in the ITS2 and 28S rDNA gene-regions, and morphological differences, the infections characterized by O’Dwyer et al. (2015) were likely those of an undescribed species. However, by adding an additional molecular marker ( COI) and performing expanded phylogenetic study of the family, we have demonstrated that the intramolluscan infections described by O’Dwyer et al. (2015) are best interpreted as representative of G. yaaji . The host, molecular and morphological differences reported by Huston et al. (2016) between their material and that of O’Dwyer et al. (2015) are clearly within the bounds of intraspecific variation for G. yaaji .
Huston et al. (2016) noted that the morphological differences between their cercarial specimens of Gorgocephalus yaaji and those of O’Dwyer et al. (2015) may have been a result of differences between ‘naturally emerged’ cercariae and cercariae excised from gastropods. O’Dwyer et al. (2015) studied live and fixed cercariae dissected from gastropods, whereas Huston et al. (2016) studied live and fixed naturally emerged cercariae. Naturally emerged cercariae are generally considered as ‘mature’, whereas cercariae obtained from dissection of a gastropod may include mature and immature individuals. As stated above, the morphometric differences observed between G. yaaji cercariae appear due to normal intraspecific variation. However, one of the key morphological differences between the material of O’Dwyer et al. (2015) and Huston et al. (2016) was the observation of distinct ‘lenses’ in the eyespots of the live cercariae from the latter study. O’Dwyer et al. (2015) studied live material from dissected gastropods and is unlikely to have missed such a distinctive feature if it was present. Huston et al. (2016) interpreted this lack of eyespot lenses as indicative of a species-level difference. However, we did not obtain naturally emerged cercariae of G. yaaji from Echinolittorina cinerea in French Polynesia, although we did study live material from dissected gastropods. We did not observe eyespot lenses in the French Polynesian cercariae. Thus, as all of these cercariae are conspecific, we interpret the presence of eyespot lenses in the cercariae from the study of Huston et al. (2016) as a feature that is likely present only in mature, naturally emerged cercariae.
Five of the six known intermediate host records for gorgocephalids are attributable to Gorgocephalus kyphosi and G. yaaji . These two species have yet to be confirmed as having overlapping intermediate host ranges, but this remains possible. We obtained naturally emerged cercariae of G. kyphosi from an infected Echinolittorina vidua from Lizard Island, GBR, the same locality from which Huston et al. (2016) obtained naturally emerged cercariae of G. yaaji from E. austrotrochoides . Although there are no clear morphometric differences between the cercariae of G. kyphosi and G. yaaji , we did not observe eyespot lenses in the cercariae of G. kyphosi . However, as naturally emerged cercariae were studied from only a few infected gastropods, at present it is difficult to have confidence in the validity of this character for species differentiation. Additionally, Huston et al. (2016) described the rediae of G. yaaji from infected E. austrochoides from Lizard Island, GBR, with a strange posterior ‘protuberance’. We observed the same feature in the rediae of G. yaaji from infected E. cinerea from Rangiroa, French Polynesia. This feature was not present in any of the rediae of G. kyphosi that we observed. We do note that this ‘protuberance’ was not reported by O’Dwyer et al. (2015), so this would seem a somewhat tenuous feature for the differentiation of species. Despite repeated attempts, we were never able to obtain naturally emerged cercariae of G. kyphosi from Bembicium auratum . Considering the cryptic morphology of gorgocephalid cercariae, and the difficulty of obtaining naturally emerged specimens, use of molecular data for the identification of intramolluscan gorgocephalid infections will be required in most cases.
As noted above, Bray & Cribb (2005) reported the number of oral sucker tentacles in G. yaaji as 14–17; based on our SEM images we believe that this species, and all known gorgocephalids, have only 14 bifid tentacles. Although Manter (1966) reported G. kyphosi from both the pyloric caeca and intestine of K. sydneyanus, Bray & Cribb (2005) found that in K. vaigiensis from Lizard Island, G. kyphosi was restricted to the pyloric caeca whereas G. yaaji was found in the intestine. Our observations agree. With the exception of G. yaaji , all species of Gorgocephalus we collected were found only in the pyloric caeca. We found G. yaaji in the upper intestine, just posterior to the pyloric caeca.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
SubClass |
Digenea |
|
Order |
|
|
SuperFamily |
Lepocreadioidea |
|
Family |
|
|
Genus |