Philodoria, Walsingham, 1907
|
publication ID |
https://doi.org/10.11646/zootaxa.4944.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:380D2F75-D4F9-4974-97E2-25E0C62CB3B0 |
|
DOI |
https://doi.org/10.5281/zenodo.4683210 |
|
persistent identifier |
https://treatment.plazi.org/id/038087CB-FFE7-0700-FF75-92FBFBA0A4AA |
|
treatment provided by |
Plazi |
|
scientific name |
Philodoria |
| status |
|
Genus PHILODORIA Walsingham, 1907 View in CoL
Hawaiian Name: Hunelele ‘elilau
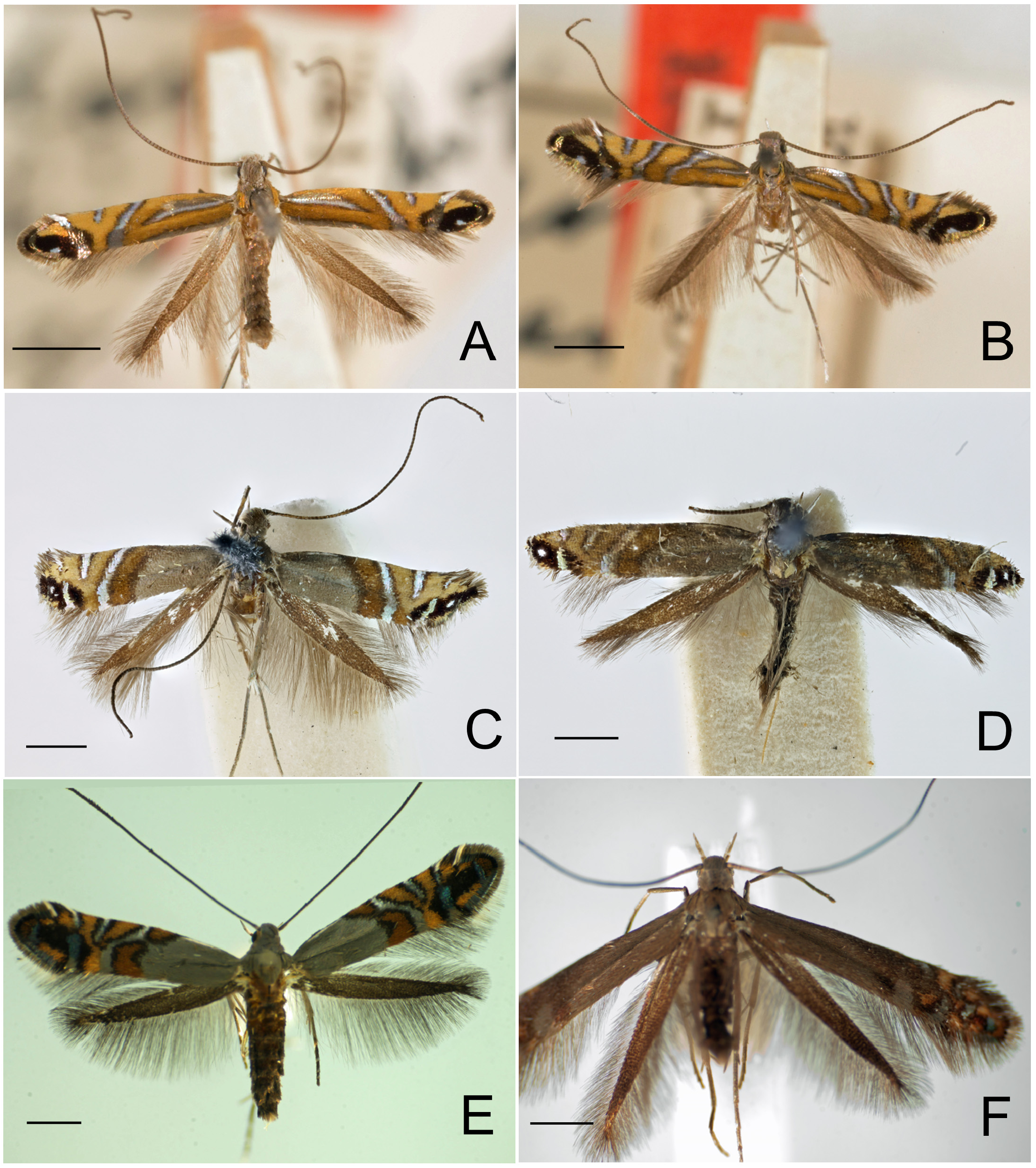
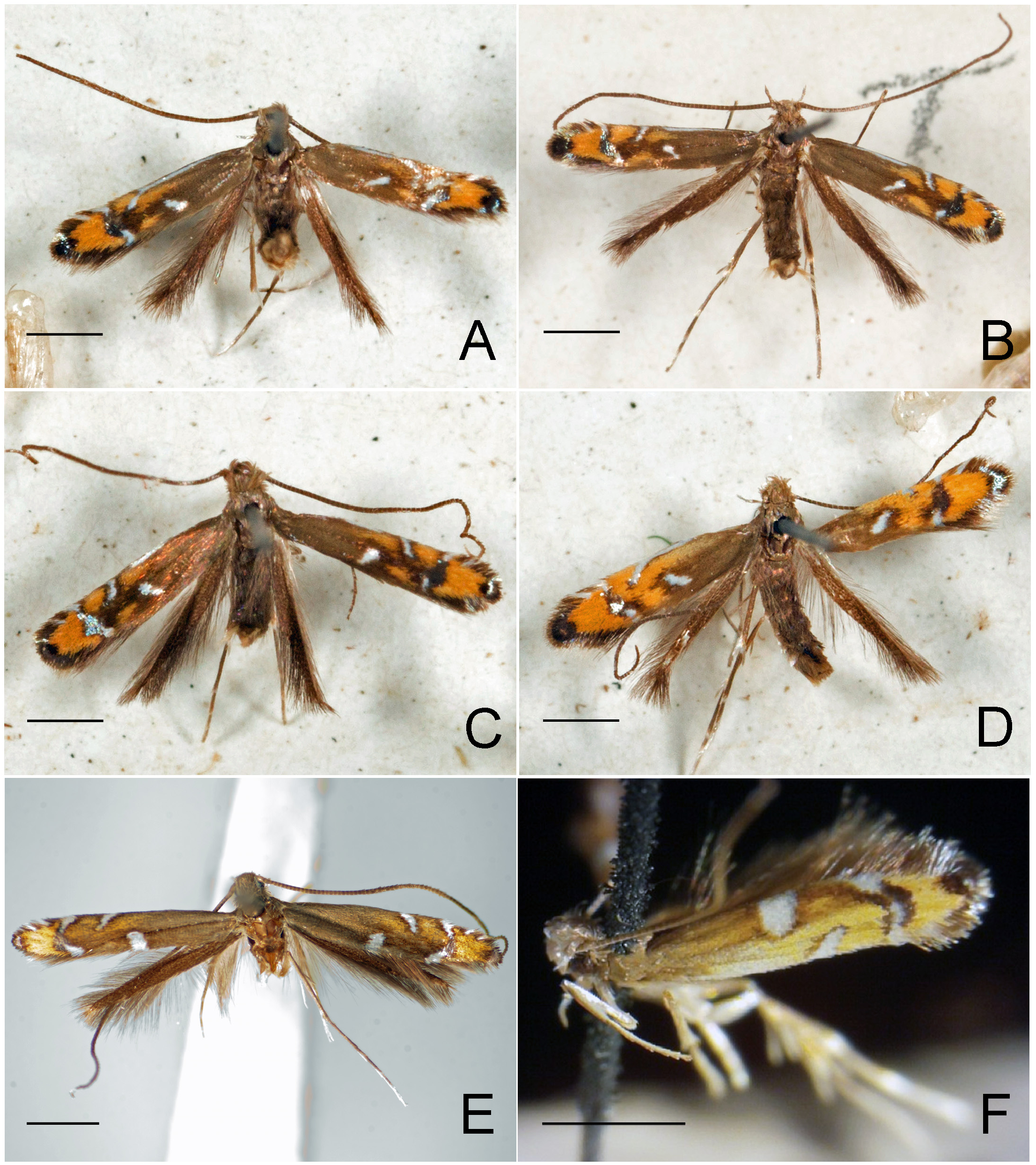
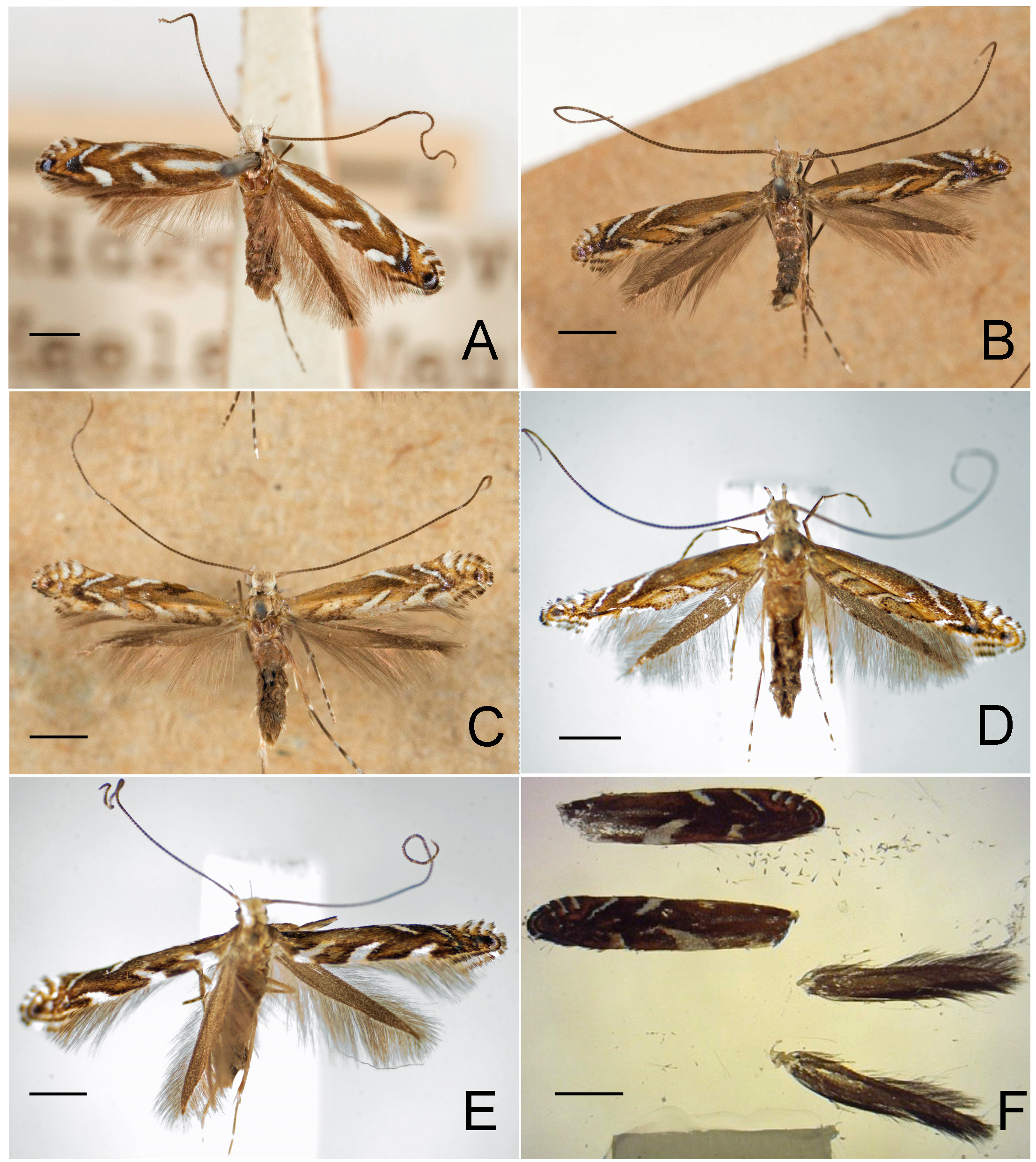
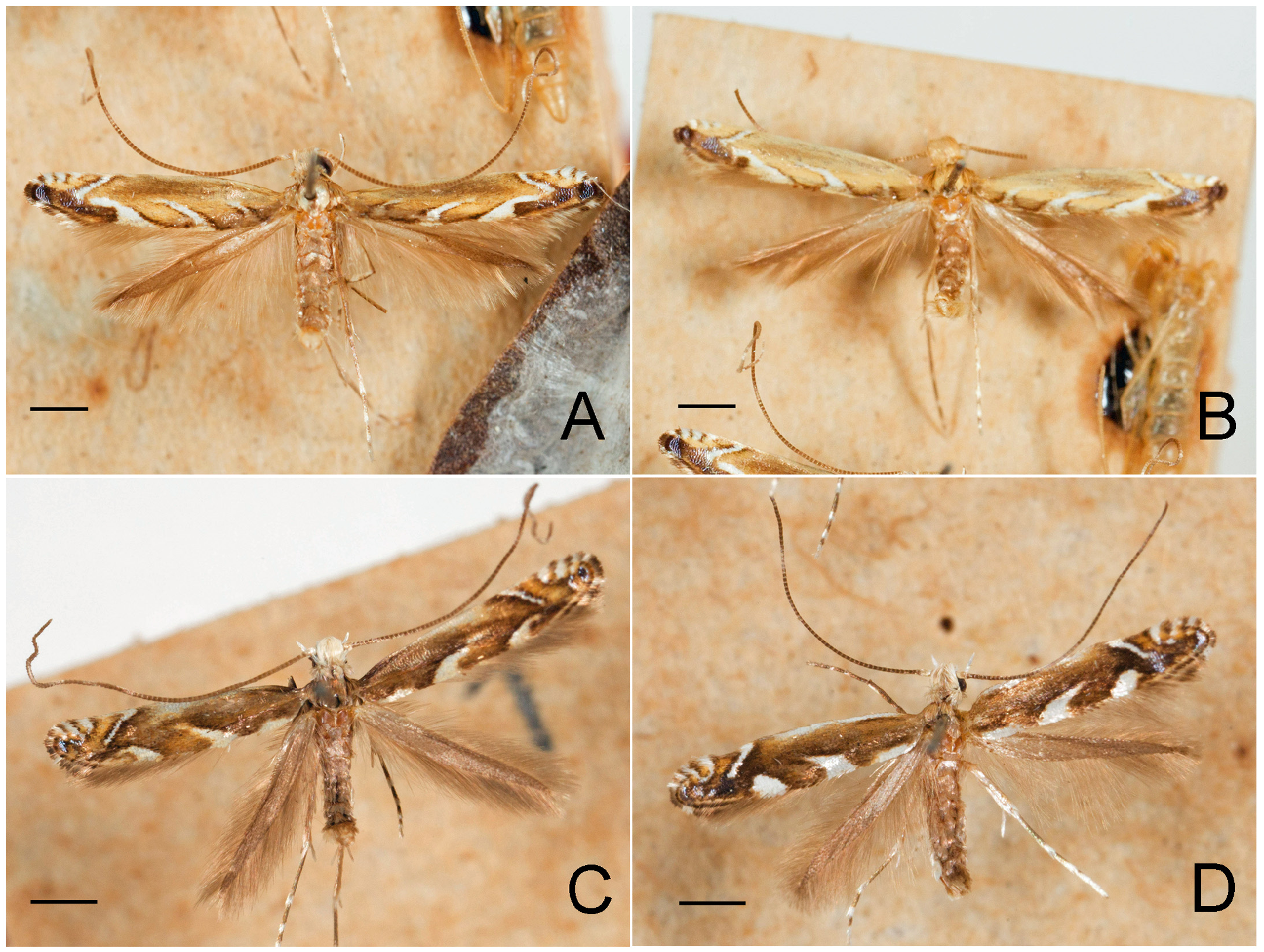
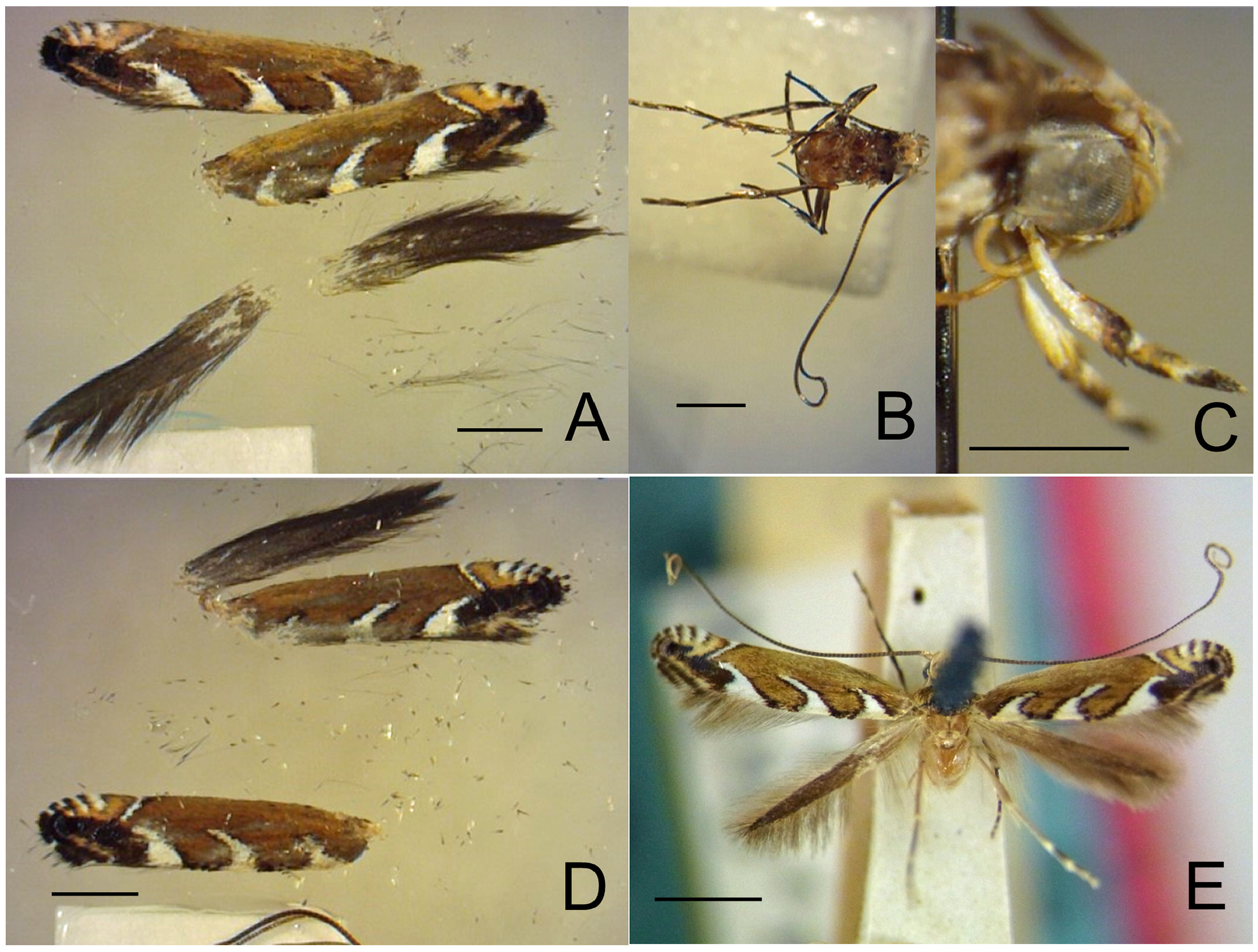
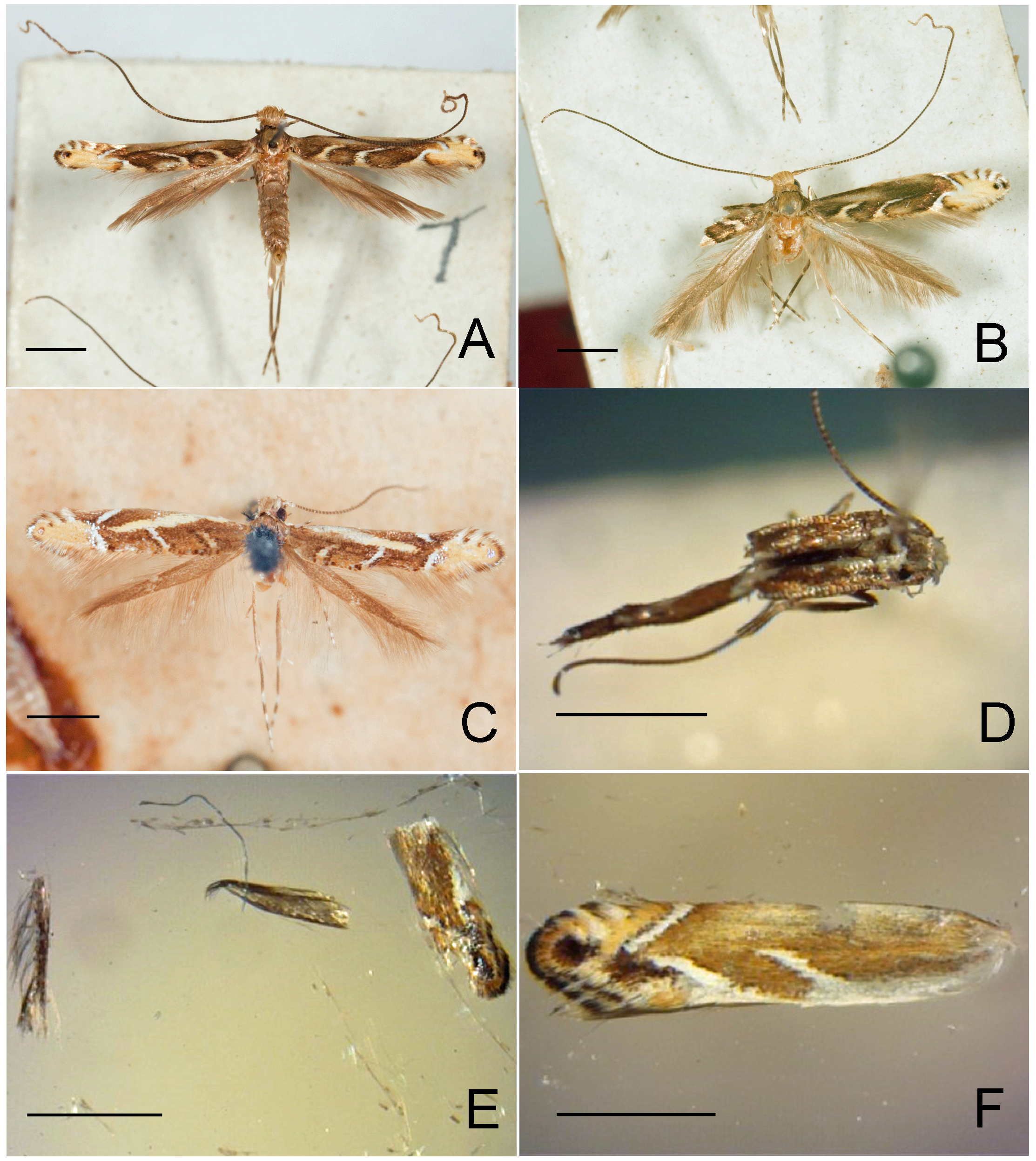
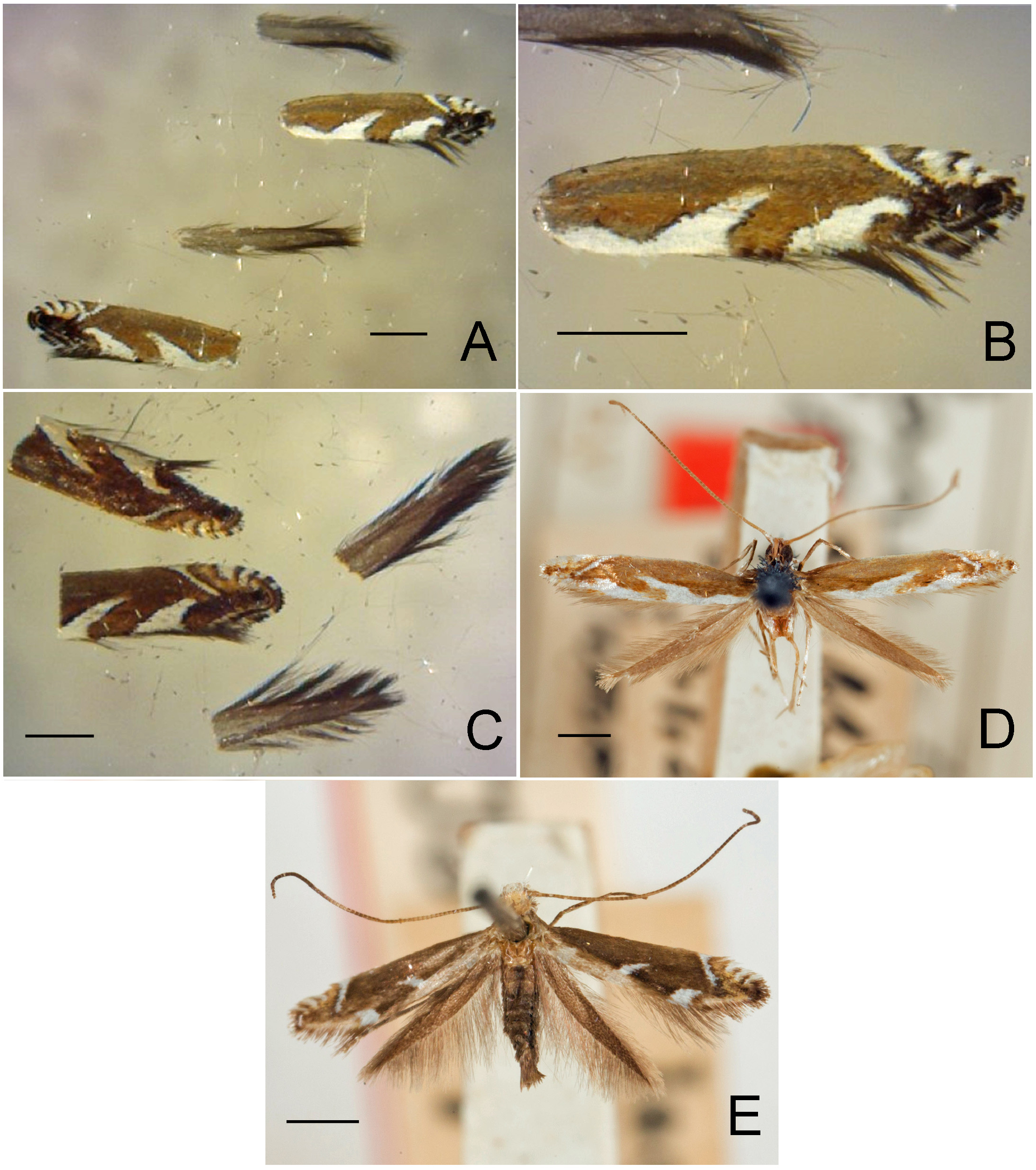
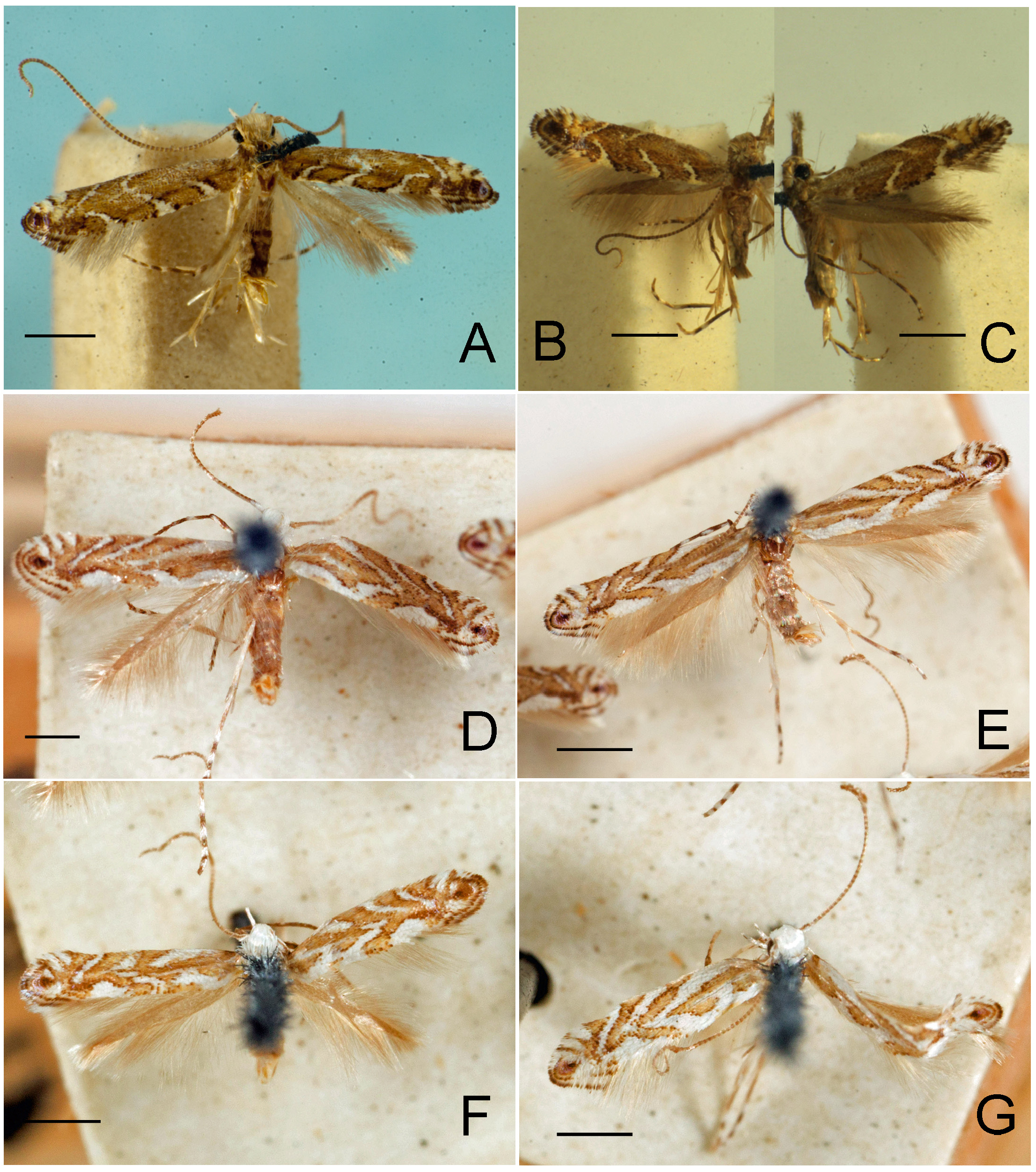
Figs. 3–95 View FIGURE 3 View FIGURE 4 View FIGURE 5 View FIGURE 6 View FIGURE 7 View FIGURE 8 View FIGURE 9 View FIGURE 10 View FIGURE 11 View FIGURE 12 View FIGURE 13 View FIGURE 14 View FIGURE 15 View FIGURE 16 View FIGURE 17 View FIGURE 18 View FIGURE 19 View FIGURE 20 View FIGURE 21 View FIGURE 22 View FIGURE 23 View FIGURE 24 View FIGURE 25 View FIGURE 26 View FIGURE 27 View FIGURE 28 View FIGURE 29 View FIGURE 30 View FIGURE 31 View FIGURE 32 View FIGURE 33 View FIGURE 34 View FIGURE 35 View FIGURE 36 View FIGURE 37 View FIGURE 38 View FIGURE 39 View FIGURE 40 View FIGURE 41 View FIGURE 42 View FIGURE 43 View FIGURE 44 View FIGURE 45 View FIGURE 46 View FIGURE 47 View FIGURE 48 View FIGURE 49 View FIGURE 50 View FIGURE 51 View FIGURE 52 View FIGURE 53 View FIGURE 54 View FIGURE 55 View FIGURE 56 View FIGURE 57 View FIGURE 58 View FIGURE 59 View FIGURE 60 View FIGURE 61 View FIGURE 62 View FIGURE 63 View FIGURE 64 View FIGURE 65 View FIGURE 66 View FIGURE 67 View FIGURE 68 View FIGURE 69 View FIGURE 70 View FIGURE 71 View FIGURE 72 View FIGURE 73 View FIGURE 74 View FIGURE 75 View FIGURE 76 View FIGURE 77 View FIGURE 78 View FIGURE 79 View FIGURE 80 View FIGURE 81 View FIGURE 82 View FIGURE 83 View FIGURE 84 View FIGURE 85 View FIGURE 86 View FIGURE 87 View FIGURE 88 View FIGURE 89 View FIGURE 90 View FIGURE 91 View FIGURE 92 View FIGURE 93 View FIGURE 94 View FIGURE 95 , 97 View FIGURE 97 .
Philodoria Walsingham, 1907: 717 View in CoL . Zimmerman 1978a: 644–718, figs. 427–481; Johns et al. 2016: 66–67.
Synonymy
Subgenus Eophilodoria Zimmerman, 1978: 659 , fig. 432. Type species: Philodoria succedanea Walsingham, 1907 .
Subgenus Philodoria Zimmerman, 1978: 659 , figs. 433–434. Type species: Philodoria marginiestrigata ( Walsingham, 1907) .
