Oxylipeurus, Mjoberg, 1910
|
publication ID |
https://doi.org/ 10.5852/ejt.2020.686 |
|
publication LSID |
lsid:zoobank.org:pub:AC52C43B-DEB7-414B-B905-18D68F1D9DD9 |
|
persistent identifier |
https://treatment.plazi.org/id/038087A3-E510-9D2D-FF01-F9340143338D |
|
treatment provided by |
Valdenar |
|
scientific name |
Oxylipeurus |
| status |
|
Preliminary key to the genera of the Oxylipeurus -complex
Characters taken primarily from Clay (1938), Clay & Meinertzhagen (1941), Carriker (1945, 1967), Emerson & Ward (1958), Kéler (1958), Elbel & Price (1970), Mey (1982, 1990, 2006, 2010), Gustafsson et al. (2020), and examinations of specimens. Additional groups deserving recognition at the genus level may exist, and many species of the complex are in need of further study and redescription. The genus Labicotes Kéler, 1939 , may also belong to this complex, based on similarities in male and female terminalia, male genitalia, and temporal chaetotaxy between this genus and Chelopistes . This needs to be confirmed by additional studies of species of Labicotes , and the genus is not included here. 1. Broad-headed, with width of head similar to, or wider than, length of head ( Figs 19–20 View Figs 19–26. 19 ); temples with elongated “horns” ( Fig. 19 View Figs 19–26. 19 ) or with prominent lateral bulges ( Fig. 20 View Figs 19–26. 19 ).................................... 2
– Slender-headed, with head clearly longer than wide ( Fig. 5 View Figs 5–12 ); temples generally rounded, never with prominent bulging.............................................................................................................................. 3
