Plectroctena Smith
|
publication ID |
https://doi.org/10.11646/zootaxa.3817.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:A3C10B34-7698-4C4D-94E5-DCF70B475603 |
|
DOI |
https://doi.org/10.5281/zenodo.5117588 |
|
persistent identifier |
https://treatment.plazi.org/id/03775906-A6D2-2C92-FF17-FE72122FFBCF |
|
treatment provided by |
Felipe |
|
scientific name |
Plectroctena Smith |
| status |
|
Plectroctena Smith View in CoL View at ENA
Fig. 39 View FIGURE 39
Plectroctena Smith, F., 1858: 101 (as genus in Poneridae). Type-species: Plectroctena mandibularis Smith, F., 1858: 101 ; by monotypy.
Cacopone Santschi, 1914: 325 (as genus). Type-species: Cacopone hastifer Santschi, 1914: 325 ; by monotypy. Bolton, 1974: 313 ( Cacopone as junior synonym of Plectroctena View in CoL ).
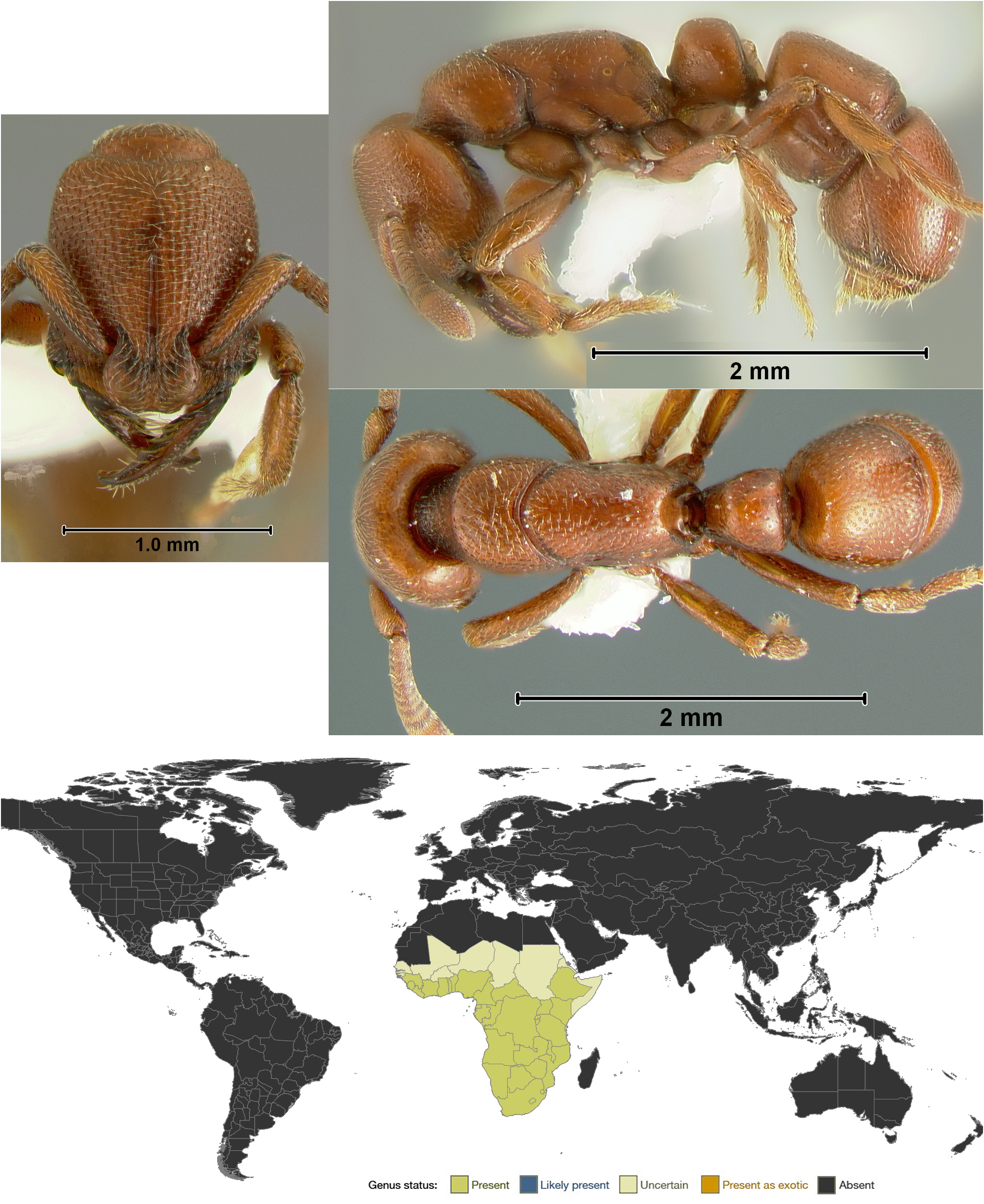
Plectroctena View in CoL is a medium sized genus (16 described species) widespread in Sub-Saharan Africa. They are cryptobiotic predators of millipedes, millipede eggs and termites.
Diagnosis. Plectroctena can be readily identified by its linear mandibles, which have dorsal longitudinal grooves, and by the anteromedial and lateral excavations of its clypeus, all of which are autapomorphic within the Ponerinae . Plectroctena is most similar to Loboponera , Boloponera , and Promyopias , which all have expanded frontal lobes and an overall similar gestalt. Plectroctena differs from Loboponera most obviously in the shape of the mandibles (triangular in Loboponera ). Boloponera and Promyopias both have linear mandibles, but they both lack the autapomorphies of Plectroctena given above, among several other differences. Plectroctena also bears some resemblance to Myopias , given their linear mandibles, but Plectroctena lacks an anteromedial projection of the clypeus and has only a single metatibial spur.