Original description: “Antennae a little longer than the forewings, simple, without pecten. Labial Palpi long, curved, drooping, divergent, smooth; terminal joint almost as long as the median. Maxillary Palpi obsolete. Haustellum well-developed. Ocelli present. Head and thorax smooth. Forewings narrow, elongate, lanceolate: neuration 12 veins all separate; 2, 5 and 6 weak; 7 to costa. Hindwings narrow, lanceolate, acute; cilia 3 1/2: neuration 7 veins (3 and 4 coincident); 2 and (3 +4) stalked; cell open between 4 and 5; 5 and 6 stalked out of 7; 8 short. Abdomen slender. Legs, hind tibiae smooth.
This genus is closely allied to Gracilaria, Hw. , but differs in the absence of the maxillary palpi.” ( Walsingham 1907: 717).
Type species. Philodoria succedanea Walsingham, 1907 View in CoL by original description.
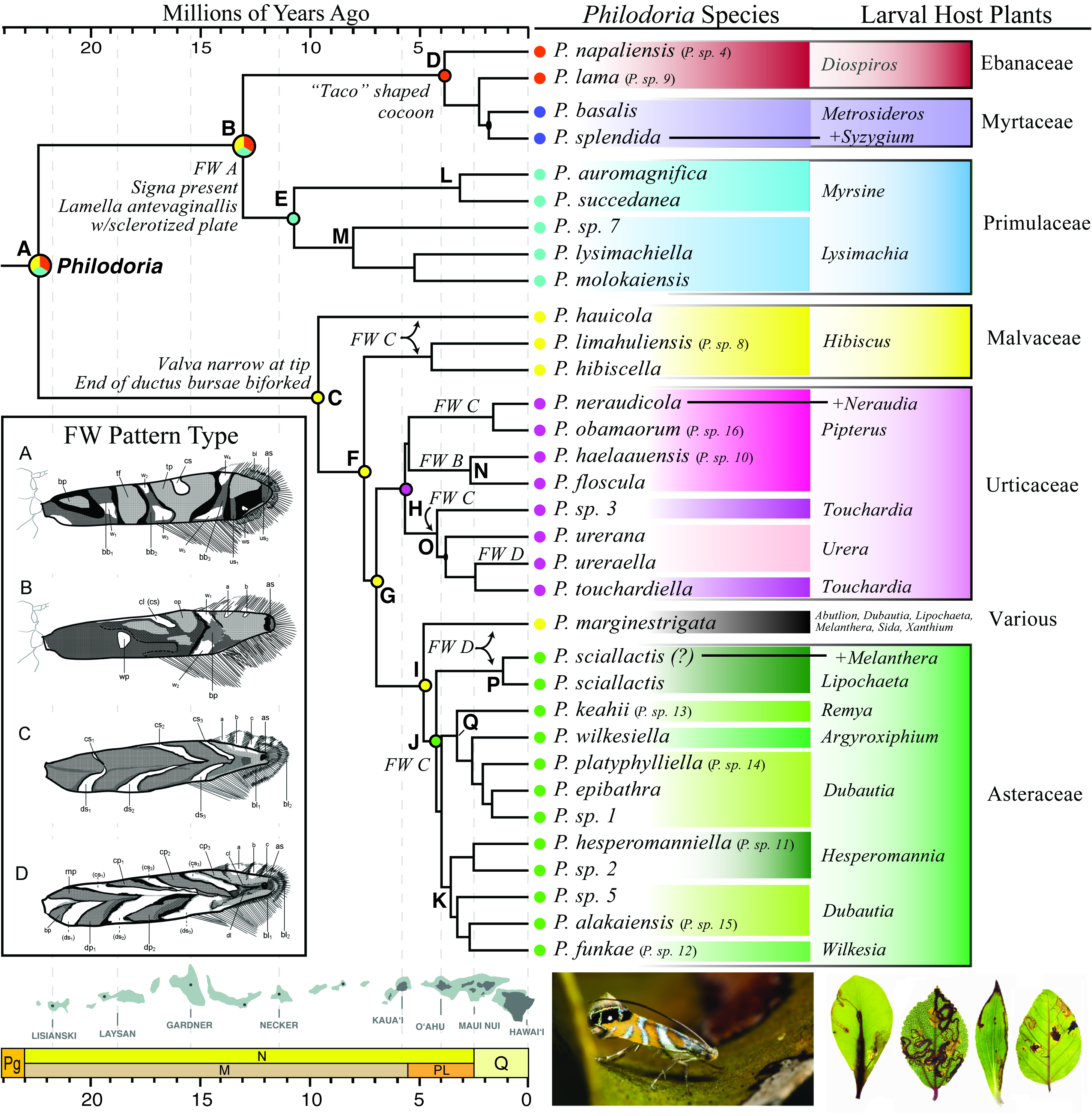
Systematic history. The systematic history of the genus was reviewed by Johns et al. (2016). In summary, the genus was placed originally in the Tineidae by Walsingham (1907), and incorrectly assigned to the Glyphipterygidae by Meyrick (1912a). Walsingham characterized the genus from others as lacking a maxillary palpus. Genera he thought were closely related, Gracillaria and Elachista , have a maxillary palpus and/or blackish forewing. Swezey (1910b, 1913bc, 1915, 1920, 1928, 1940 & 1946) and Meyrick (1928) similarly assigned species within Philodoria with a developed maxillary palpus to other genera, i.e. Parectopa and Gracillaria . Meyrick (1928) placed some Philodoria species described by Walsingham as Gracillaria in the Gracillariidae . Zimmerman (1978a) taxonomically reviewed Hawaiian Gracillariidae and placed some species assigned to other genera in Philodoria . Zimmerman also divided the genus into two subgenera, Philodoria ( Eophilodoria) and Philodoria ( Philodoria) based on the length of the maxillary palpus. Recently, Johns et al. (2016) synonymized the subgenus Eophilodoria Zimmerman 1978 with the genus Philodoria Walsingham, 1907 . Philodoria has been treated as a genus of the Parectopa group, subfamily Gracillariinae ( Kumata et al. 1988; Davis & Robinson 1998; De Prins & De Prins 2005). Recently, Kawahara et al. (2017) placed Philodoria in the subfamily Ornixolinae Kuznetzov & Baryshnikova, 2001 based on a phylogeny that utilized 22 genes and 94 gracillariid species. Johns et al. (2018) published the first molecular phylogeny of Philodoria utilizing 507 loci and 33 Philodoria species. They estimated the origin of the genus to the now partially sunken Hawaiian islands of Laysan or Lisianski, approximately 21 Ma, and hypothesized that the ancestral moth was likely associated with host plants in the families Ebenaceae , Malvaceae or Primulaceae . While the study of Johns et al. (2018) was a major breakthrough in understanding the evolutionary patterns of Philodoria , many of the species included in that study did not have formally assigned names and species boundaries were never formally examined. Two species of Philodoria were described by Kobayashi et al. (2018) but many more remained undecribed until the present revision.