2. Temporal setae mts1–2 macrosetae ( Fig. 20 View Figs 19–26. 19 )...................................... Trichodomedea Carriker, 1946
– Temporal setae mts1–2 microsetae ( Fig. 19 View Figs 19–26. 19 ) ................................................. Chelopistes Kéler, 1939
3. Dorsal preantennal suture present ( Figs 5 View Figs 5–12 , 21 View Figs 19–26. 19 ) .................................................................................. 4
– Dorsal preantennal suture absent or if present only visible around aperture of ads and not extending medianly ( Fig. 22 View Figs 19–26. 19 ) ........................................................................................................................... 10
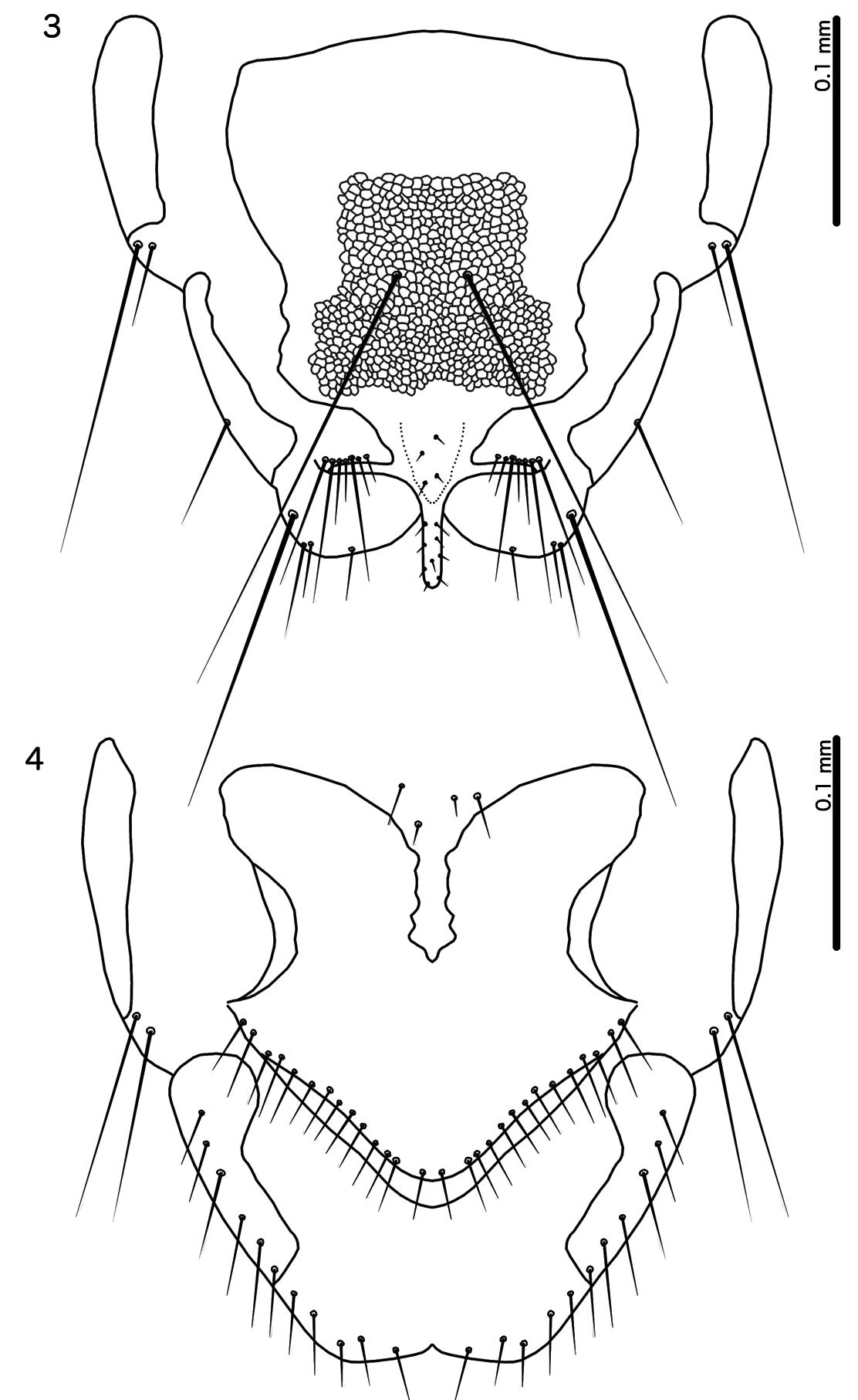
4. Dorsal preantennal suture as median, elongated oval, not expanded laterally ( Fig. 5 View Figs 5–12 ); female terminalia with marginal mesosetae distributed more or less equally around distal margin ( Fig. 4 View Figs 3–4 ); eye very large and preocular nodus absent ( Fig. 5 View Figs 5–12 ) ..................................... Calidolipeurus gen. nov.
– Dorsal preantennal suture transversal, normally reaching apertures of ads ( Fig. 21 View Figs 19–26. 19 ); female terminalia with marginal setae gathered in the same area ( Fig. 23 View Figs 19–26. 19 ); eye not very large ( Fig. 21 View Figs 19–26. 19 ), and preocular nodus present ..................................................................................................................................... 5
5. Clypeo-labral suture present ( Fig. 24 View Figs 19–26. 19 ); stylus expanded distally, with small “hooks” on lateral margins ( Fig. 25 View Figs 19–26. 19 )........................................................................ Gallancyra Gustafsson & Zou, 2020
– Clypeo-labral suture absent ( Fig. 5 View Figs 5–12 ); stylus differing in shape, but never with lateral ‘hooks’......... 6
6. Dorsal preantennal suture with postero-lateral elongations (“epistomal suture” sensu Kéler 1958) extending towards preantennal nodi ( Fig. 26 View Figs 19–26. 19 ); hyaline margin present ( Fig. 26 View Figs 19–26. 19 ) ....................................................................... Splendoroffula Clay & Meinertzhagen, 1941
– Dorsal preantennal suture without such extensions ( Fig. 21 View Figs 19–26. 19 ); hyaline margin absent ( Fig. 21 View Figs 19–26. 19 ) ....... 7
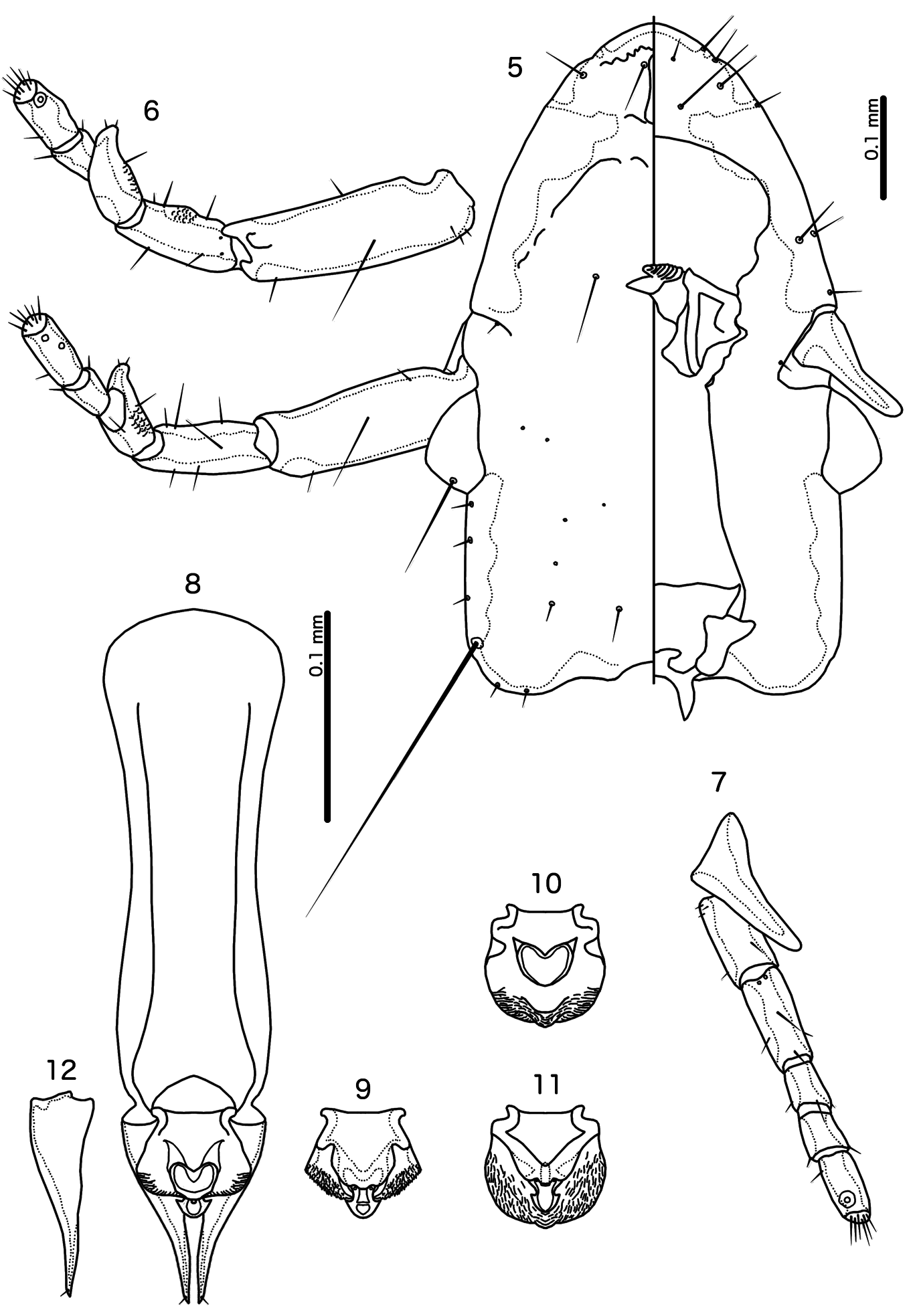
7. Dorsal postantennal suture present ( Fig. 27 View Figs 27–37. 27 ); male genitalia asymmetrical, with mesosome much reduced ( Fig. 28 View Figs 27–37. 27 )...................................................................................... Oxylipeurus Mjöberg, 1910
– Dorsal postantennal suture absent ( Fig. 21 View Figs 19–26. 19 ); male genitalia symmetrical, with prominent mesosome (variable, but similar to Figs 8–11 View Figs 5–12 ).................................................................................................... 8
8. Coni elongated (similar to Fig. 5 View Figs 5–12 ); male mesosome with prominent V- or Y-shaped thickening in distal half ( Fig. 29 View Figs 27–37. 27 ); proximal margin of mesosome with rounded lateral lobes ( Fig. 29 View Figs 27–37. 27 ); frons convergent to median point in most species (similar to Fig. 27 View Figs 27–37. 27 ).............. Megalipeurus Kéler, 1958 .