Synoptic description. Worker. Medium to very large (TL 5.6–23.5 mm; Bolton, 1974) ants with the standard characters of Ponerini . Mandibles linear, crossing each other apically when closed, edentate or with one or two teeth, and with a dorsal longitudinal groove and a basal groove. Clypeus excavated anteromedially and with a lateral excavation near each mandibular articulation. Frontal lobes greatly expanded, closely approximated, and overhanging the anterior clypeal margin. Eyes small to absent, located far anterior on the sides of the head. Mesopleuron divided by a transverse groove, the anepisternum apparently fused to the mesonotum and metapleuron. Metanotal groove usually absent, occasionally vestigial. Propodeum broad dorsally, the posterolateral margins expanded into lamellae. Propodeal dorsum rarely with a weak longitudinal groove. Propodeal spiracles round. Metapleural gland orifice opening laterally. Meso- and metafemora with a dorsal longitudinal groove. Metatibial spur formula (1p). Anteroventral articulatory surface of petiole long and broad, with a narrow median groove. Petiole nodiform. Gaster with a strong constriction between pre- and postsclerites of A4. Head and body shining, punctate, with striations on the sides of the mesosoma, minimal pilosity, and no pubescence. Color red to black.
Queen. Usually alate, but ergatoid in some species. Alate queens are similar to workers but slightly larger, with larger eyes and with ocelli. Ergatoids are similar but at most have only vestigial ocelli ( Bolton, 1974; Bolton & Brown, 2002).
Male. See description in Bolton (1974); also discussed in Bolton & Brown (2002).
Larva. Described for P. mandibularis by Wheeler & Wheeler (1989).
Geographic distribution. Plectroctena ranges throughout most of Sub-Saharan Africa, from Sierra Leone to Ethiopia and south to South Africa ( Bolton & Brown, 2002).
Ecology and behavior. More is known about the habits of Plectroctena than of any other genus in the Plectroctena group, but data on most species are still scarce. Like other members of the group, Plectroctena are primarily cryptobiotic, nest in soil or rotting wood, and forage in these same microhabitats as well as among leaf litter ( Arnold, 1915; Bolton, 1974; Bolton et al., 1979; Peeters & Crewe, 1988; Bolton & Brown, 2002; Déjean et al., 2002). They have also been found nesting in abandoned termitaries (Déjean et al., 1996). Very little is known about the social and reproductive behavior of Plectroctena . Colony sizes are unknown for most species but colonies of P. lygaria , P. mandibularis and P. minor are reported to have about 300 or fewer workers ( Bolton et al., 1979; Déjean et al., 2001, 2002; Wilkins et al., 2006).
Most Plectroctena species have winged queens, but at least four species have ergatoid queens ( Bolton, 1974), and at least one of these ( P. mandibularis ) is facultatively polygynous ( Wilkins et al., 2006). In laboratory conditions, ergatoid queens of P. mandibularis successfully captured prey and were able to rear brood without the assistance of workers, suggesting that colony foundation in this species is semiclaustral ( Villet, 1991a; confirmed in natural conditions by Villet, 1999), in contrast to most ants with ergatoid queens. Mating behavior by P. mandibularis is also unusual in that virgin females leave the nest and apparently call for males using a pheromone; in most ponerines with ergatoid queens, mating occurs in the natal nest of the queen ( Villet, 1999). In P. mandibularis , ergatoid queens apparently inhibit reproduction by workers but orphaned workers of P. mandibularis will begin laying eggs and can successfully rear male brood ( Peeters & Crewe, 1988).
Plectroctena are primarily specialist predators of millipedes or millipede eggs, but they also prey to a lesser extent on termites and other arthropods, including other ants ( Arnold, 1915; Fletcher, 1973; Bolton et al., 1979; Lévieux, 1983; Peeters & Crewe, 1988; Schatz et al., 2001; Bolton & Brown, 2002; Déjean et al., 2002). Workers typically forage individually but may hunt in small groups ( Bolton, 1974; Peeters & Crewe, 1988), and sometimes recruit nestmates to help with large prey (see below). Foraging behavior has been extensively studied in P. minor , which specializes to a large degree on millipedes. In cafeteria experiments, P. minor workers overwhelmingly preferred millipedes, but also accepted centipedes, termites, isopods, grasshoppers, and beetle larvae ( Suzzoni et al., 2000; Schatz et al., 2001). Queens foraging shortly after colony foundation, on the other hand, ignored large millipedes and preferred smaller, more easily captured prey such as isopods or termites ( Déjean & Suzzoni, 1991; Suzzoni et al., 2000). The presence of millipedes in the diet of a P. minor colony is required for it to produce reproductive females and significantly enhances the production of workers, but is not required for production of male brood ( Suzzoni et al., 2000).
Déjean & Suzzoni (1991) and Déjean et al. (2001) studied the capture of millipedes by P. minor . Workers of this species use their linear mandibles, paralyzing sting, and nestmate recruitment to capture and retrieve millipedes of a wide range of sizes, including very large individuals. Workers demonstrate significant flexibility in their foraging behavior, depending on the size and location of their prey. Their mandibles are able to grasp millipedes under 4 mm in diameter, which are stung repeatedly until paralyzed. Larger millipedes pose more of a problem and require creative strategies for capture and retrieval, including use of mandibular snapping (see below) and recruitment of two to five nestmates via use of a chemical trail. Large millipedes are either cut up or collectively transported whole, while smaller prey are retrieved by single workers. Individual P. minor foragers are able to retrieve millipedes weighing more than 100 times their own weight, the largest ratio of prey to worker weight known for any ant ( Déjean et al., 2001). Like P. minor , foragers of P. mandibularis recruit nestmates to assist in prey retrieval ( Fletcher, 1973), and also lay chemical trails from the pygidial gland for individual orientation and recruitment during nest emigrations ( Villet et al., 1984; Wilkins et al., 2006).