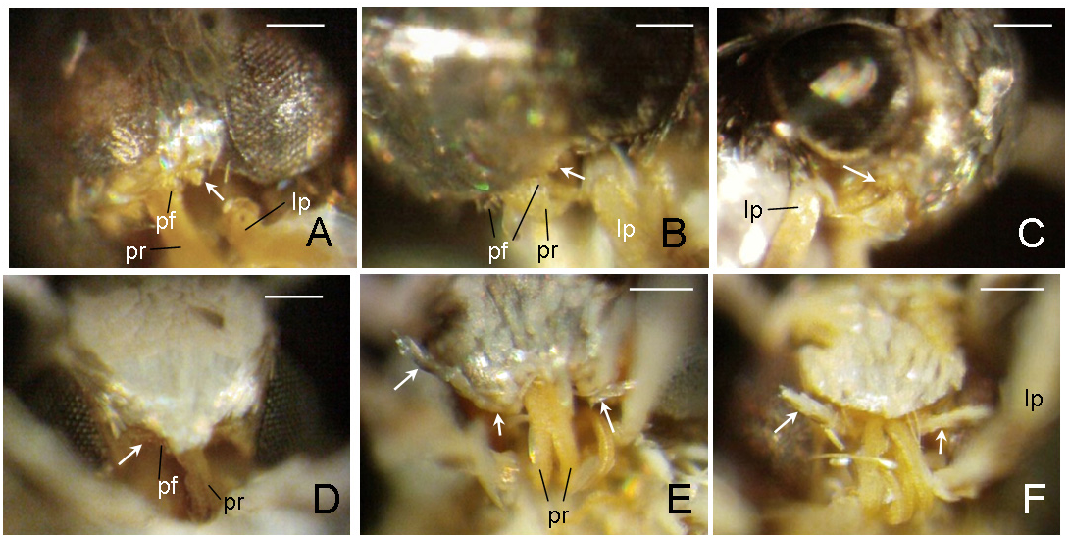
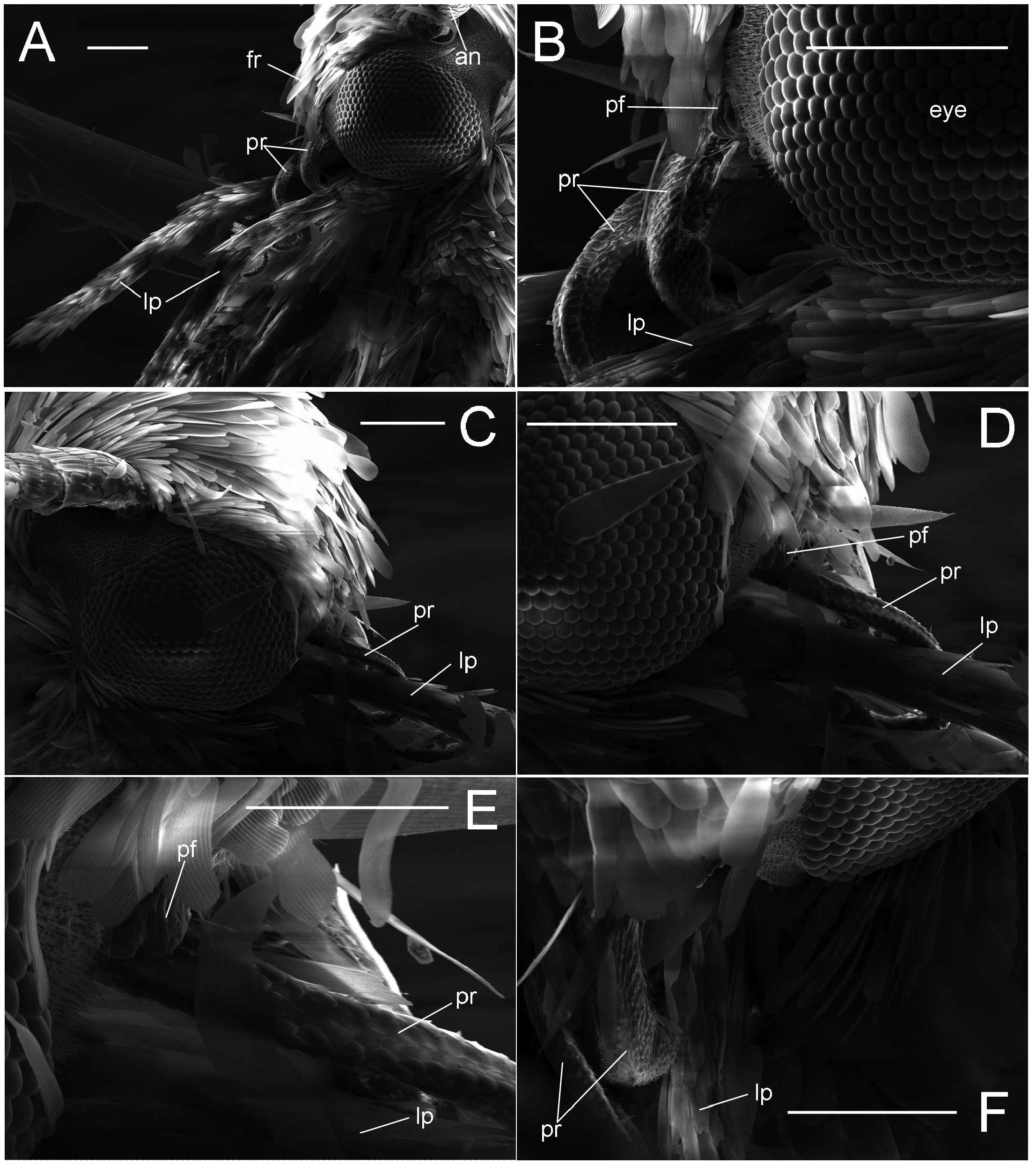
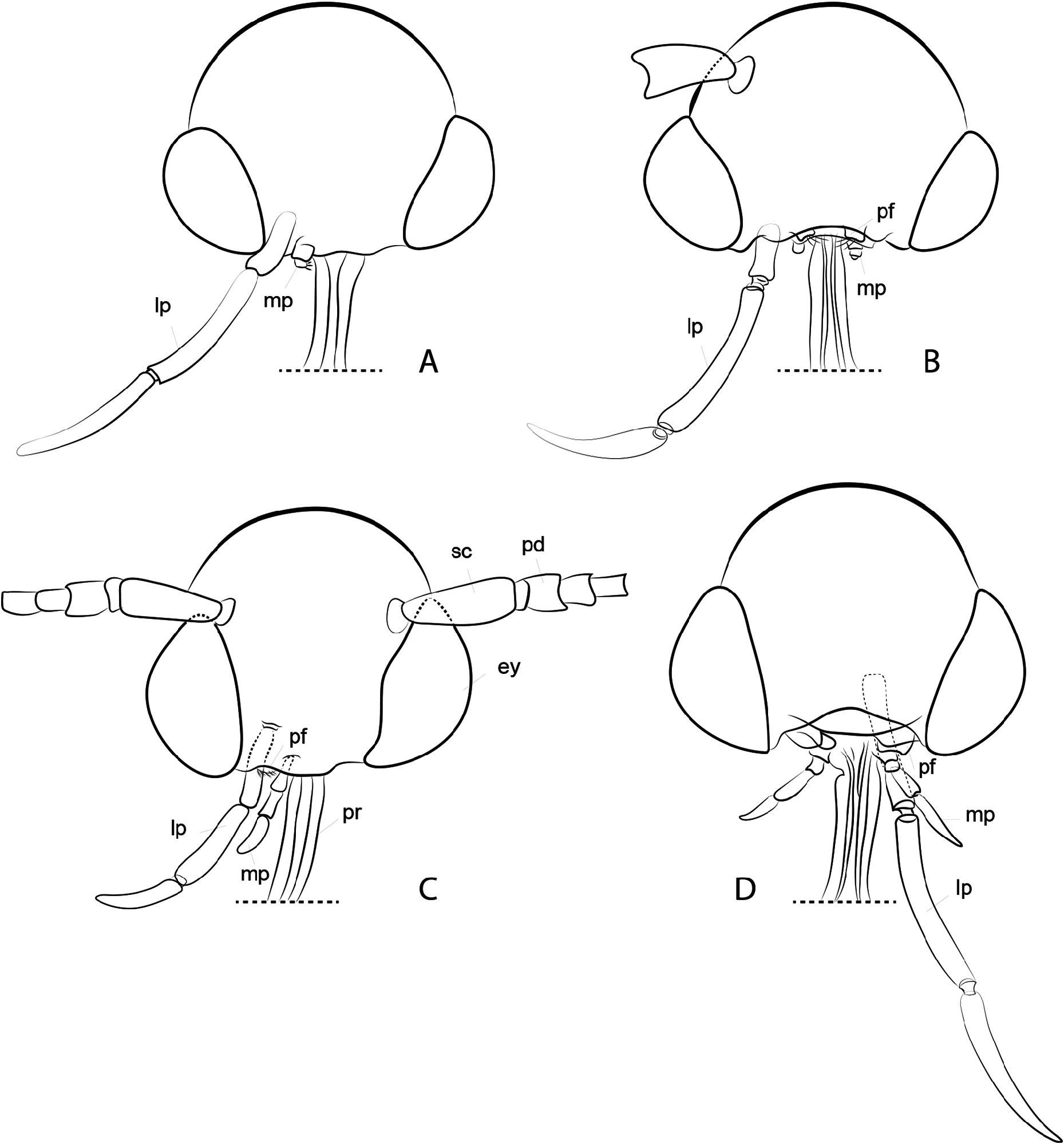
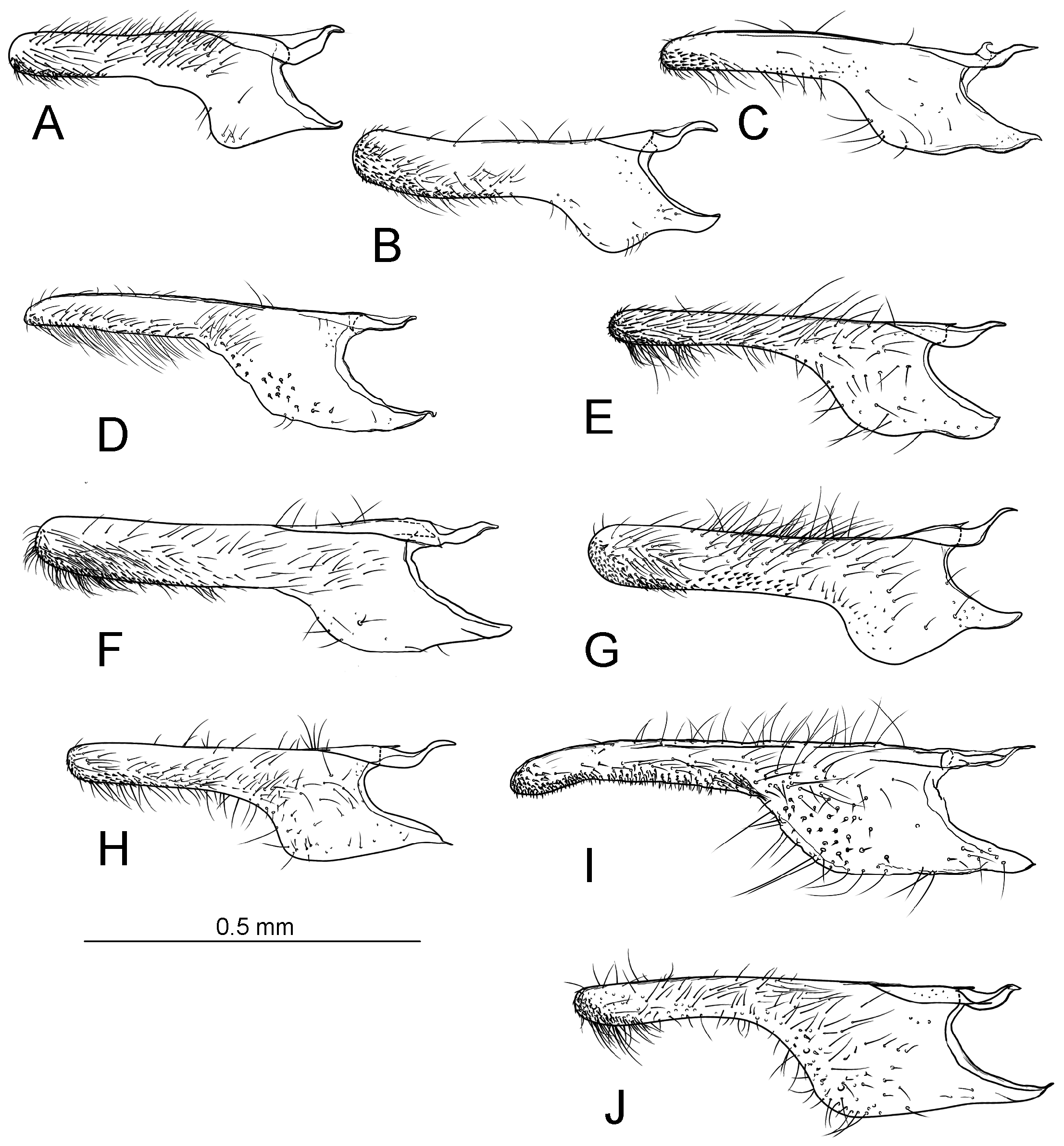
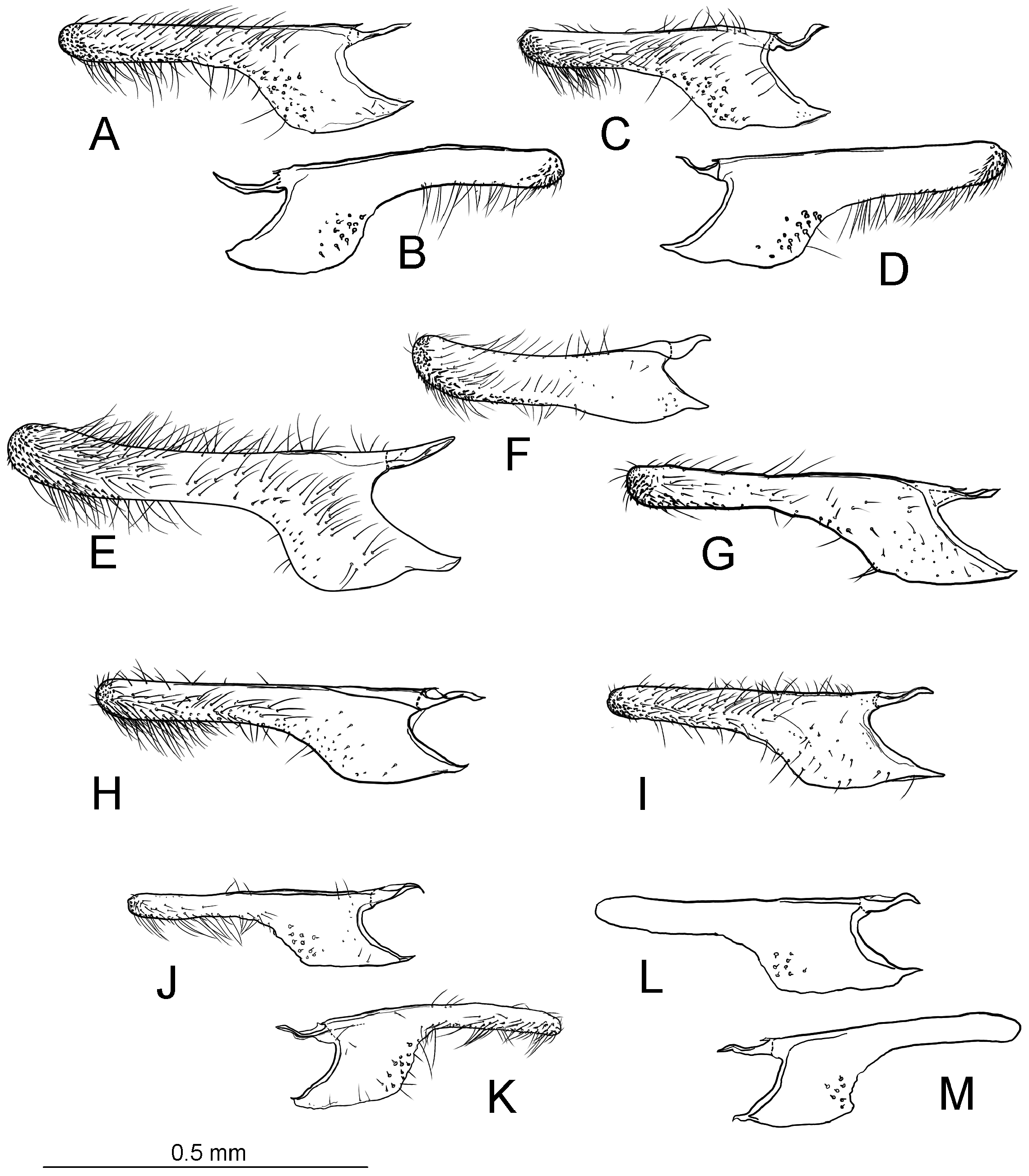
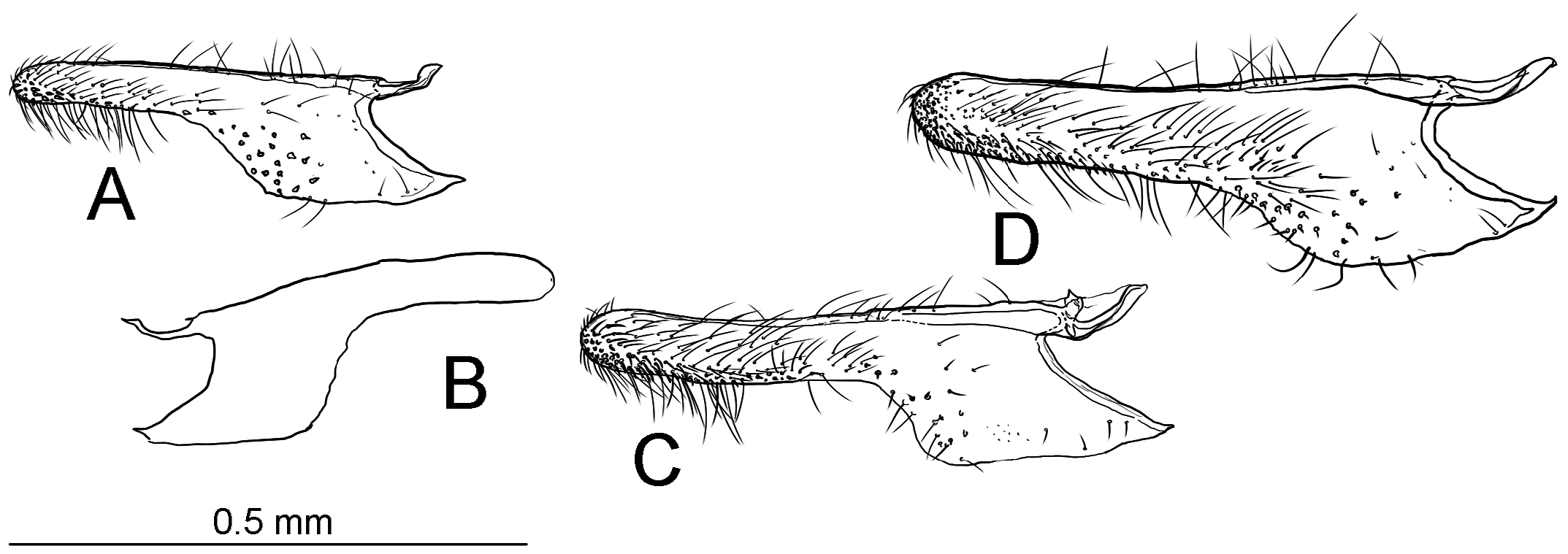
Diagnosis. Adults. The association of this genus to Ornixolinae is substantiated by the presence of three autapomorphies for the subfamily: 1) the presence of moderately long, four-segmented maxillary palpi; 2) forewing with three branches of the medial vein and two branches of the cubital vein ( Fig. 38 View FIGURE 38 ); and 3) an ostium bursae opening at the seventh sternum in female ( Fig. 40E, F View FIGURE 40 ). However, 17 Philodoria species possess very reduced four-segmented maxillary palpi, of which the apical two segments are very small and indistinct ( Figs. 34A,D View FIGURE 34 , 37A, B View FIGURE 37 ) or nearly absent ( Figs. 34B, C View FIGURE 34 , 35 View FIGURE 35 ).
Diagnostic features of the genus include: forewing with lustrous and metallic apical portion; hindwing with small frenular costal bristles; in the male, a dorsal flap extending tergum VIII; in the female, terminus of ductus bursae tubular and sclerotized; corpus bursae with a pair of lateral signa that are either a pair of spines or series of small spines; inception of ductus seminalis at the posterior part of corpus bursae. Larvae and pupae were not examined in the present study and therefore do not include a diagnosis for them..
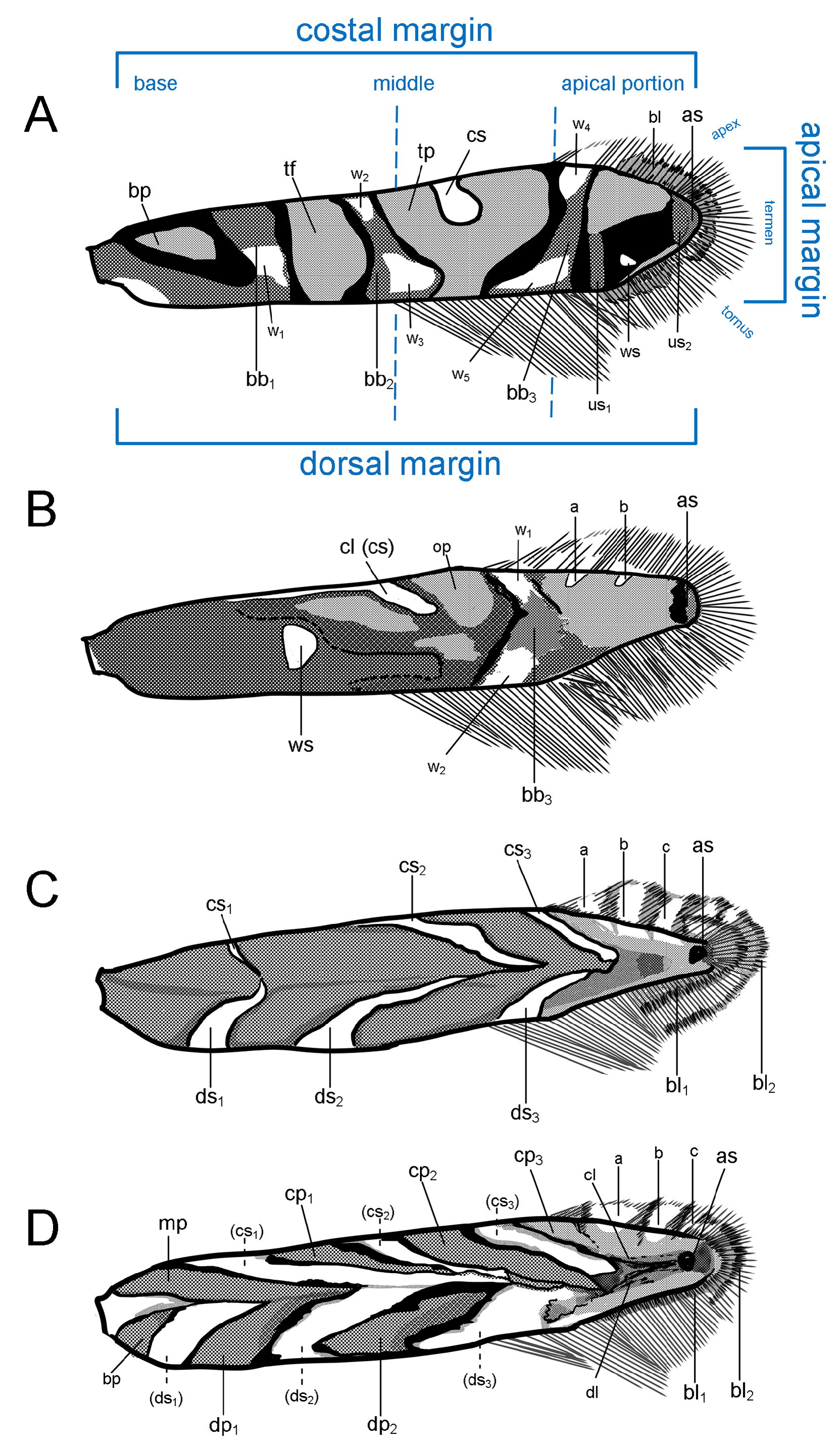
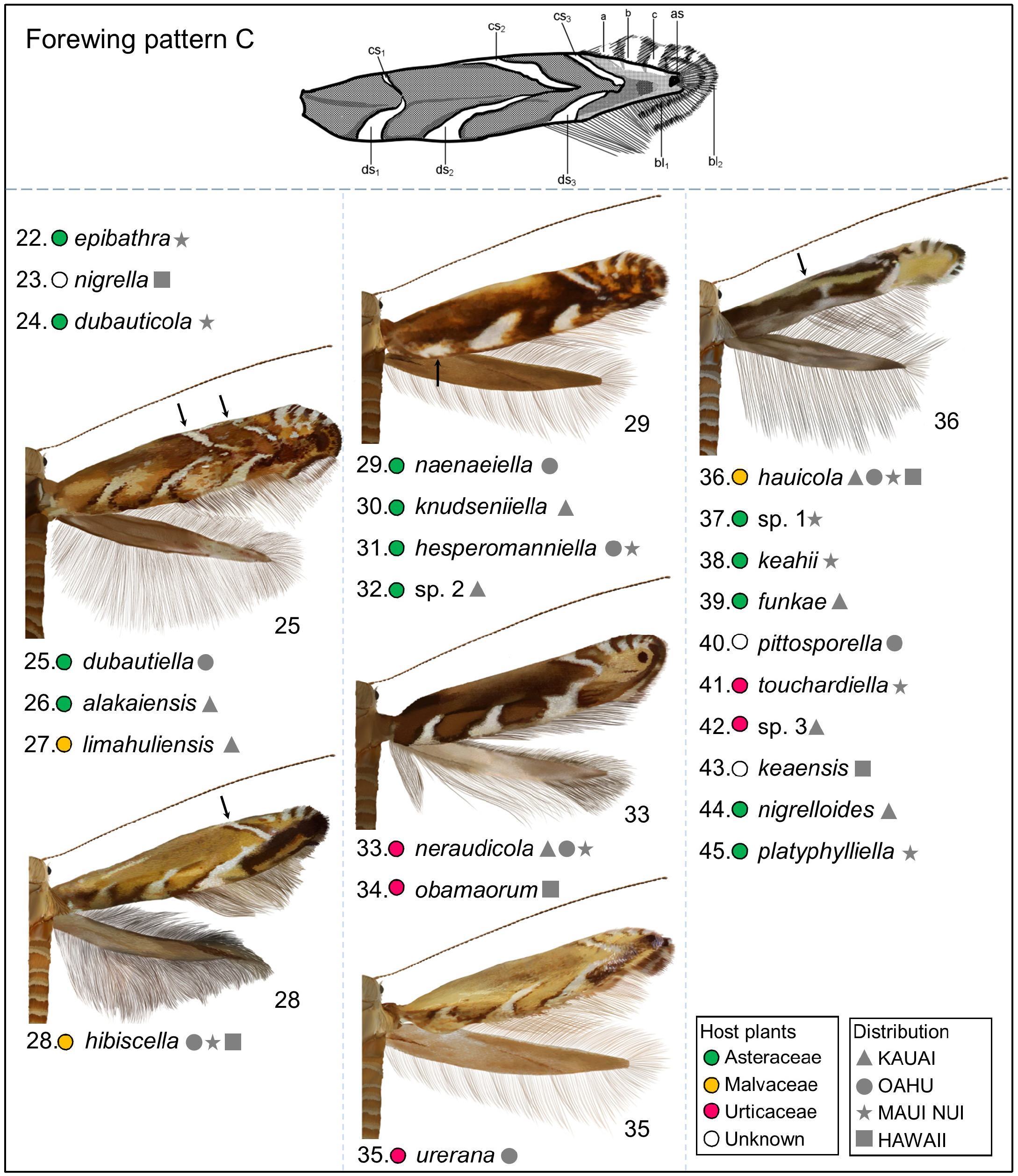
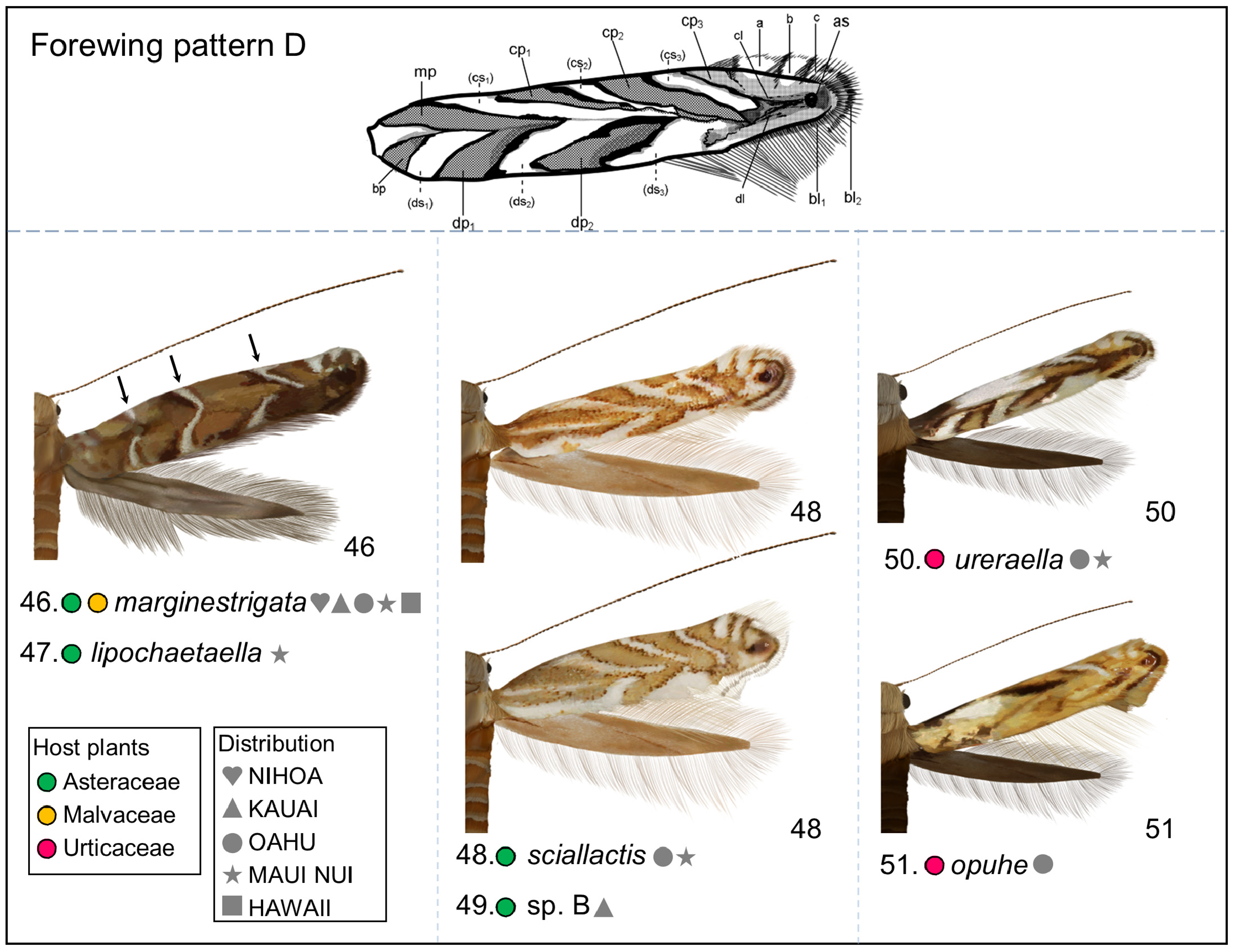
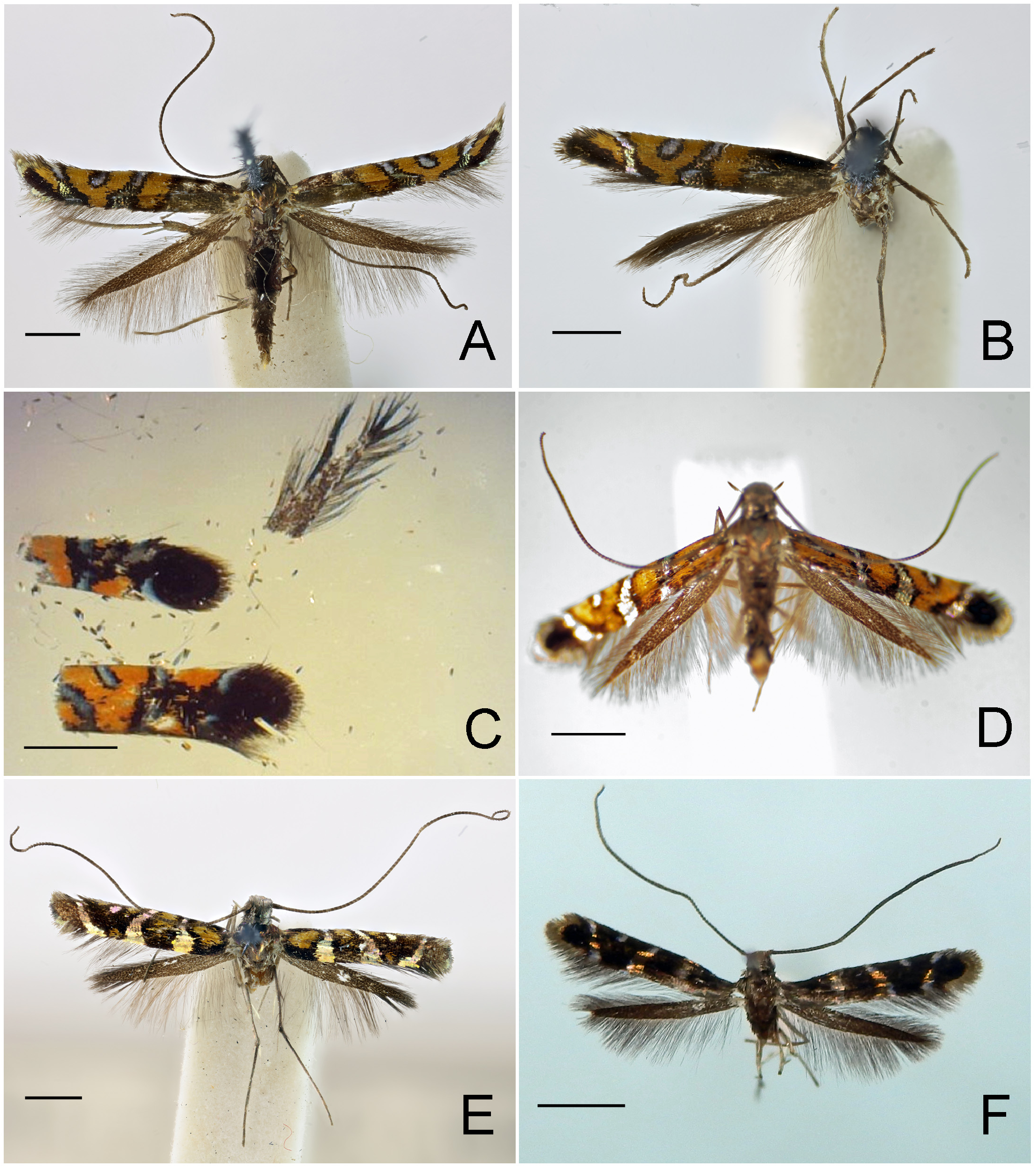
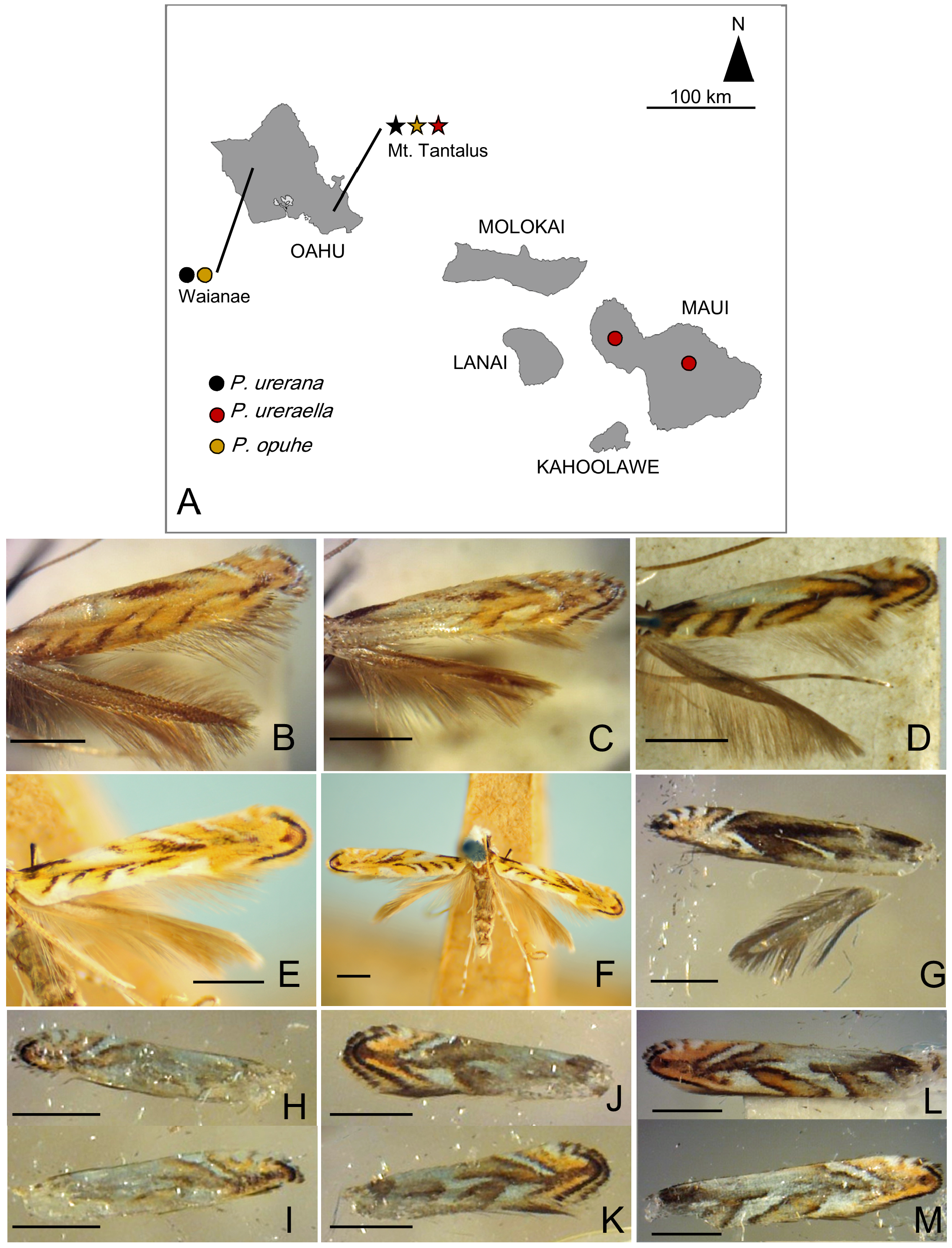
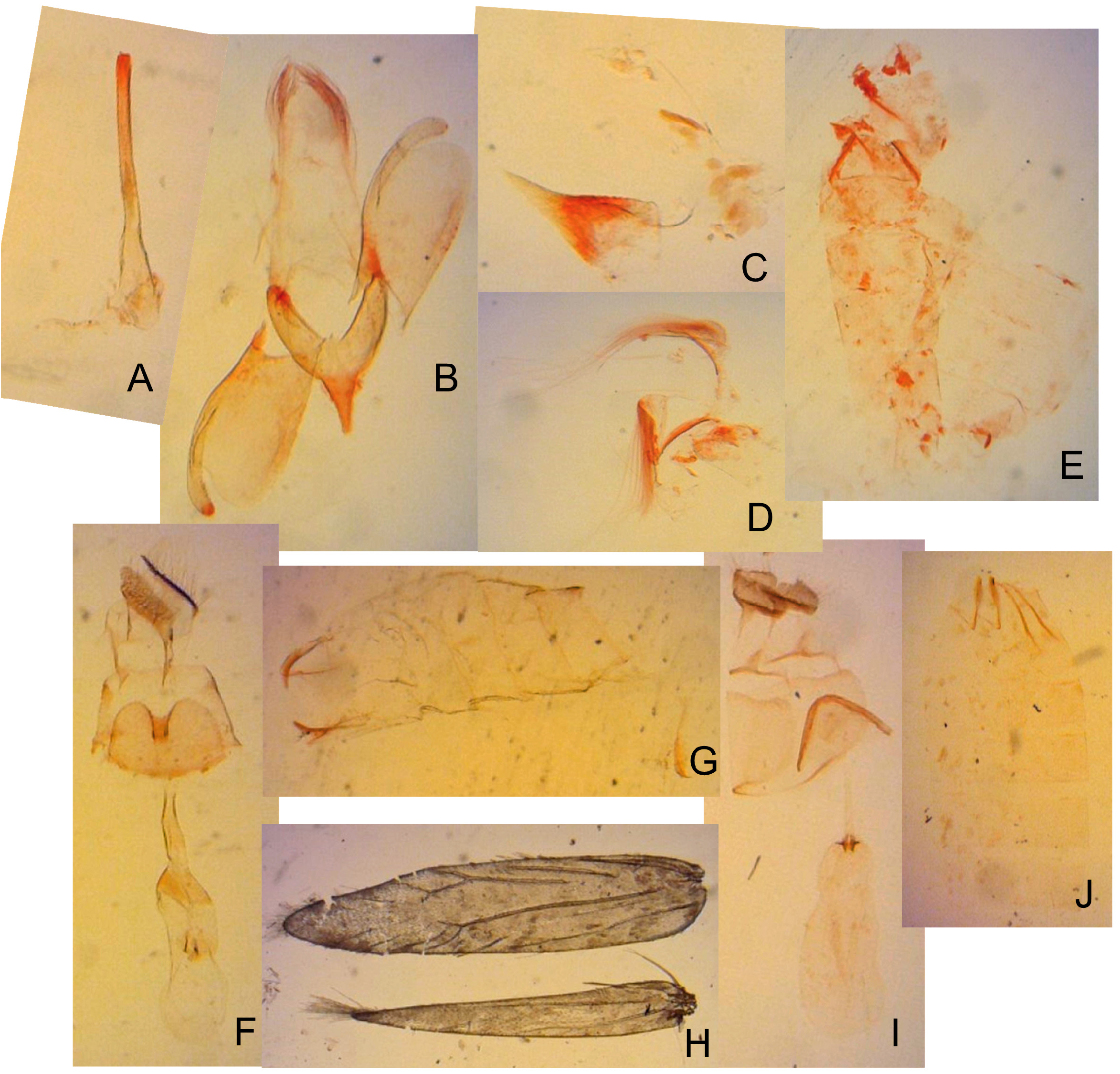
Redescription: Adult ( Figs. 3–6 View FIGURE 3 View FIGURE 4 View FIGURE 5 View FIGURE 6 , 34–40 View FIGURE 34 View FIGURE 35 View FIGURE 36 View FIGURE 37 View FIGURE 38 View FIGURE 39 View FIGURE 40 ). Head and frons smooth. Haustellum well-developed. Ocelli and chaetocemata absent (despite Walsingham’s [1907] claim that ocelli are present, Philodoria lack ocelli). Maxillary palpus developed, 4-segmented ( Figs. 34E, F View FIGURE 34 , 36 View FIGURE 36 ) or reduced 2-segmented and obscure small 3rd and 4th segments ( Fig. 34A, D View FIGURE 34 ), sometimes greatly reduced, vestigial, obsolete ( Figs. 34B, C View FIGURE 34 , 35 View FIGURE 35 ). Labial palpus developed, upcurved, 3-segmented. Antenna about 1.0–1.5 length of forewing. Thorax smooth. Legs slender, smooth. Basal color of forewing dark lustrous and metallic, fuscous or brown. Forewing pattern ( Fig. 3 View FIGURE 3 ) consisting of orange ocherous patches with white bands or spots ( Fig. 3A, B View FIGURE 3 ) or oblique white to brown streaks ( cs 1–3 and ds 1–3) ( Fig. 3C, D View FIGURE 3 ). Hindwing a little shorter in length than forewing.
Wing venation ( Figs. 38 View FIGURE 38 , 62H View FIGURE 62 ). Forewing lanceolate, with thirteen veins ( Fig. 38A View FIGURE 38 ). Sc along costal fold, ending at one-third of costa; R1 from base to ending at the middle of costa; branched R2 at two-third of the wing; R3, R4 and R5 from end of cell to costa; M1, M2, M3 and CuA1 from end of cell to tornus; CuA2 rather week, at about two-thirds of the wing; CuP weak, from base and reaching dorsal area; A1+2 from base to beyond midde of dorsum. Hindwing lanceolate, with eight veins ( Fig. 38B View FIGURE 38 ); Sc along anterior margin, ending at one-fourth of costa with about four small frenulums; R1 weak, along anterior margin, ending at near the middle of the wing; RS from base to reaching apex; branched M1 and M2; CuA from base to ending at the middle of dorsum; branched M3; CuP from base to ending at one-fourth of dorsum.
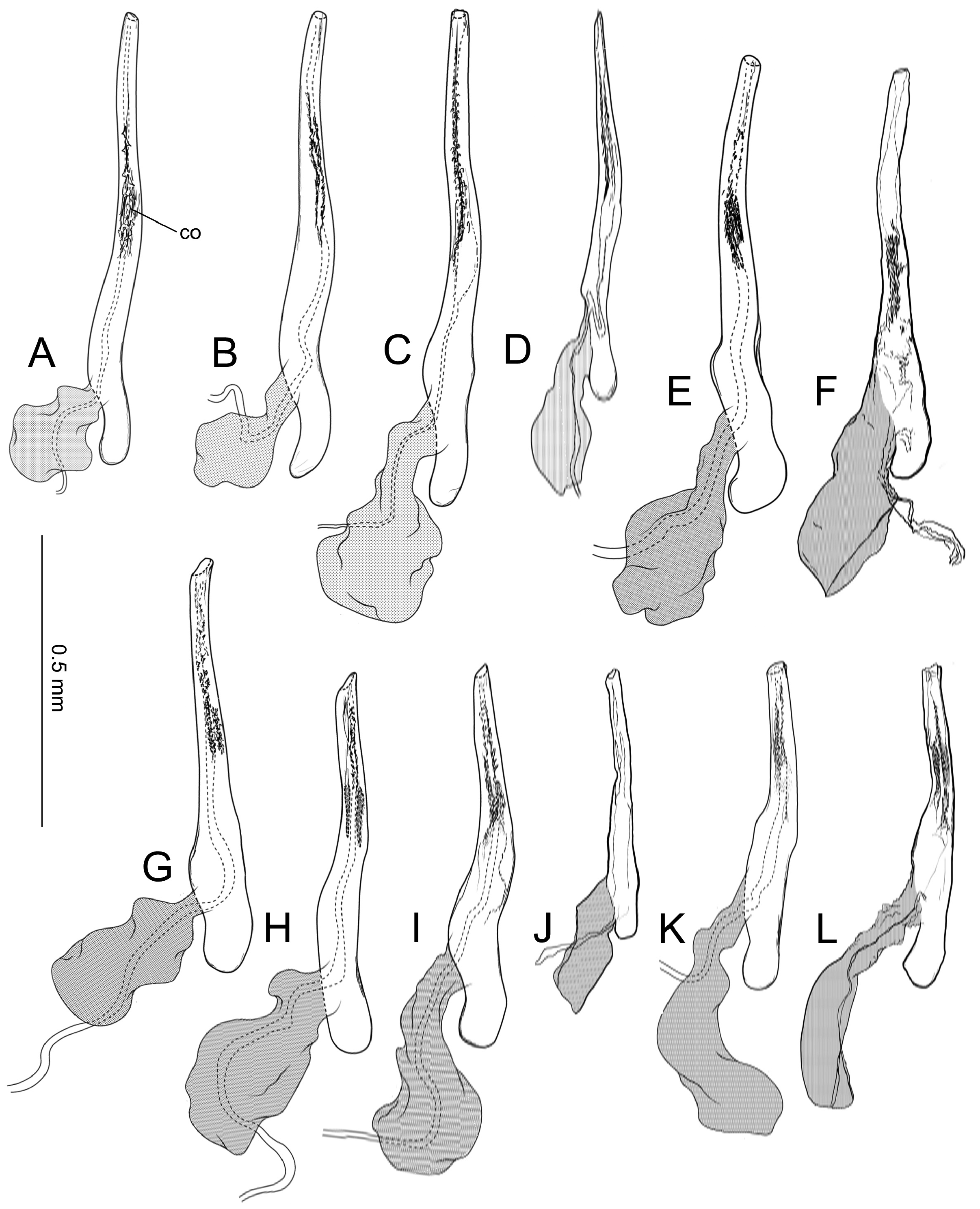
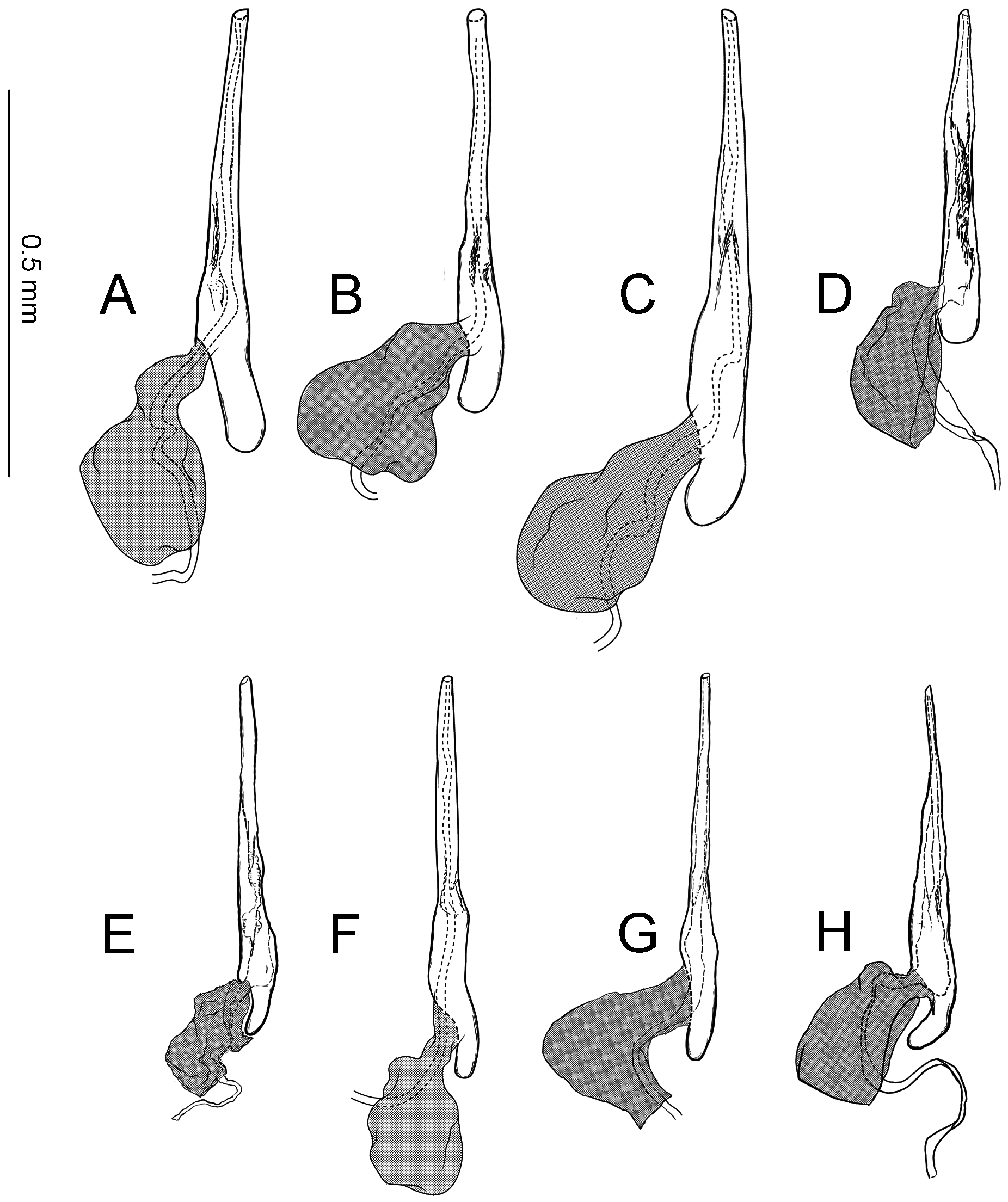
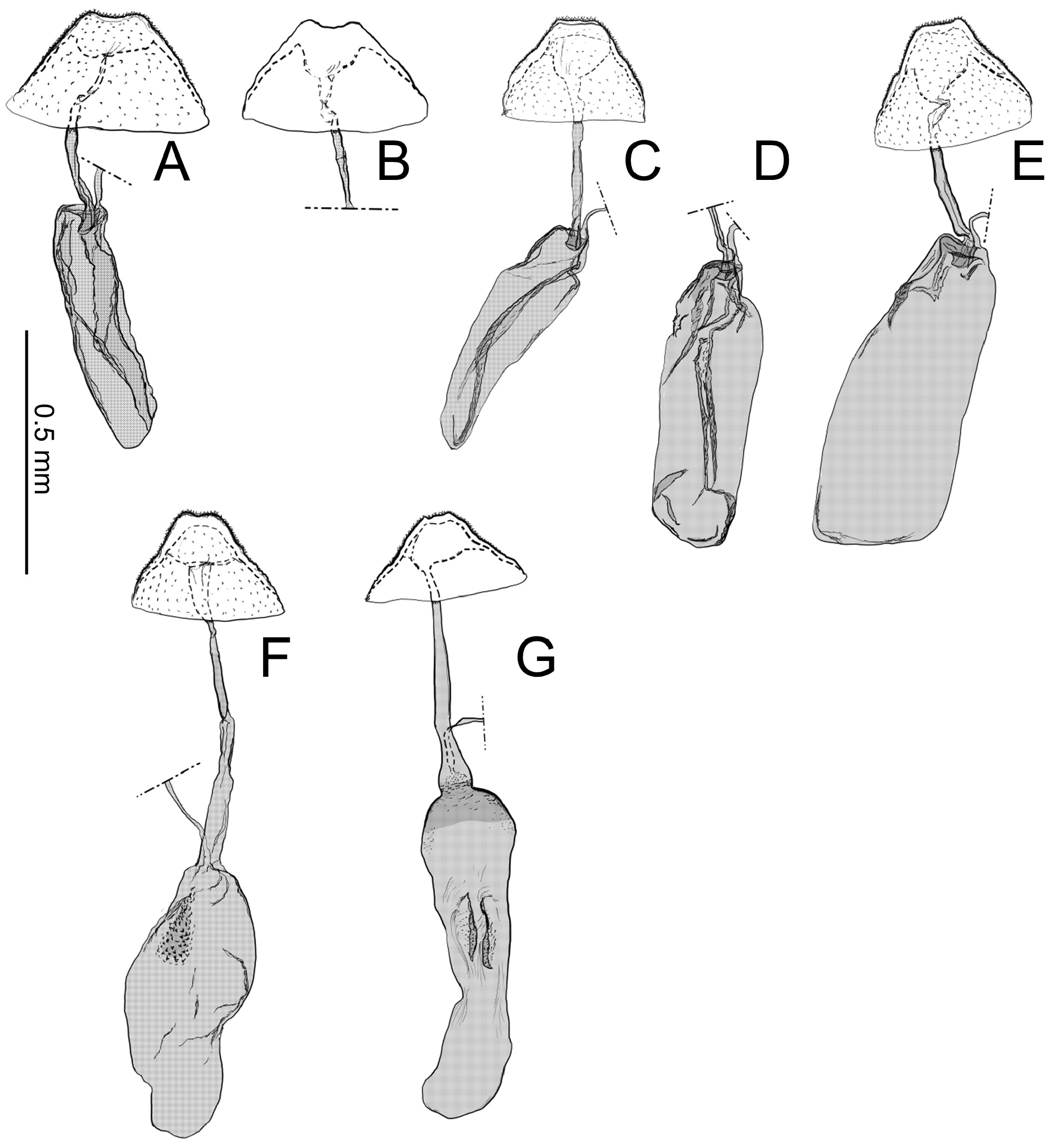
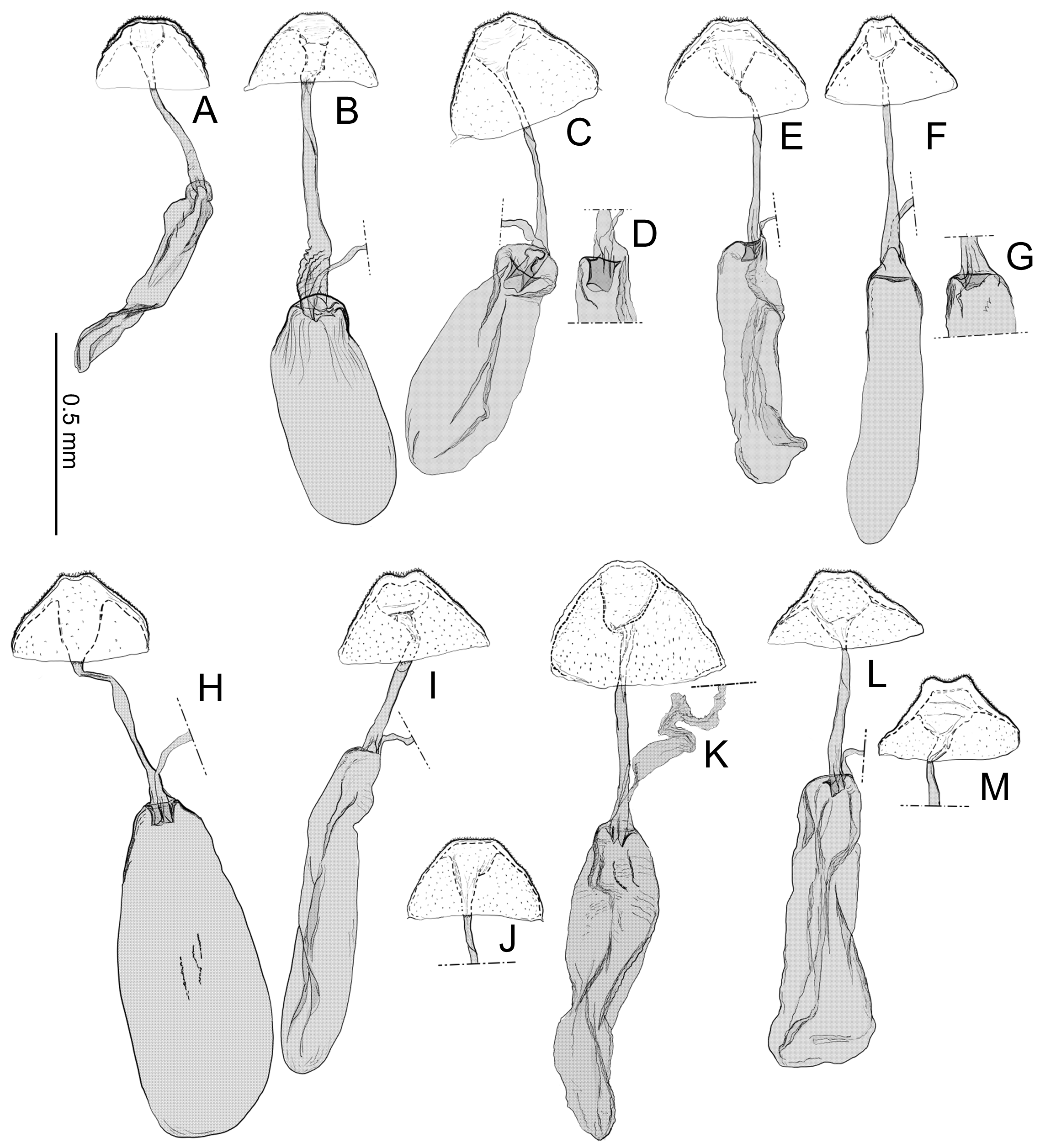
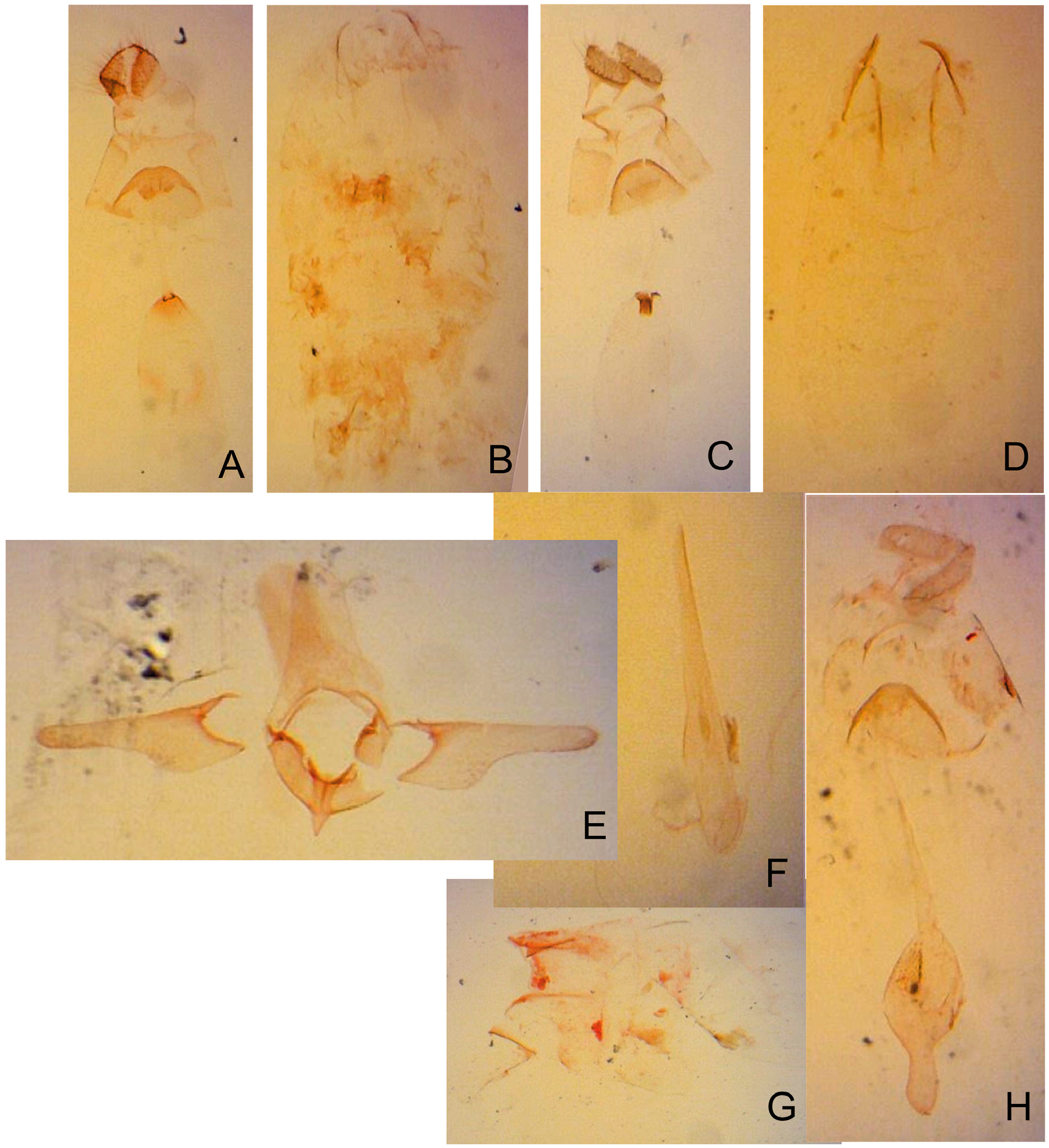
Abdomen. Tergum smooth without spiniform setae. Male 8th abdominal segment ( Fig. 39 View FIGURE 39 ) with one or two pairs of coremata; a dorsal flap extending tergum VIII, elongate, triangular, covered with slender, round and flat scales ( Fig. 39 View FIGURE 39 , 40D View FIGURE 40 ).
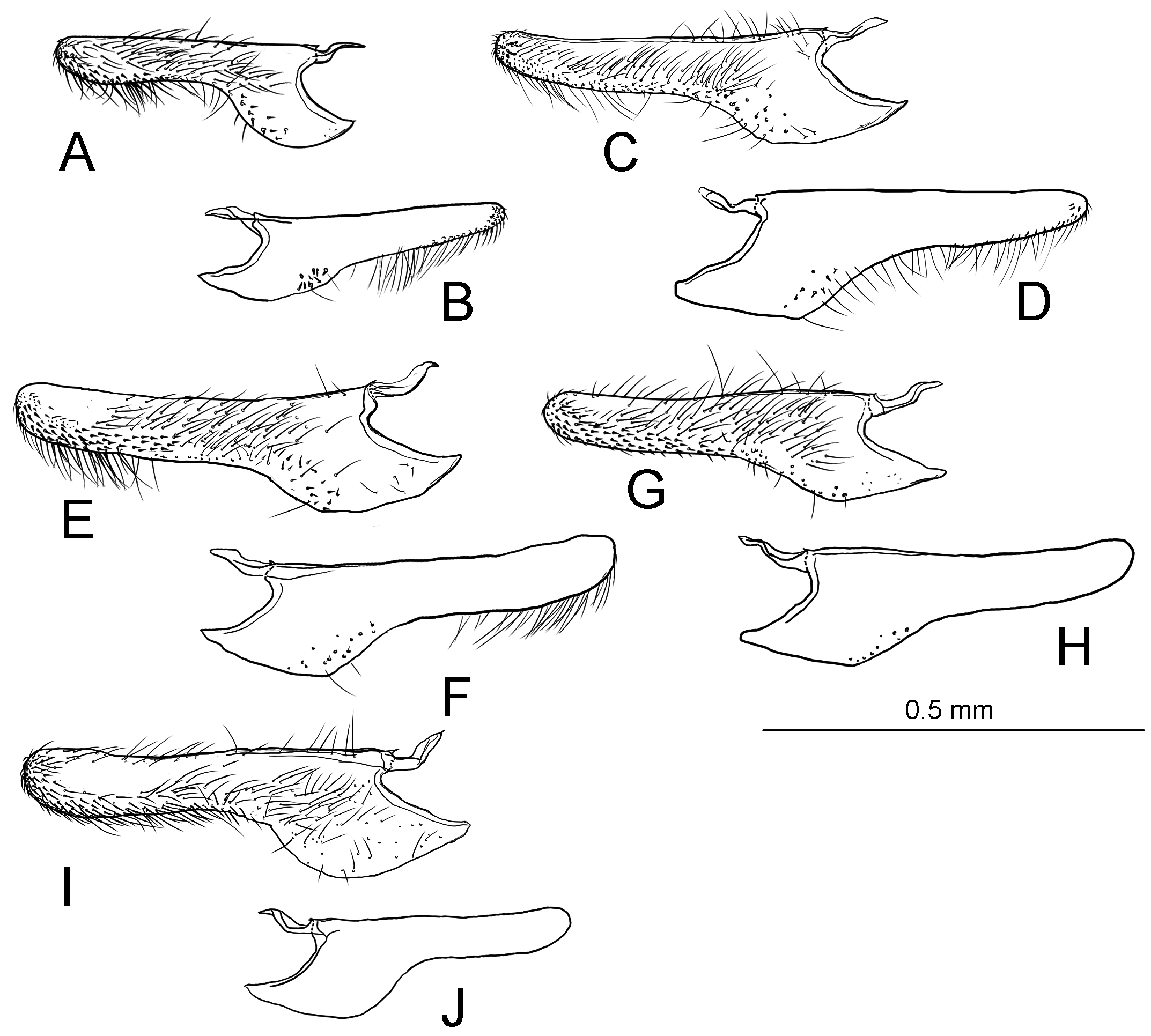
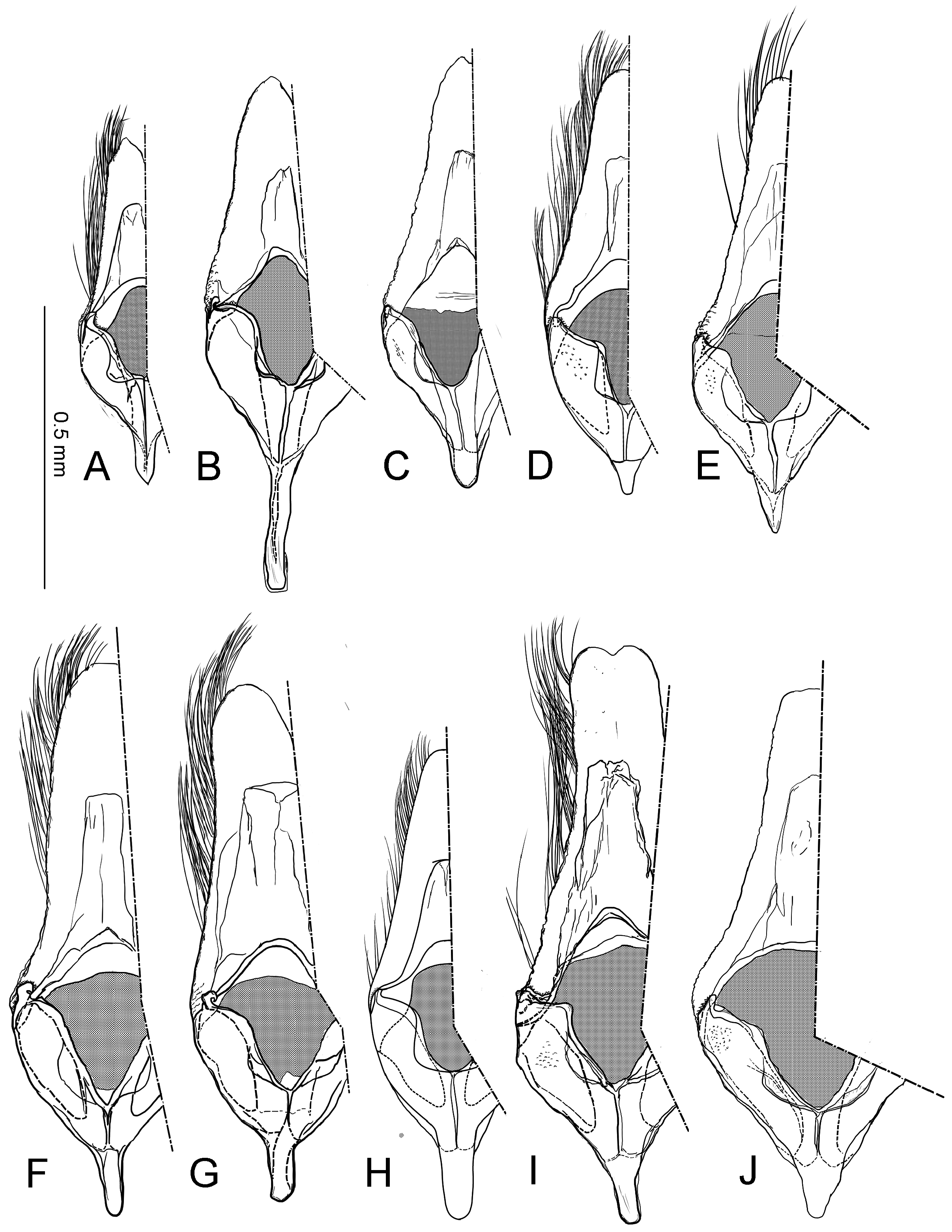
Male genitalia ( Fig. 40A–C View FIGURE 40 ) Uncus absent. Tegumen as long as valva in length ( Fig. 40B View FIGURE 40 ). Tuba analis membranous with weakly sclerotized subscaphium; gnathos V-shaped transverse band, terminal margin weakly joining subscaphium and anterior process connecting ventral base of tegumen ( Fig. 40B View FIGURE 40 ). Valva elongate, at the middle, tapering along costal margin ( Fig. 40C View FIGURE 40 ), some species have broad valva with a process; small spines at the apex, surrounded by a set of long setae; a set of short median sized spines arranged on the basal region. Vinculum U-shaped; saccus developed. Phallus tubular, slender; vesica with cornuti; coecum developed ( Fig. 40A View FIGURE 40 ).
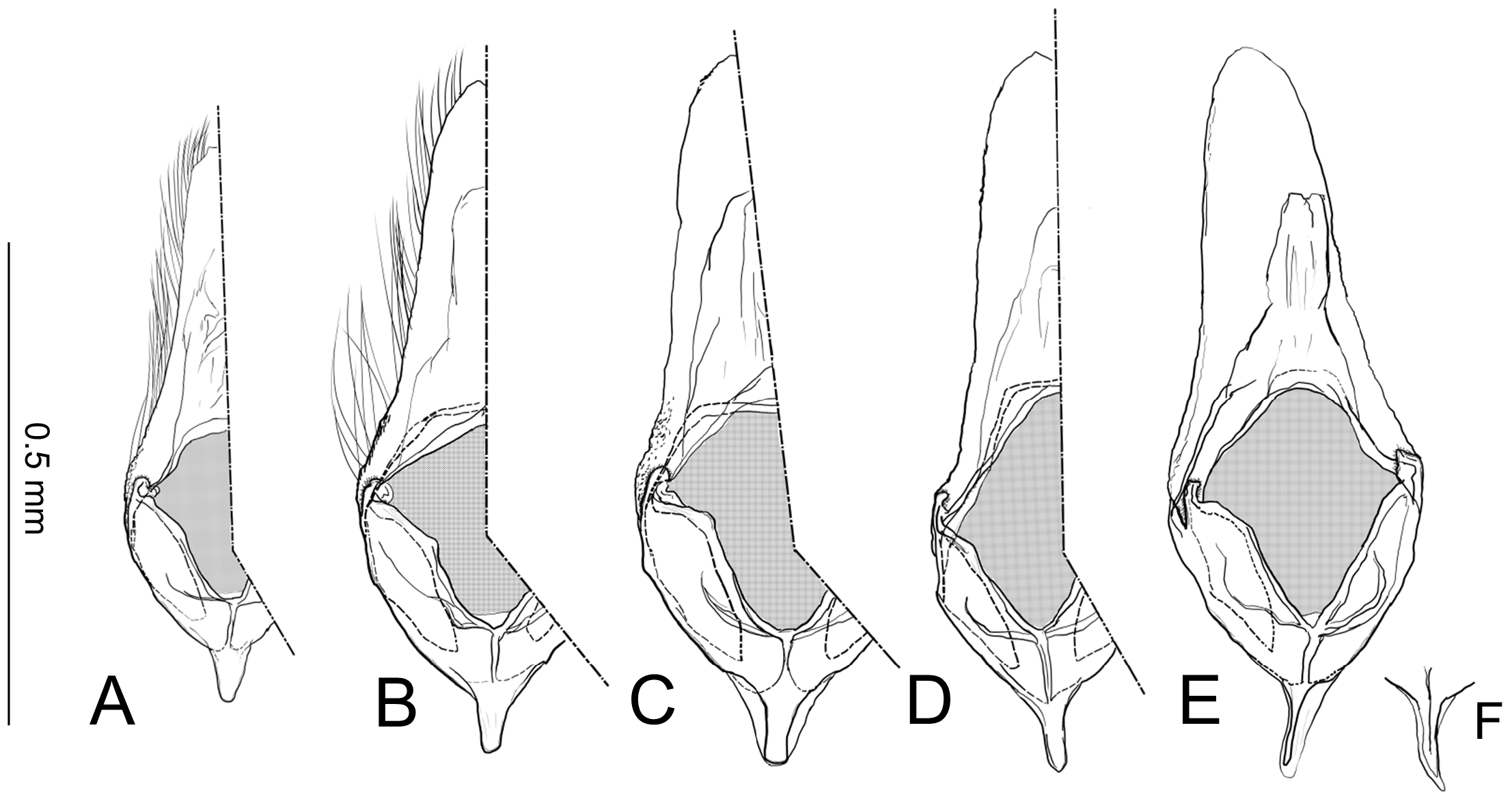
Female genitalia ( Fig. 40E–H View FIGURE 40 ) Apophyses anteriores and apophyses posteriores slender. Ostium bursae broad, opening at the middle of 7th abdominal segment ( Fig. 40E View FIGURE 40 ); antrum cup-shaped with a pair of lateral lobes; lamella antevaginalis semicircular and sometimes sclerotized; ductus bursae slender, tubular, posterior section very slender and membranous, curved inside of body and median section weakly sclerotized and flattened ( Fig. 40E, F View FIGURE 40 ); anterior section tubular sclerotized, terminus of ductus bursae biforked in ventral view and flanked pairs of sclerotized wrinkles reaching anterior end of corpus bursae; inception of ductus seminalis on the posterior part of ductus bursae; corpus bursae pyriform; signa both lateral, a pair of spines or many series of small spines ( Fig. 40G, H View FIGURE 40 ).
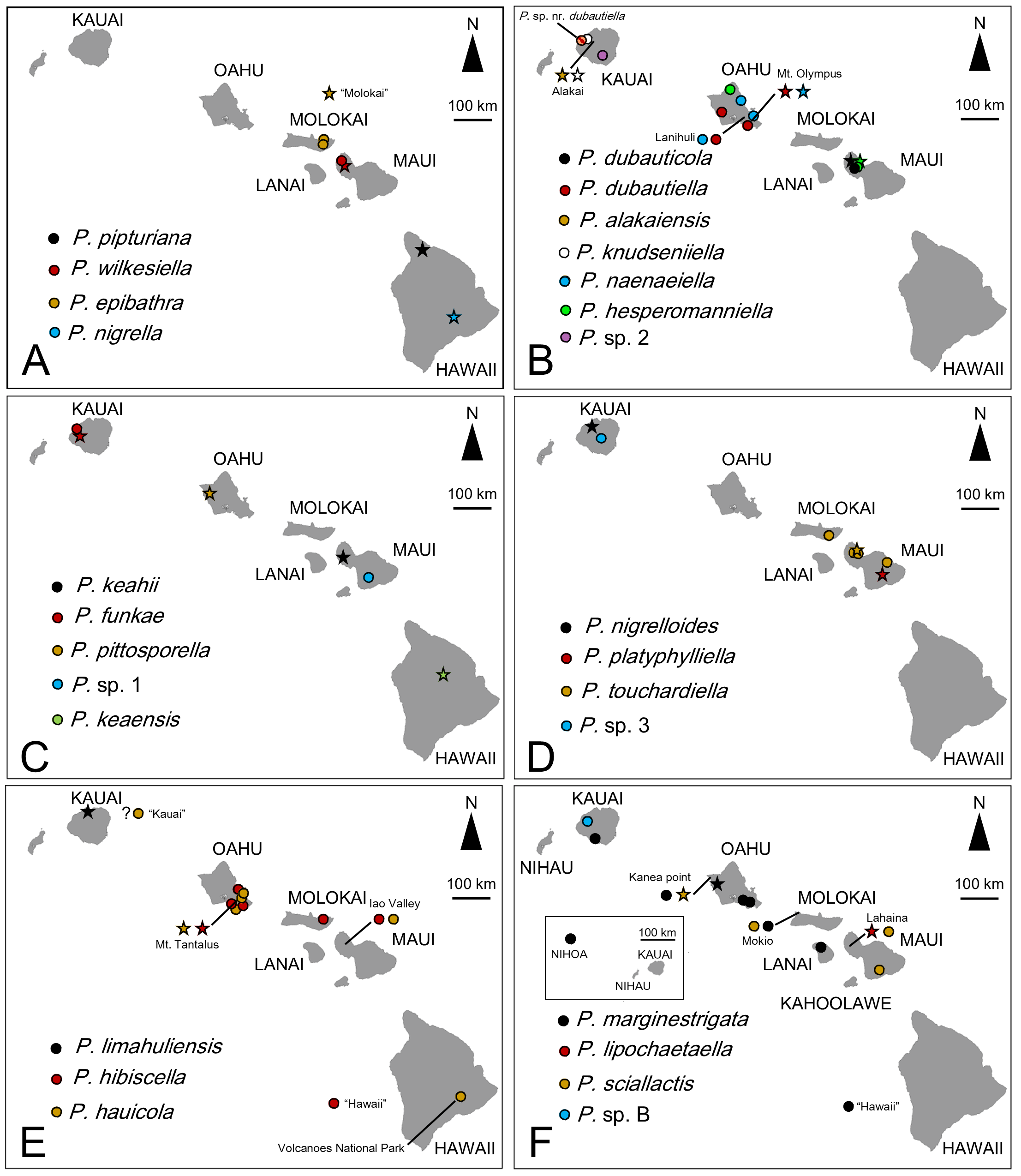
Distribution. United States: Hawaiian Islands (Nihoa, Kauai, Oahu, Molokai, Lanai, Maui and Hawaii [Big Island]).
Host plants. Asteraceae , Ebanaceae, Malvaceae , Myrtaceae , Primulaceae and Urticaceae .
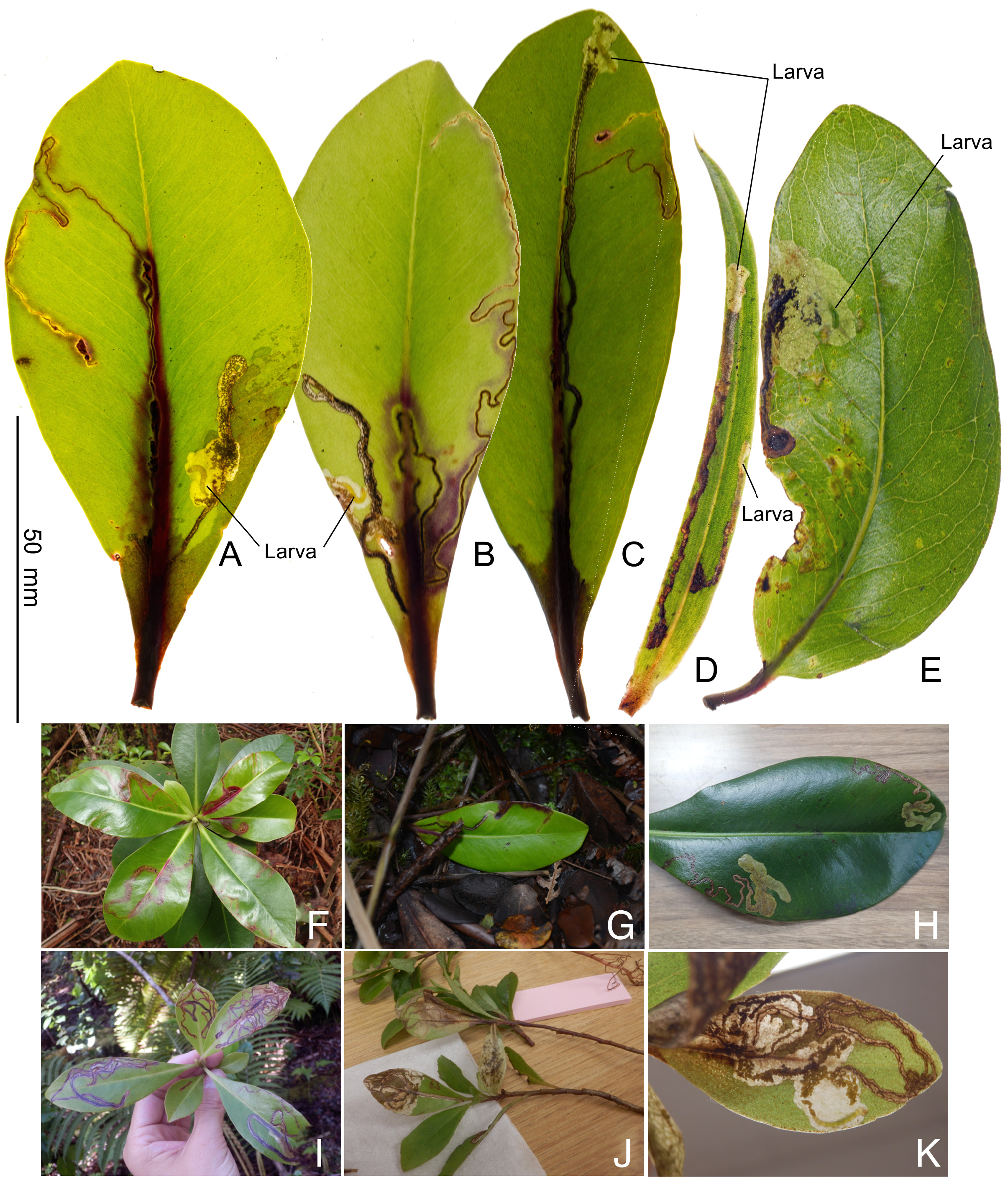
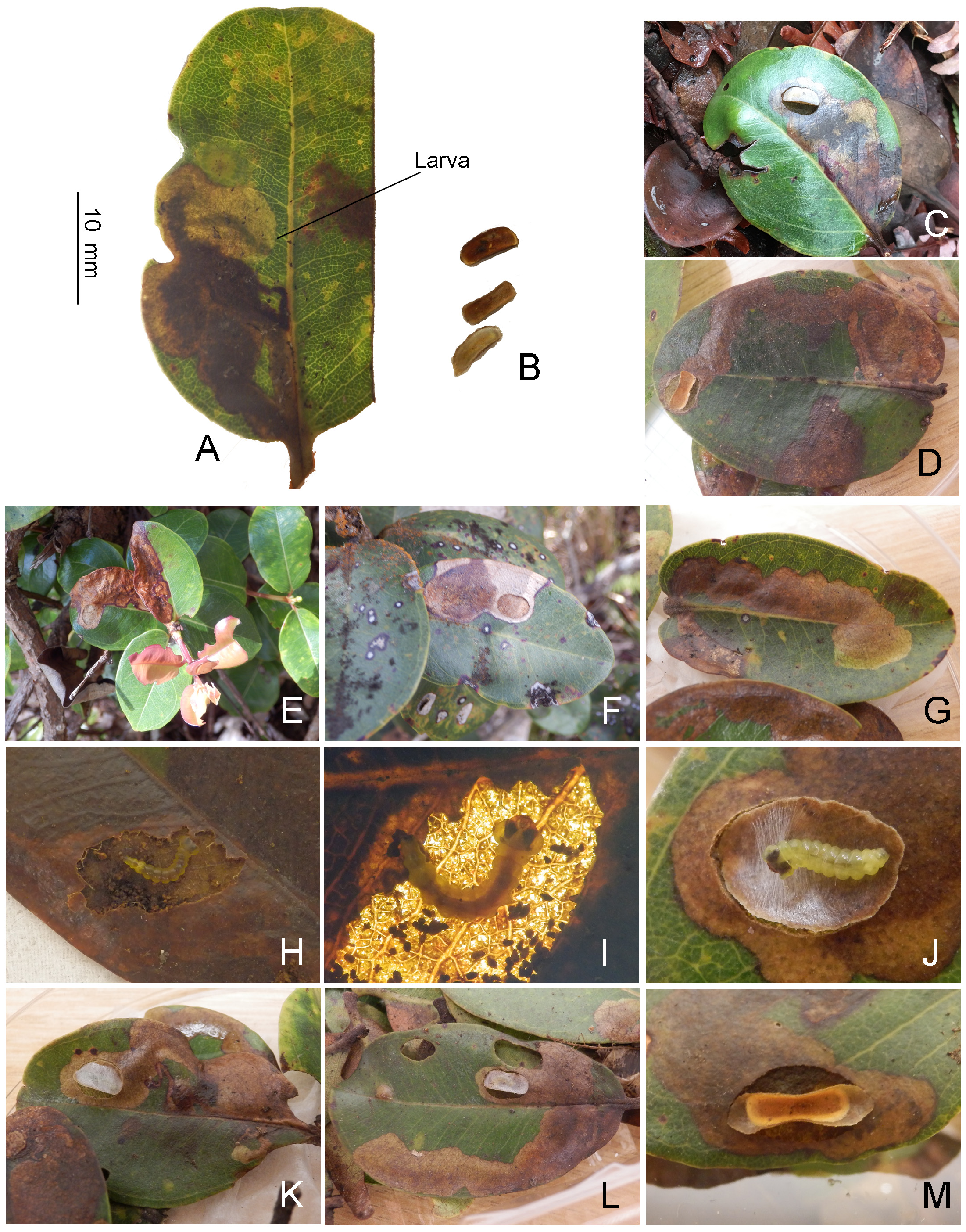
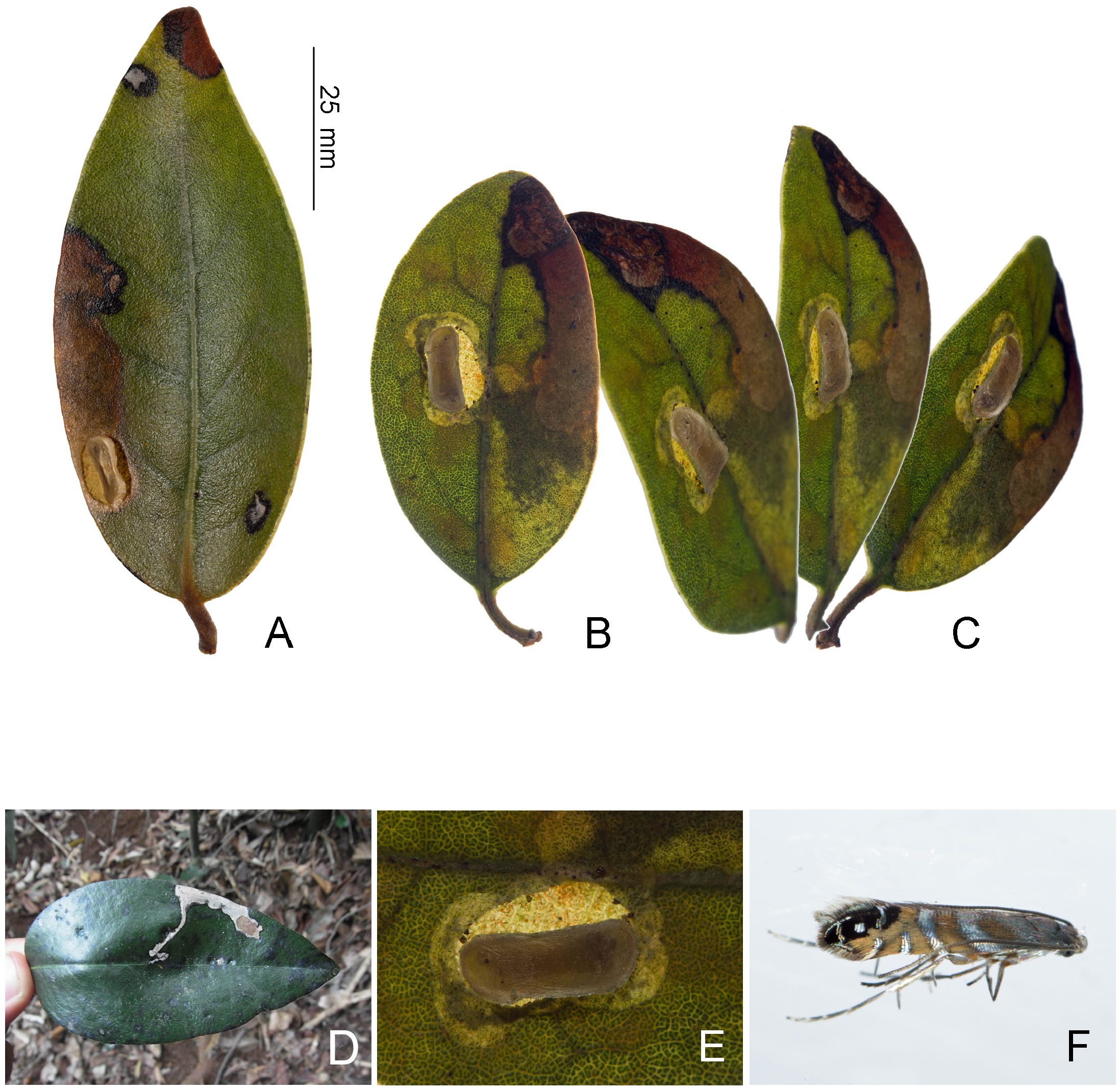
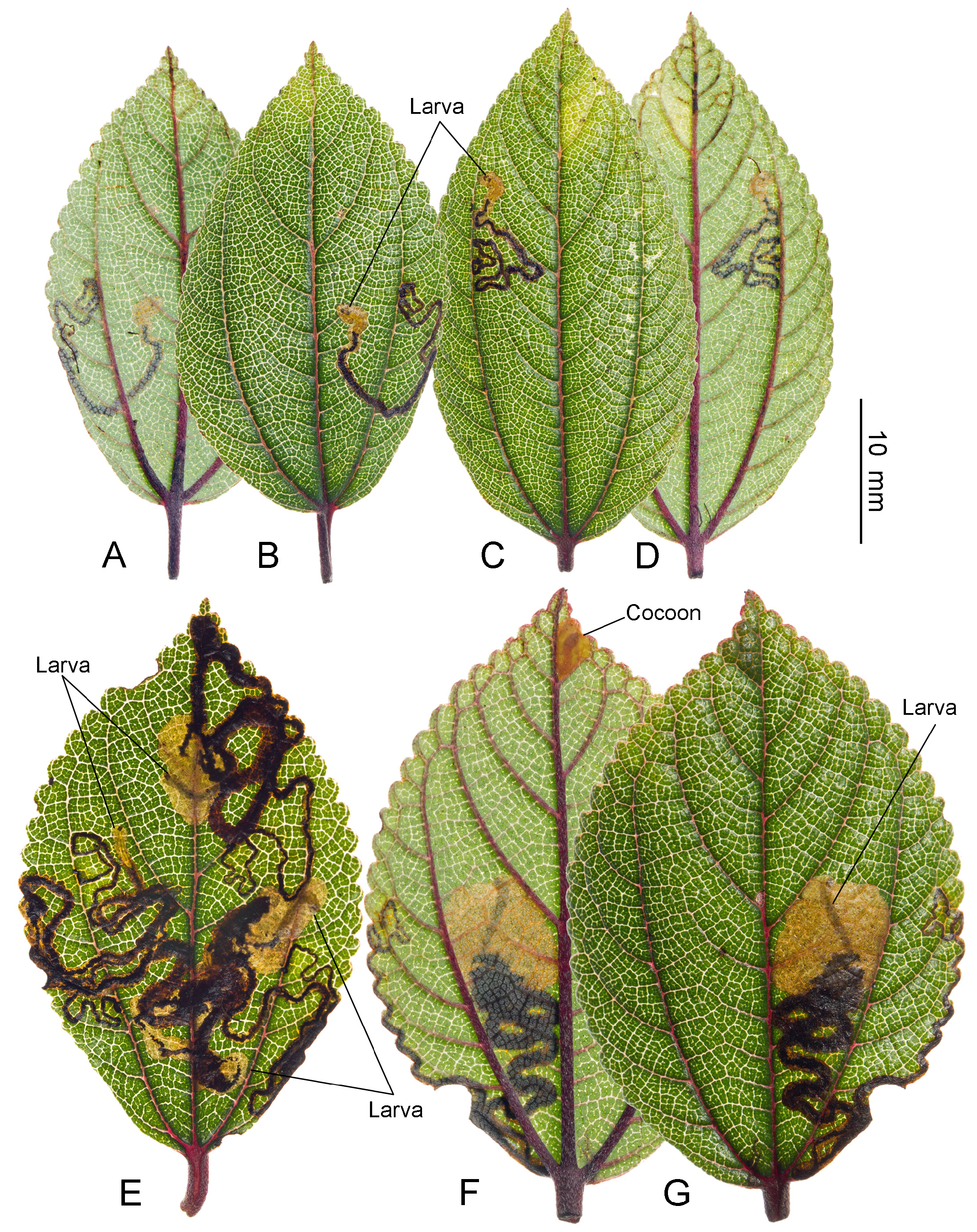
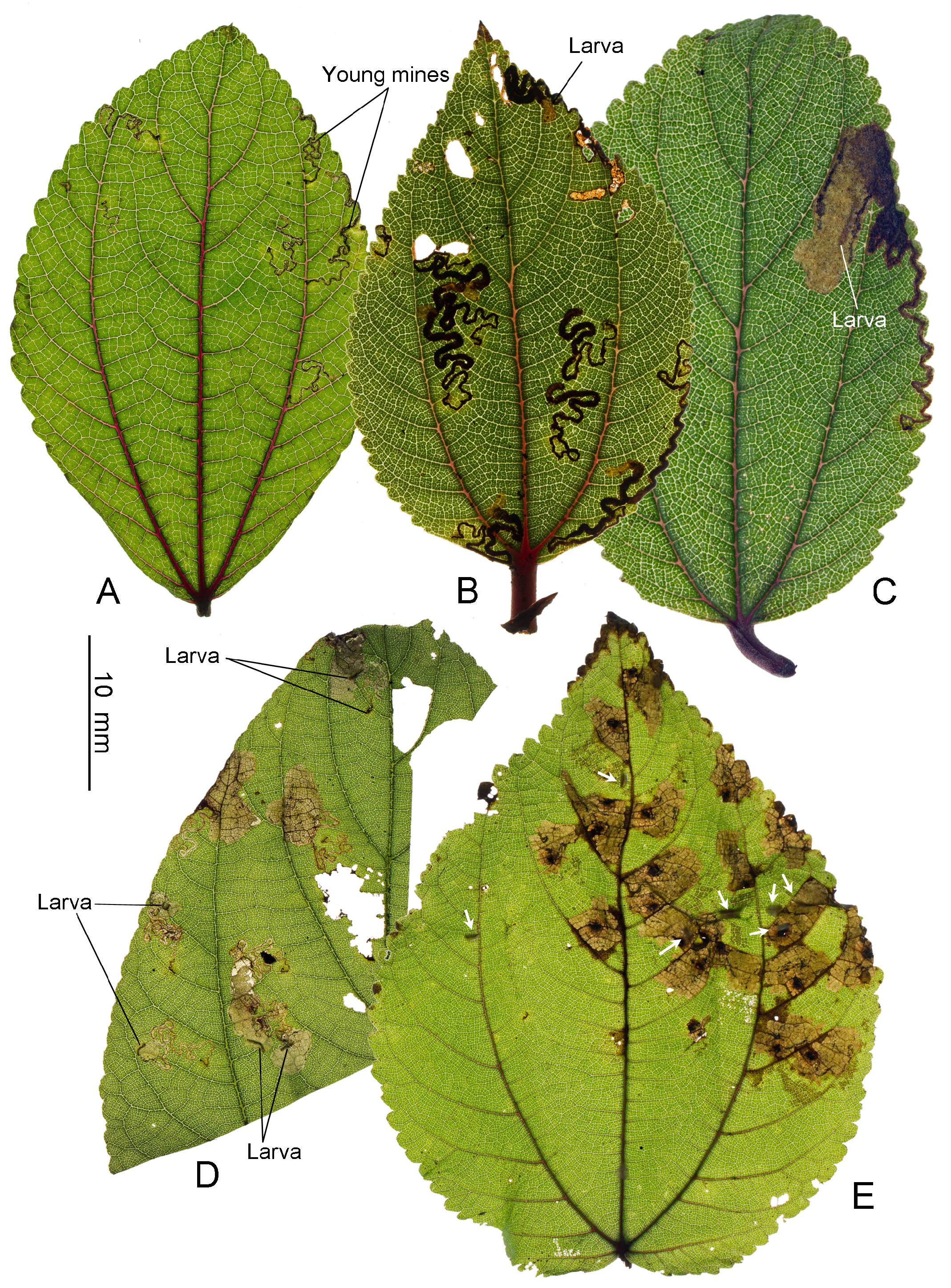
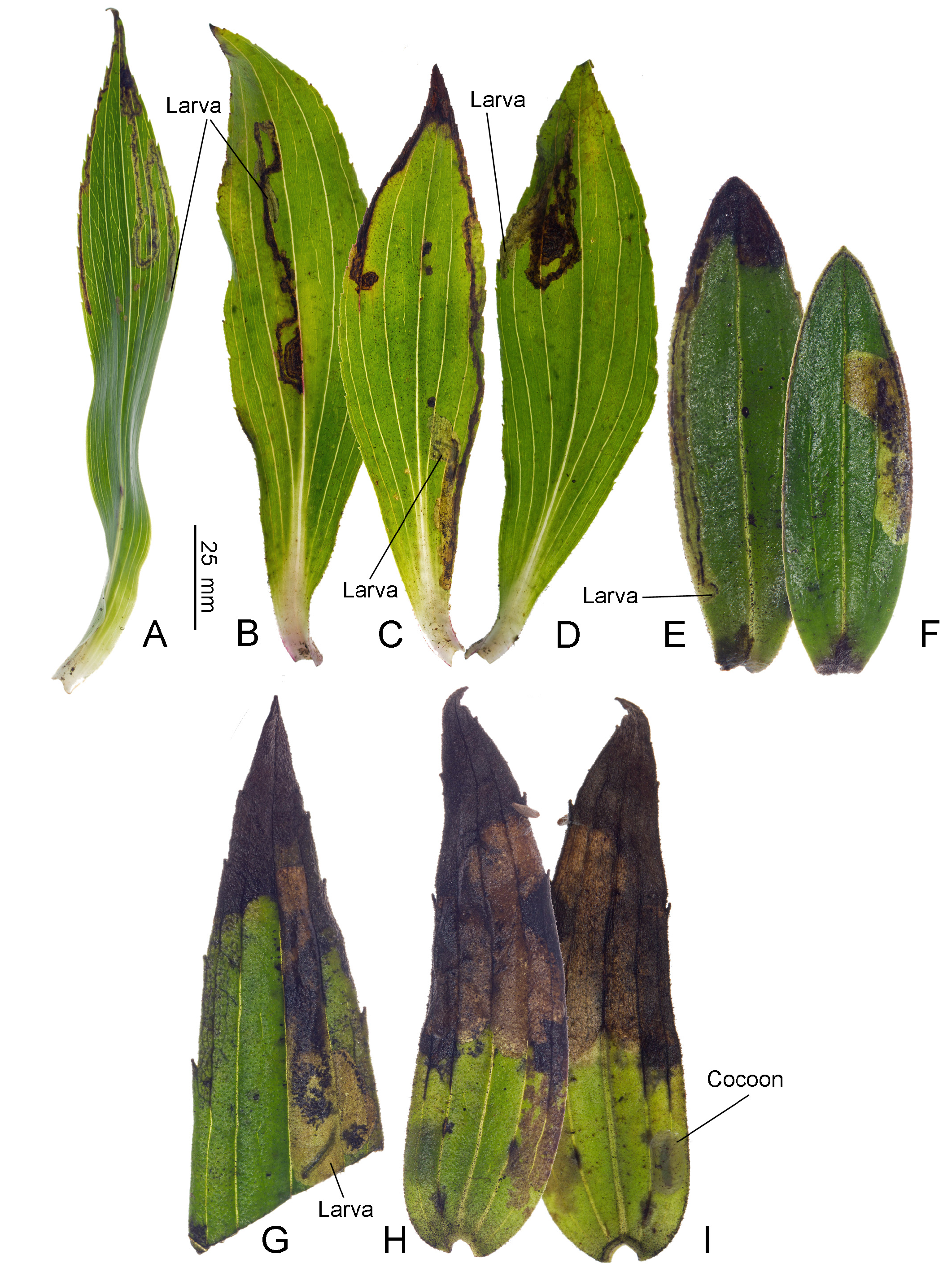
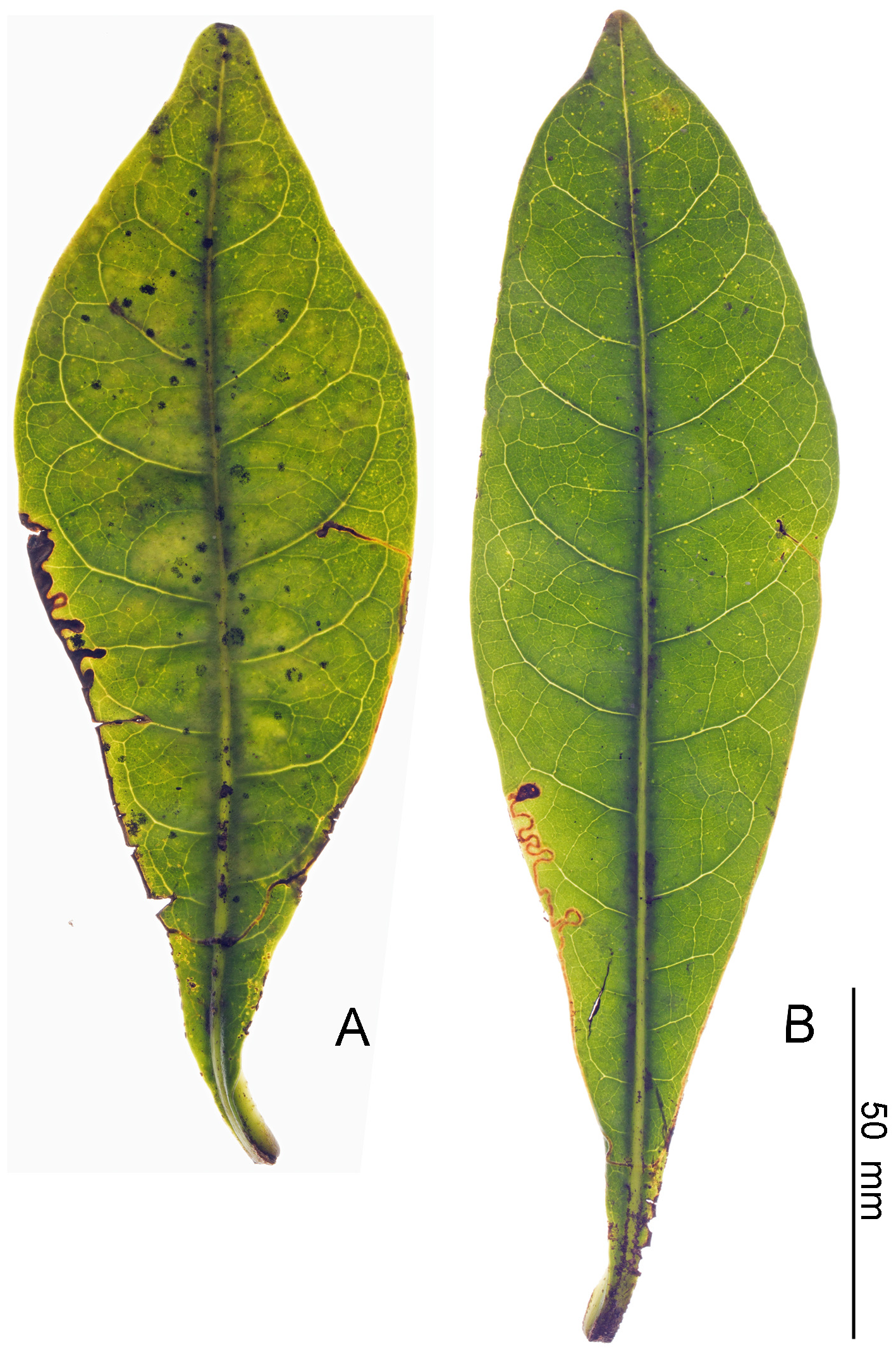
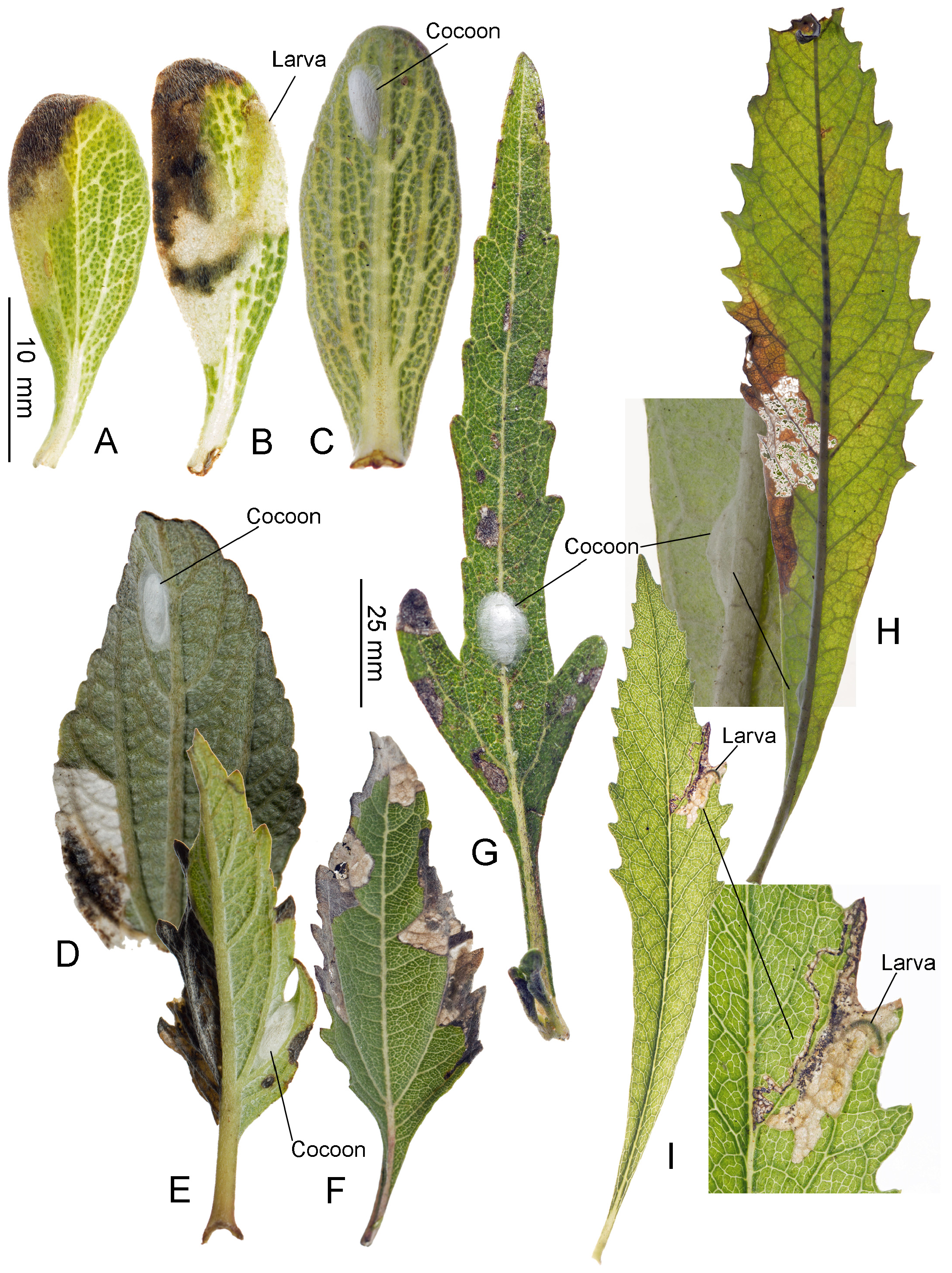
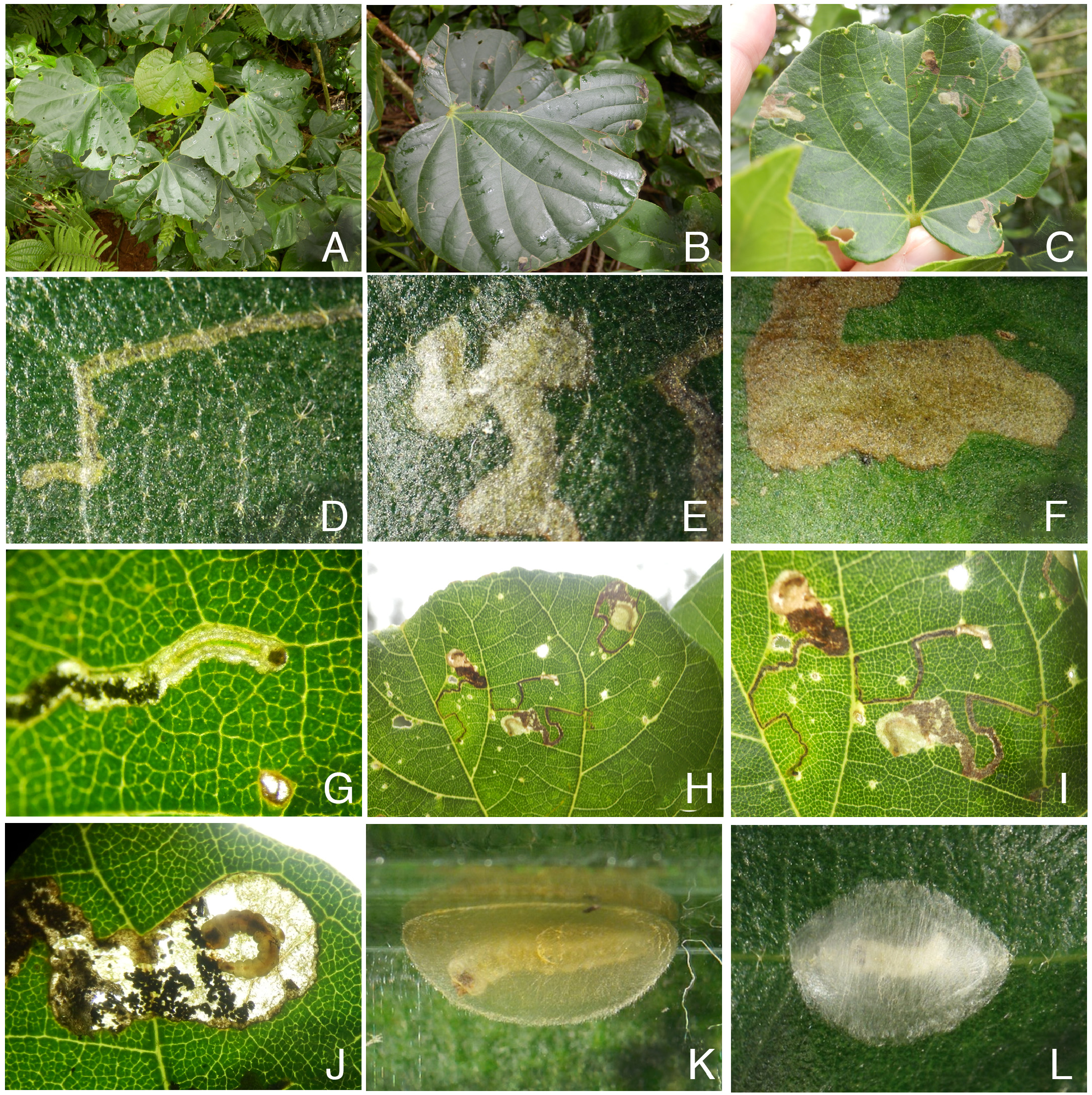
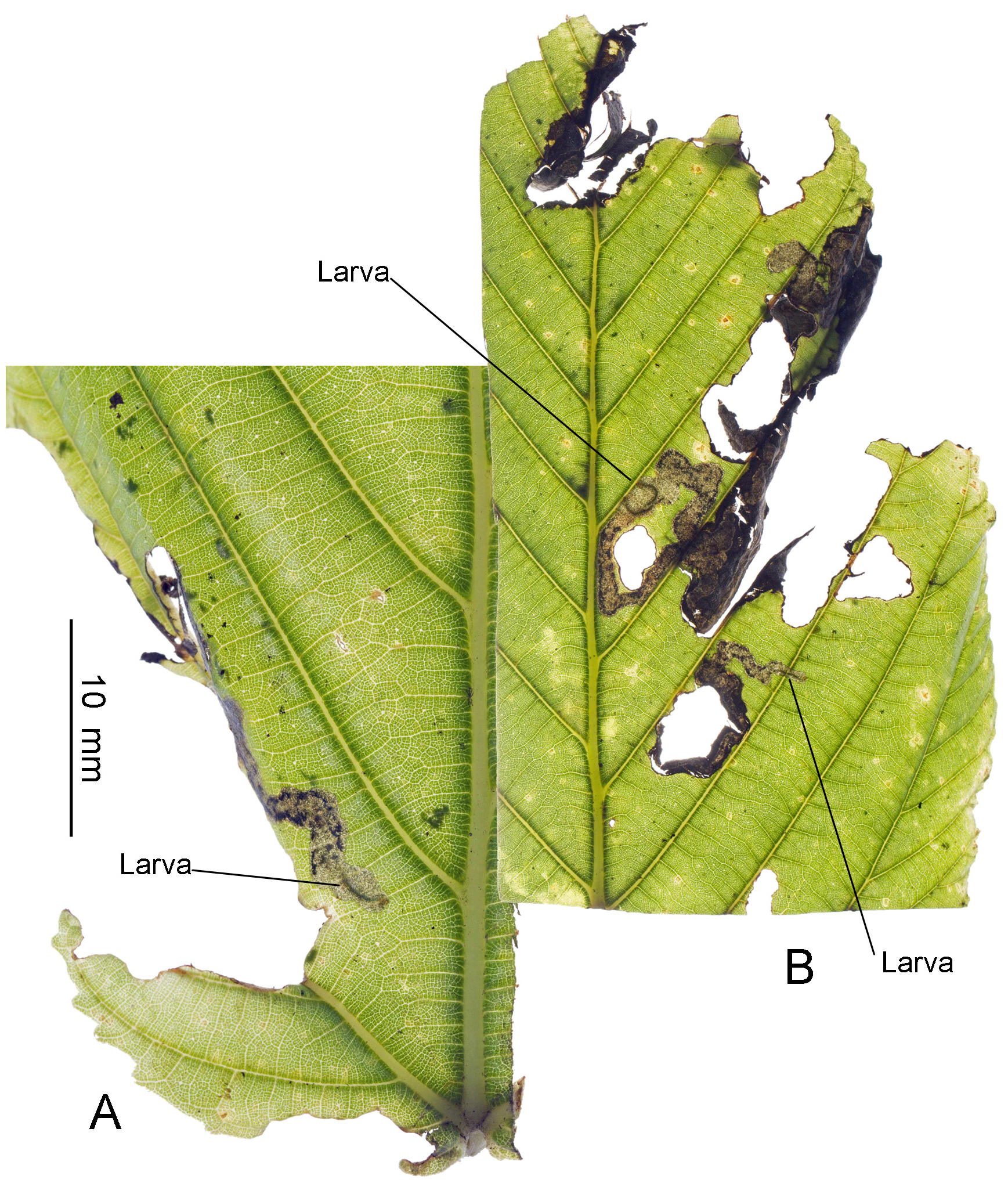
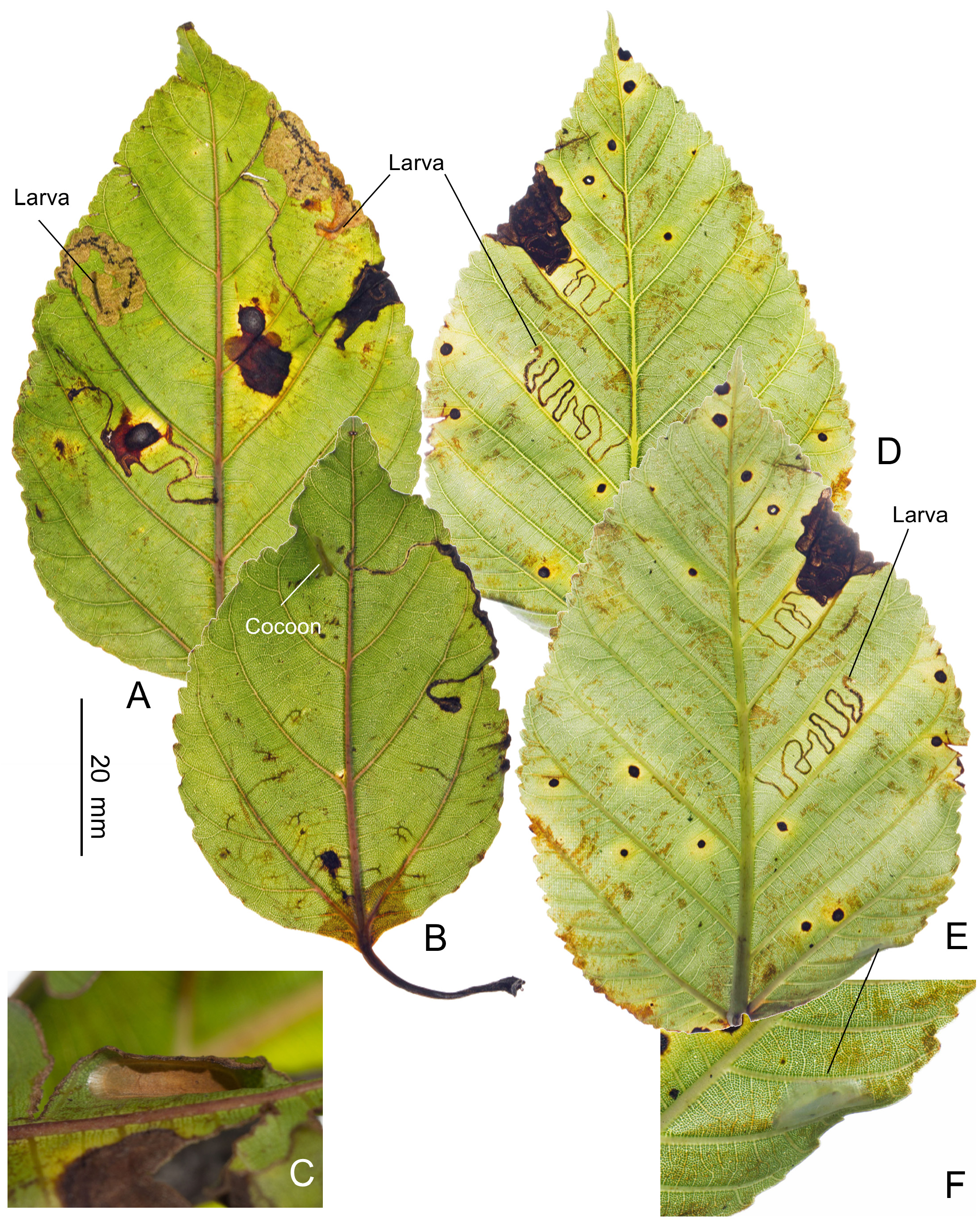
Biology. ( Figs. 79–95 View FIGURE 79 View FIGURE 80 View FIGURE 81 View FIGURE 82 View FIGURE 83 View FIGURE 84 View FIGURE 85 View FIGURE 86 View FIGURE 87 View FIGURE 88 View FIGURE 89 View FIGURE 90 View FIGURE 91 View FIGURE 92 View FIGURE 93 View FIGURE 94 View FIGURE 95 ). Philodoria larvae mine leaves of Hawaiian endemic plants in at least 20 genera and 52 species. Of these, 16 plant species are herbaceous. The larva forms a slender linear or serpentine mine on the adaxial side of the host leaf, and gradually expands its mine as it feeds and the mine later becomes the form of a blotch. Larvae of nearly all Philodoria species are presumed specialists on one plant genus (for example, 16 Philodoria species were recorded from one plant species), except for P. neraudicola P. sciallactis and P. splendida , that utilize two genera in one plant family. Philodoria marginestrigata is unusual in that it utilizes five genera in two plant families. Twenty-seven species are known to create a cocoon outside of the mine, usually pupating on the surface of the leaf. Three species, P. dubautiella , P. marginestrigata , and P. wilkesiella pupate within larval mines ( Fig. 88A View FIGURE 88 ; Swezey 1913: 279; Zimmerman 1978a: 685).
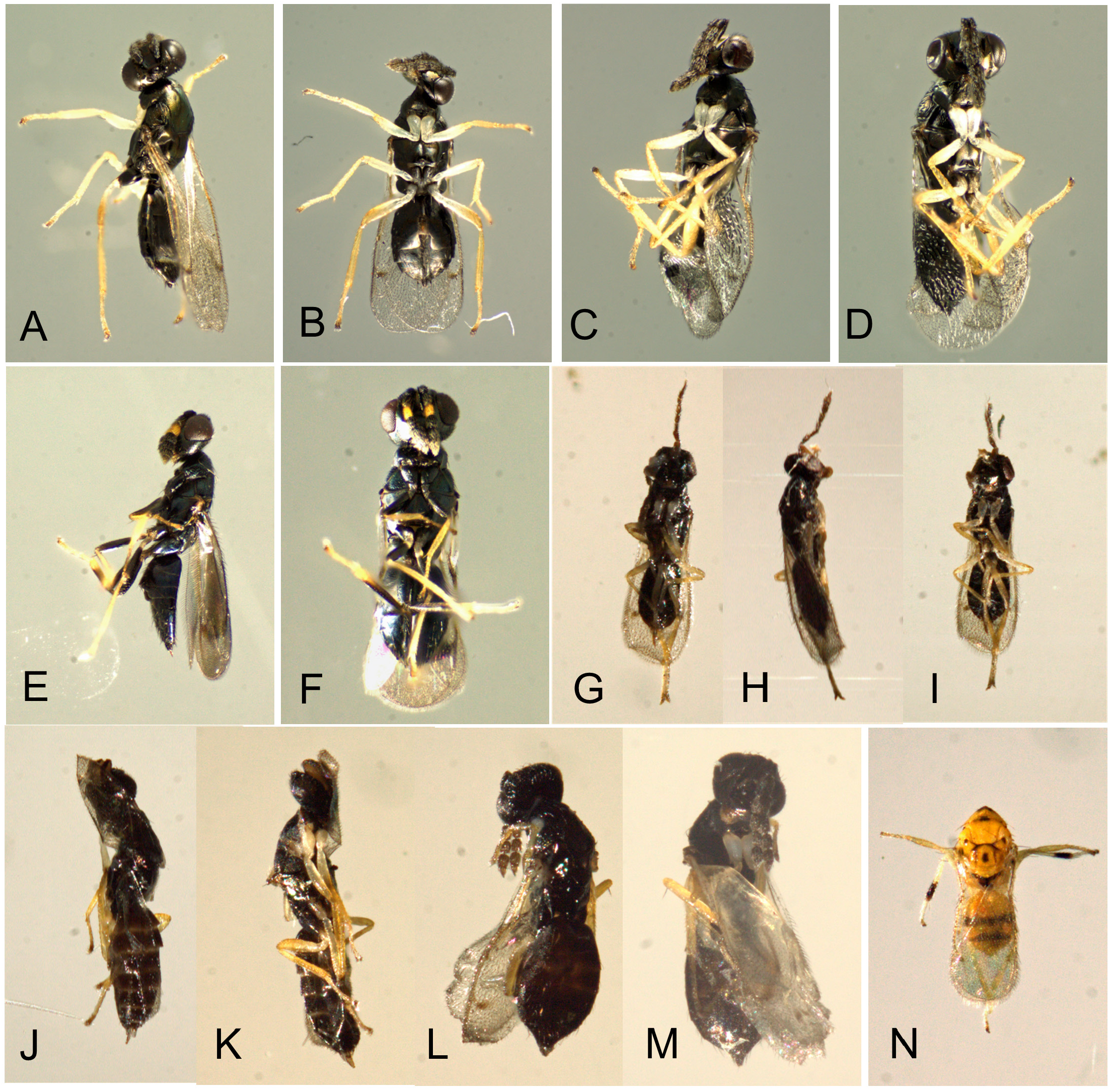
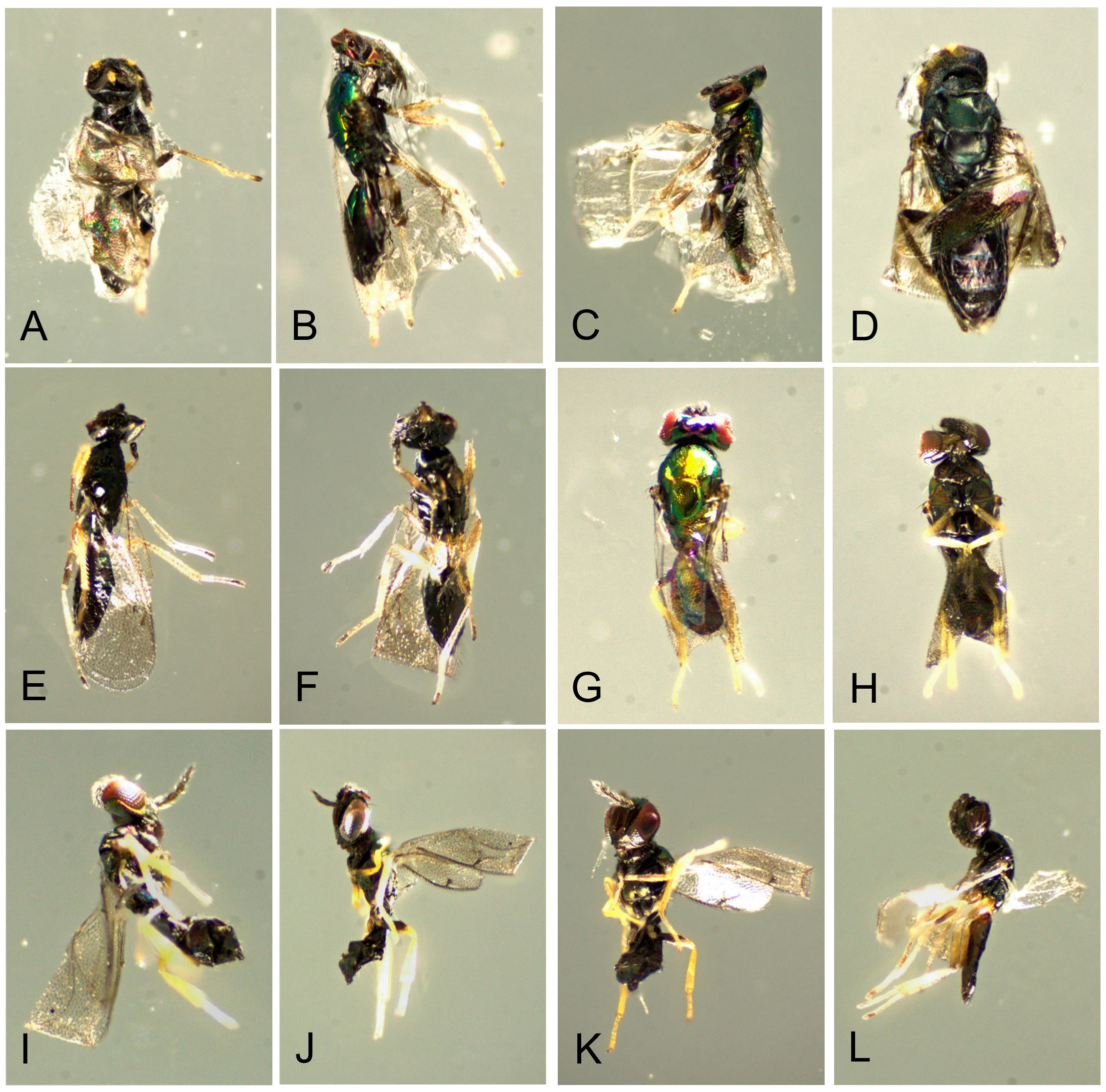
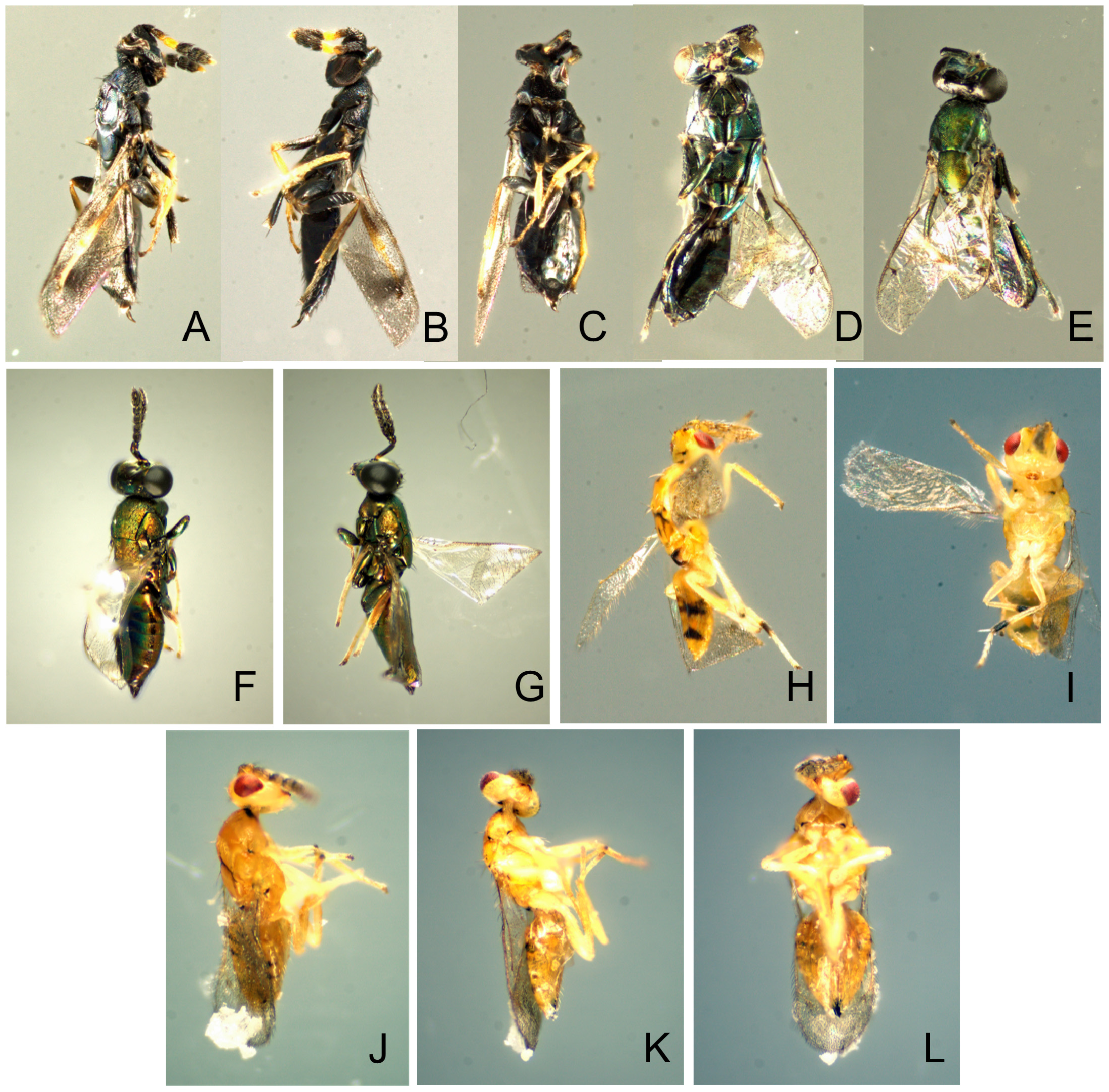
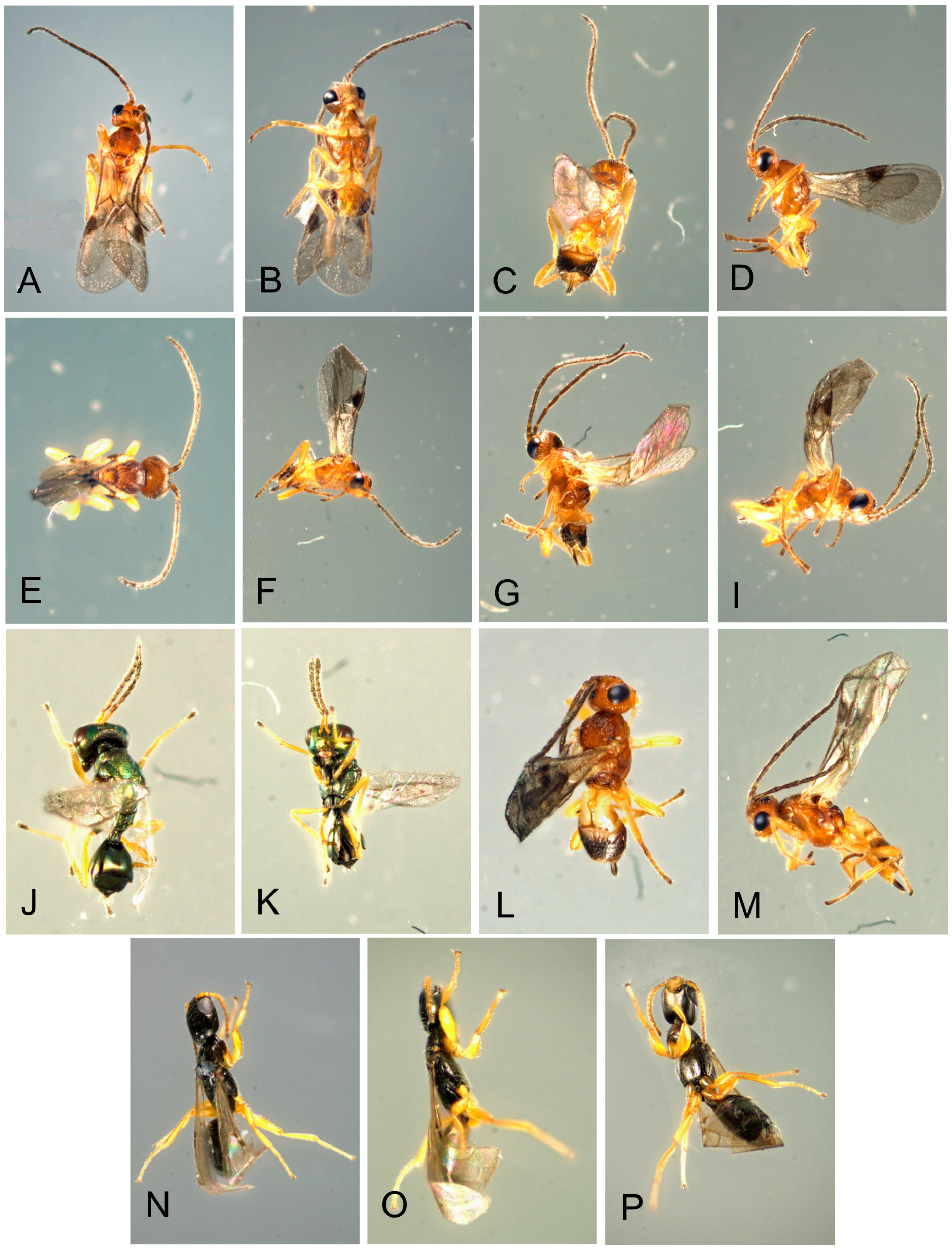
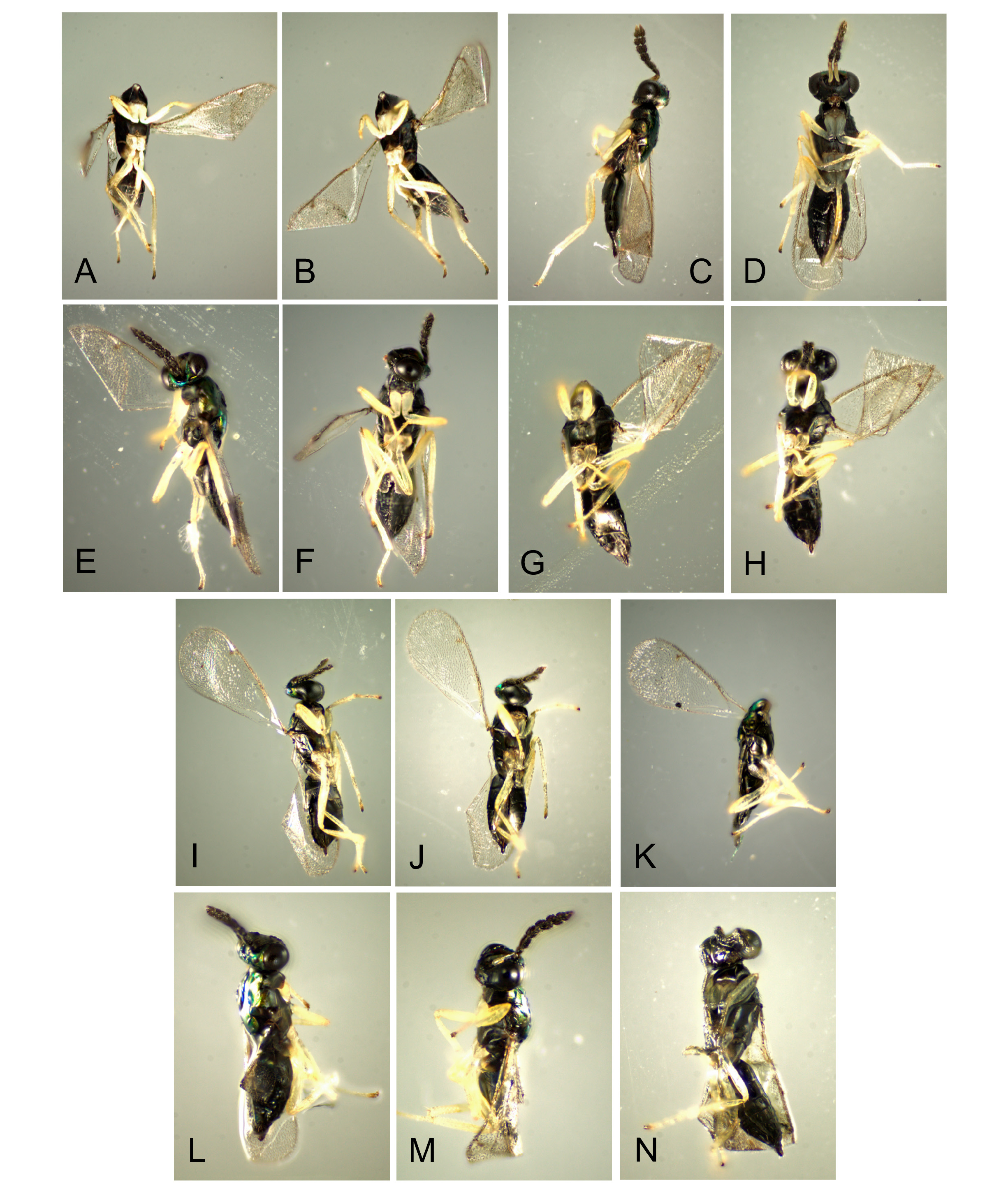
Natural enemies. ( Table 3 View TABLE 3 , Figs. 98–110 View FIGURE 98 View FIGURE 99 View FIGURE 100 View FIGURE 101 View FIGURE 102 View FIGURE 103 View FIGURE 104 View FIGURE 105 View FIGURE 106 View FIGURE 107 View FIGURE 108 View FIGURE 109 View FIGURE 110 ). Natural enemies, other than parasitoids, are not well known. Eighteen parasitoid species from four families have been recorded from 14 Philodoria species ( Yoshimoto 1965; Zimmerman 1978a), these are: Bethylidae : Sierola planiceps , S. philodoriae , S. pulchra , Sierola sp.; Braconidae : Mirax sp., Pholetesor bedelliae ; Eulophidae : Cirrospilus sp., Closterocerus sp., Diglyphus begini , Diglyphus sp., Euderus metallicus , Neochrysocharis formosus , Pauahiana maculatipennis , P. metallica , Pnigalio externa , Sympiesis vagans , Zagrammosoma flavolineatum ; Pteromalidae : Lyrcus tortricidis . We reared and examined 98 parasitoid wasps that emerged from 15 Philodoria species.
Remarks. A total of 51 species are described or redescribed in the present study (See Table 4 and discussion). In addition, some potentially unnamed species have been reported, for example, a “ Philodoria ( Philodoria) species” on Maui associated with Clermontia (Campanulaceae) ( Zimmerman 1978a), and two unidentified “ Philodoria ( Eophilodoria) species” on the island of Hawaii (Big Island) associated with Myoporum (Scrophulariaceae) (Mines by Swezey 1954:136) and Pisonia (Nyctaginaceae) (See Swezey 1954:167) respectively. We observed small minelike markings on Pisonia on Maui and Oahu, and window-like leaf mines in Clermontia on East and West Maui, but these mines contained no larvae and it is unclear whether these are made by Philodoria . We also could not observe Philodoria leaf mines on Myoporum , despite there being records of Philodoria on this plant. Myoporum leaves from Maui and Hawaii (Big Island) had leaf mines, but those were made by agromyzid flies.
Etymology: According to Walsingham (1907), the scientific name “ Philodoria ” has the meaning “bounteousness.” It remains unknown what aspect of the moths this word refers to. We present a Hawaiian common name because there are many Hawaiian names for endemic plants and animals, yet this group of moths lacks one. Early Western entomologists in Hawaii did not record Hawaiian names of the many living plants and animals nor did they often take advantage of native guides in their entomological explorations. Roughly translated, hunelele means "tiny flier " ( hune + lele) which reflects the microlepidopteran nature of these moths. The second part ʻelilau means "leaf excavator" and alludes to the leaf mining habits of the larval form. We encourage anyone who works with Philodoria species to use this name when referring to the genus in the vernacular.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
SubFamily |
Ornixolinae |
Philodoria
| Kobayashi, Shigeki, Johns, Chris A. & Kawahara, Akito Y. 2021 |
Philodoria
| Johns, C. A. & Moore, M. R. & Kawahara, A. Y. 2016: 66 |
| Zimmerman, E. C. 1978: 644 |
| Walsingham, Thomas 1907: 717 |