Aeolothrips tenuicornis Bagnall, 1926
|
publication ID |
https://doi.org/10.11646/zootaxa.4446.3.3 |
|
publication LSID |
lsid:zoobank.org:pub:339D34DB-ED59-4F9C-9FA0-2C07B21DA2BB |
|
DOI |
https://doi.org/10.5281/zenodo.5950120 |
|
persistent identifier |
https://treatment.plazi.org/id/0380CC59-C57F-C01D-2D95-A626FB9CD2B7 |
|
treatment provided by |
Plazi |
|
scientific name |
Aeolothrips tenuicornis Bagnall, 1926 |
| status |
|
Aeolothrips tenuicornis Bagnall, 1926 View in CoL
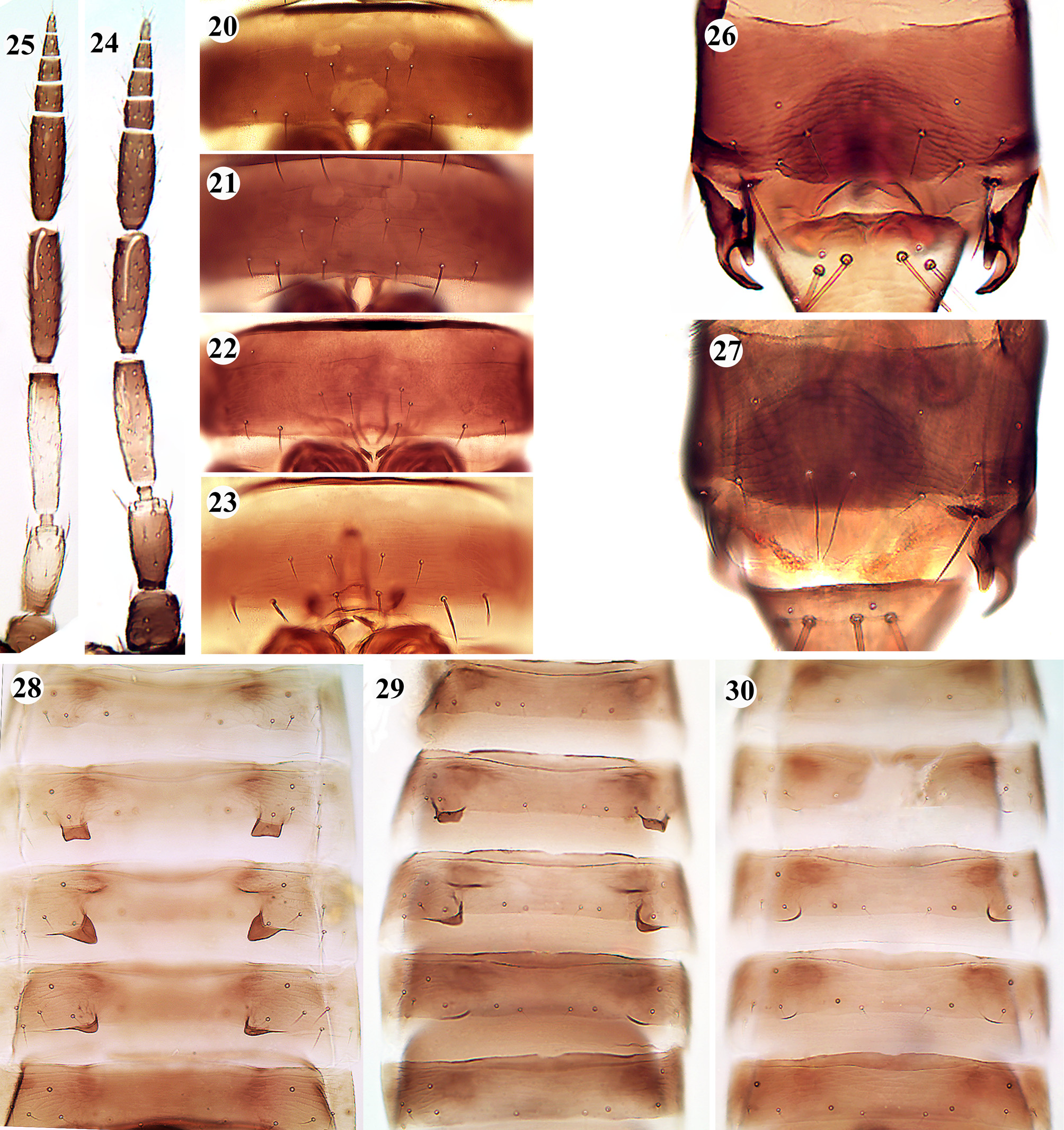
( Figs 20–30 View FIGURES 20–30 )
This species is widespread in Europe and the Mediterranean to the Middle East (zur Strassen 2003), as well as Iran ( Bhatti et al. 2009; Minaei 2013a). Four synonyms are listed in ThripsWiki (2018), primarily due to the brief descriptions by earlier workers such as Bagnall (1926), and the lack of comparisons with the type species. Character states used by Bagnall (1933, 1934) emphasizing length of antennal segments III–IV, sensorium length, as well as the colour of the antennae and pronotum are here recognized as unsatisfactory for separation of Aeolothrips species.