Plectroctena workers are able to snap their mandibles to stun or kill enemies or prey ( Déjean and Suzzoni, 1991; Déjean et al. 2001, 2002). This behavior is unique among ponerines but also occurs in the ambyloponine genus Mystrium and some termites ( Gronenberg et al., 1998). The forceful snapping of the mandibles is used in territorial aggression, defense, and prey capture. In the study of Déjean et al. (2002), P. minor foragers almost always snapped their mandibles when confronted with termite soldiers (which are potentially dangerous) or large prey, while smaller prey were usually captured without snapping. Déjean et al. (2002) suggest that the snapping mechanism is an adaptation to hunting in tight spaces, though it is also an effective weapon against other ants and is readily employed when other ponerines (especially other Plectroctena ) are encountered in the vicinity of the nest.
Phylogenetic and taxonomic considerations. Plectroctena was erected by F. Smith (1858) to house the species P. mandibularis . Emery (1911) placed it in his new subtribe Plectroctenini along with Psalidomyrmex and Myopias (and its synonym Trapeziopelta ), based on similarities in sculpturing, pubescence, and tibial spurs.
Plectroctena has a single junior synonym, Cacopone , which was erected by Santschi (1914) to hold the single species C. hastifer (now Plectroctena hastifera ). Oddly, Santschi initially stated that Cacopone was somehow related to Myopias and Psalidomyrmex , but did not mention Plectroctena despite their obvious similarities. He did make this connection in his revision of Plectroctena , however, but continued to separate them based on supposed differences in mandibular and clypeal structure ( Santschi, 1924). Bolton (1974) synonymized Cacopone under Plectroctena after noting mistakes in Santschi’s description and the discovery of a new species with mandibles intermediate between the two genera.
Schmidt's (2013) molecular phylogeny of the Ponerinae clearly places Plectroctena far from Myopias , as predicted by Bolton (1974). Among the taxa sampled in Schmidt’s (2013) phylogeny, Plectroctena is resolved as sister to Loboponera , though it is possible that Boloponera is the true sister to Plectroctena (see the discussion of relationships within the Plectroctena genus group, above).
Bolton (1974) divided Plectroctena into three species groups, of which two (the P. mandibularis and P. minor groups) are included in Schmidt’s (2013) phylogeny. The third species group (the P. hastifera group) represents Cacopone , and though it is not included in the phylogeny, we see no reason to withdraw it from Plectroctena and therefore retain it as a junior synonym of that genus.
Species of Plectrocten a
Bolton (1974) revised Plectroctena , and Bolton & Brown (2002) provided a species key (which lacks P. thaui , described since).
P. anops Bolton, 1974 : Ghana
P. cristata Emery, 1899 : Cameroon
P. cryptica Bolton, 1974 : Ghana
P. dentata Santschi, 1912 : Angola
P. gestroi Menozzi, 1922 : Principe Island
P. hastifera ( Santschi, 1914) : Ghana
P. laevior Santschi, 1924 : Tanzania
P. latinodis Santschi, 1924 : DRC
P. lygaria Bolton, Gotwald & Leroux, 1979 : Ivory Coast
P. macgeei Bolton, 1974 : Nigeria
P. mandibularis Smith, F., 1858 : South Africa
P. minor Emery, 1892 : Ivory Coast
P. strigosa Emery, 1899 : South Africa
P. subterranea Arnold, 1915 : Zimbabwe
P. thaui Fisher, 2006 : Cameroon
P. ugandensis Menozzi, 1933 : Uganda
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
SubFamily |
Ponerinae |
|
Tribe |
Ponerini |
Plectroctena Smith
| Schmidt, C. A. & Shattuck, S. O. 2014 |
Cacopone
| Bolton, B. 1974: 313 |
| Santschi, F. 1914: 325 |
| Santschi, F. 1914: 325 |