– Coni short ( Fig. 21 View Figs 19–26. 19 ); male mesosome without thickening in distal half; proximal margin variable, but never with rounded lateral lobes; frons rounded ............................................................................... 9
9. Male abdominal segments IX and IX+X with prominent postero-lateral extensions (“claspers” sensu Carriker 1945) ( Fig. 30 View Figs 27–37. 27 )............................................................ Eiconolipeurus Carriker, 1945
– Male abdomen without such structures .................................................... Reticulipeurus Kéler, 1958
10. Frons convergent to median point ( Fig. 27 View Figs 27–37. 27 ) .....................................................................................11
– Frons rounded ( Fig. 21 View Figs 19–26. 19 ) ................................................................................................................... 12
11. Male tergopleurites II–VII medianly continuous with no median indentations of anterior margin; male abdominal segments IX +X and XI fused into roughly triangular cone ( Fig. 31 View Figs 27–37. 27 ); stylus elongated and slender, in the shape of a posterior extension of the male subgenital plate ( Fig. 31 View Figs 27–37. 27 ); female terminalia without “claspers”, vulval margin more or less straight ............................... Afrilipeurus Mey, 2010
– Male tergopleurites II–VII either divided medianly, or with median indentation of anterior margin; male tergopleurites IX +X and XI separate, posterior margin concave (similar to Fig. 1 View Figs 1–2 ); stylus short and blunt ( Fig. 32 View Figs 27–37. 27 ); female terminalia with “claspers”, vulval margin deeply concave ( Fig. 33 View Figs 27–37. 27 ) .................................................................................................... Talegallipeurus Mey, 1982
12. Male parameres strongly S-curved ( Fig. 34 View Figs 27–37. 27 ); stylus arising centrally on abdominal segment IX +X ( Fig. 35 View Figs 27–37. 27 ) ..................................................................................... Sinolipeurus Gustafsson et al., 2020
– Male parameres not S-curved ( Fig. 36 View Figs 27–37. 27 ); stylus varying in shape, but always arising terminally or subterminally on subgenital plate (similar to Fig. 3 View Figs 3–4 )....................................................................... 13
13. Male genitalia simple, with parameres fused to basal apodeme and mesosome much reduced ( Fig. 37 View Figs 27–37. 27 ) ...................................................................................................... Epicolinus Carriker, 1945
– Male genitalia with parameres articulating with basal apodeme, and mesosome not reduced (similar in structure but not shape to Fig. 8 View Figs 5–12 ) ................................................................................................. 14
14. Lateral margins of postantennal head with secondary, ventral carina between antennal socket and site of mts2 or mts3 ( Fig. 38 View Figs 38–40. 38 ); area between margin of head and secondary carina, densely reticulated, including ventral surface of eye ( Fig. 38 View Figs 38–40. 38 ); male parameres with pst1–2 situated close together apically; male gonoporal complex does not reach distal margin of mesosome; female subgenital plate divided medianly ( Fig. 39 View Figs 38–40. 38 )..................................................... Valimia Gustafsson & Zou, 2020
– Lateral margins of postantennal head without secondary carina and without extensive ventral reticulation (similar to Fig. 5 View Figs 5–12 ); male parameres with pst1–2 separated, and only pst2 apical; male gonoporal complex reached to or beyond distal margin of mesosome; female subgenital plate medianly continuous ( Fig. 40 View Figs 38–40. 38 )........................................ Cataphractomimus Gustafsson et al., 2020
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
SubOrder |
Ischnocera |
|
Family |