Although, zur Strassen (1996) stated that sternite VII setae S1 being closer to each other than to setae S2 is not a constant character for A. tenuicornis , he used this character to distinguish that species along with seven others in the identification key to Aeolothrips of Europe and the Mediterranean (zur Strassen 2003, couplet 18). Moreover, according to couplet 19 in that key, the sensorium on antennal segment IV extends scarcely to the apical half or less, while in most of the individuals from Iran, the sensorium extends to beyond of basal half of this segment. The same problem is true for other keys, such as by Priesner (1948, 1965).
Titschack (1964), was the first to mention the existence of great variation in A. tenuicornis , and placed, A. anthyllidis and A. clavicornis , as synonyms of this species. Later, two further species, A. ghabni and A. bucheti , were considered as synonyms of A. tenuicornis (zur Strassen 1996). Moreover, zur Strassen (1996) claimed that the degree of variation in the Mediterranean populations is more significant than that found north of the Alps, however the males are less affected than females.
It seems likely that there are differences between populations from Iran and other places in certain characters in A. tenuicornis . In almost in all specimens of this species from Iran, the distance between setae S1–S1 on abdominal sternite VII is equal ( Fig. 20 View FIGURES 20–30 ) or slightly more than the distance between setae S1–S2 ( Fig. 21 View FIGURES 20–30 ), whereas zur Strassen (1996, 2003) states this distance is clearly less in Central European specimens, and is usually about equal in specimens from Mediterranean and southern populations ( Fig. 23 View FIGURES 20–30 ). This is confirmed after studying the specimens from Europe listed below. In only one female among about 190 from different parts of Iran, was the separation between S1–S1 less than between S1–S2 ( Fig. 22 View FIGURES 20–30 ).
Antennal segments III–IV in females of this species from Iran are at most 4.5 and 4 times as long as broad, respectively ( Fig. 24 View FIGURES 20–30 ), while in females from Europe these ratios are as much as 5.8 and 5 ( Fig. 25 View FIGURES 20–30 ). Moreover, the ratio of the length of antennal segment V to lengths VI–IX in females is different between Iranian and European populations; often this ratio is somewhat less than 1 (or equal) in Iranian individuals, while in specimens from Europe it is more than 1. In the Mediterranean area, a tendency towards lighter pronotum is more common than in central European individuals (zur Strassen 1996) while in the Iranian specimens the pronotum is almost always dark brown.
In males, chaetotaxy of tergite IX differs significantly between the Iranian and European populations; so that, in Iranian individuals the paired innermost setae S1 of dark plate on tergite IX are shorter, somewhat straight, and far from each other, and somewhat closer to lateral setae S2 ( Fig. 26 View FIGURES 20–30 ), while in European individuals they are longer, curved, distinctly close to each other, and far from lateral setae S2 ( Fig. 27 View FIGURES 20–30 ). In this regard, Egyptian males appear to have an intermediate condition between Iranian and European males (see Priesner 1938, Fig. 1 View FIGURES 1–11 ), whereas Iranian females are more similar to that of Egyptian in many characters (see Priesner 1938). In European individuals dorsal tubercles of tergites IV–VI in male are clearly longer than those in Iranian individuals ( Fig. 28 View FIGURES 20–30 ). The length of the tubercles is also varied between Iranian individuals, and in some rather cases, they are almost vestigial ( Figs 29–30 View FIGURES 20–30 ).
The differences in length of antennal segments III and IV, and the colour of antennal segments II and III are the most important variations in various populations of Iran; in females, length to width ratio of antennal segment III varies between 3.5 and 4.5, and for antennal segment IV varies between 3 and 3.6. The distal yellow part of antennal segment II may occupy wide range between 0.2 to 0.8 as long as the length of segment. Antennal segment III is usually light brown, lighter in basal half, with gradually darkness in distal half, but degrees of more brightness are commonly observed in Iranian individuals.
Specimens studied from Europe (specimens from Iran excluded). FRANCE, Roussillon, St. Cyprien Sud , 1 male, von Onagra grandiflora , ix.1995, M. Ulitzka . PORTUGAL, Algarve, Castro Marim, 2 females, an weisser Hauswand , vi.2013, A. Dobrindt ; 1 male, same data, Auf der Haut bei Stechen. A. Dobrindt . SPAIN, Islas Canarias, La Palma , 1 female, from flowers, 21.iii.2011, S. Kobro.
FIGURES 37–51. Aeolothrips species. Antenna. Female (37–50): (37) A. gloriosus , (38) A. wittmeri , (39) A. cursor , (40) A. versicolor , (41) A. iranicus , (42) A. albithorax , (43) A. melaleucus , (44) A. laurencei , (45) A. desrticola , (46) A. collaris , (47) A. fasciatus , (48) A. mongolicus , (49) A. intermedius , (50) A. eremicola . Male: (51) A. eremicola .
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |