Phytoptus atherodes, Chetverikov, Philipp E., 2011
|
publication ID |
https://doi.org/ 10.5281/zenodo.200512 |
|
DOI |
https://doi.org/10.5281/zenodo.6183875 |
|
persistent identifier |
https://treatment.plazi.org/id/FC139970-4F21-8240-FF73-FB25FD53FD56 |
|
treatment provided by |
Plazi |
|
scientific name |
Phytoptus atherodes |
| status |
sp. nov. |
Phytoptus atherodes sp. n.
( Fig. 1 View FIGURE 1 ; Table 3 View TABLE 3 )
Protogyne female (n=10). Body vermiform, whitish, 331 (270–350) long, 63 (55–66) wide. Prodorsal shield semicircular with five distinct entire longitudinal lines (median, two admedians and two submedians-I) extending from the rear to the anterior shield margin. Admedian lines are parallel to median line in anterior third of shield; in rear two thirds of shield they diverge, forming a figure resembling reverse Greek letter “ψ”. Two short lines are present between median and admedian lines near rear shield margin. Submedian-I lines straight in anterior half of shield and curved in rear half. Two short lines present between admedian and submedian-I lines in anterior third of shield. Submedian-II lines from anterior shield margin running back past inner side of tubercules of ve and ending well in front of tubercules of sc. These lines may consist of 2 to 3 fragments or may be entire with a small curve at the rear. Three short lines are present on lateral sides of shield. The surface of prodorsal shield anterior to ve, with small granulations. Prodorsal shield 40 (35–41) long, 43 (34–46) wide; ve 18 (15–20) long, directed forward, tubercles 25 (21–25) apart; sc 2 (1–3) long, directed backward, tubercles 23 (20–24) apart. Distance between tubercules ve and sc 22 (22–23). Gnathosoma 26 (24–26) long. Dorsal pedipalp genual setae d 16 (15-18) long. Leg I 36 (32– 36) long, tibia 8 (6–8), l' 3 (2–3), tibial solenidion φ 8 (8–10), tarsus 7 (6–7), ω 14 (11–15) long, without knob, empodium 9/8-rayed, 15 (13–15) long. Leg II 31 (29–34) long, tibia 7 (6–7), l' absent, tarsus 6 (6–7) long, ω 15 (12–15) long, without knob, empodium 9/8-rayed, 14 (13-16) long. Setae bv present. Short longitudinal line near tubercule of bv present on both femora. Coxae with numerous thin short lines and oval microtubercules. Subcapitular plate extremely subtle, indistinct in all measured specimens. Setae 1b 24 (18–24) long, 16 (14–17) apart; 1a 34 (15–40) long, 12 (10–12) apart; 2a 63 (45–63) long, 31 (20–32) apart. Epigynium smooth, 15 (11–15) long, 23 (19–23) wide; 3a 13 (9–13) long. Opisthosoma with 90 (82–92) microtuberculated annuli. 4 (3-5) annuli present before epigynium. Setal lengths: c1 120 (100–125), c2 66 (45–66), d 36 (30–38), e 9 (9–15), f 50 (40–62), h1 4 (3– 6); 6 (6–7) annuli between rear shield margin and tubercules of c1, 7 (6–7) annuli anterior to c2; 16 (14–16) annuli situated between c2 and d; 29 (24–29) annuli situated between d and e; 27 (21–28) annuli situated between e and f, 11 (10–11) annuli situated between f and h1.
Male. In comparison to females, males are smaller in size, have shorter legs and opisthosomal setae and possess a 7/6-rayed empodium. The design of the male prodorsal shield is similar to that of the female. Measurements of males are given in Table 3 View TABLE 3 .
* Characteristics of the samples are given in the Table 2 View TABLE 2 .
Host plant. Carex atherodes Spreng. 1826 (= C. orthostachys C.A. Mey 1833 ) ( Cyperaceae ).
Relation to the host. Mites live inside leaf sheaths, causing no damage.
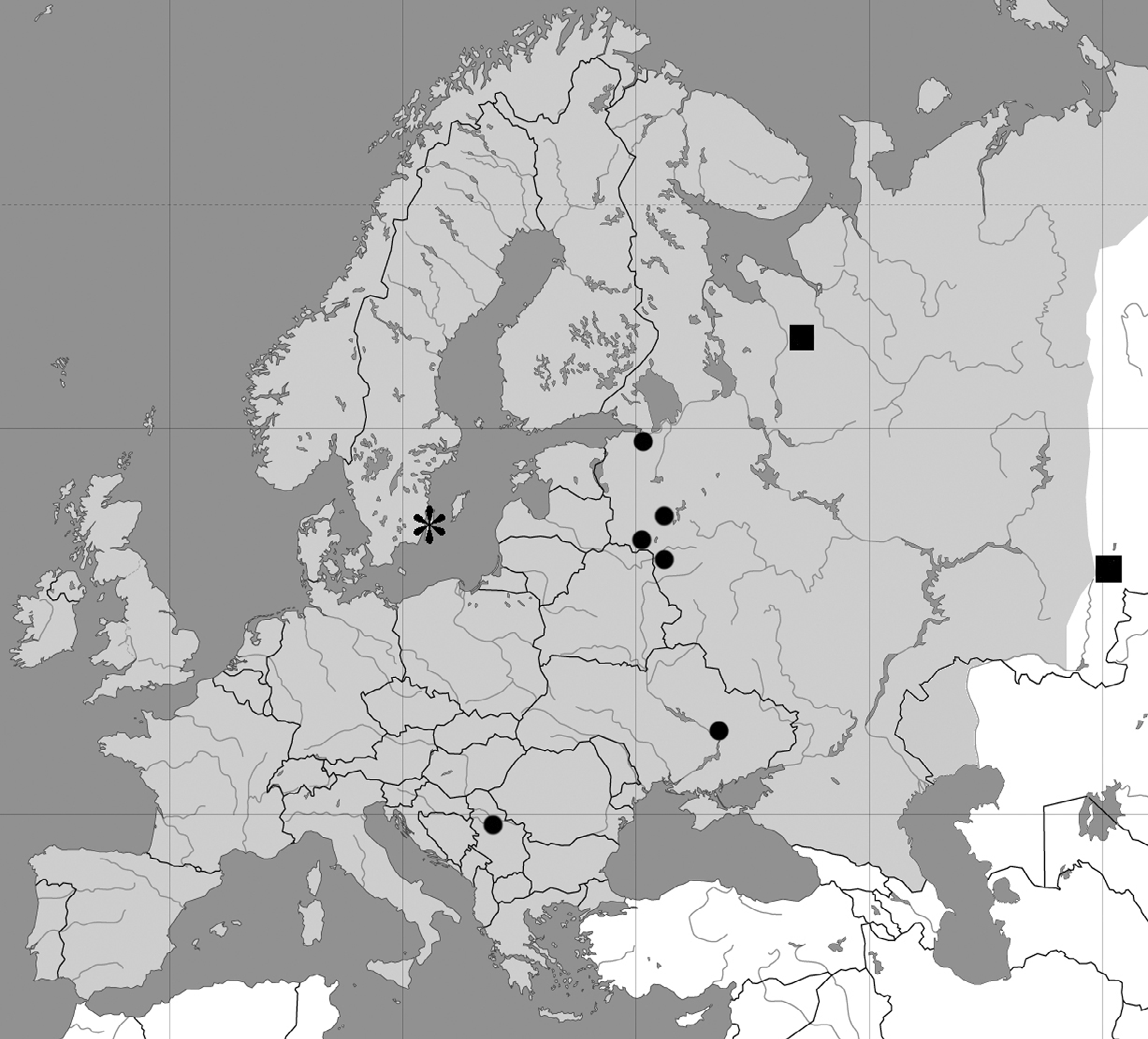
Type locality. RUSSIA: Arkhangelsk Prov., Plesetsk area, meadow near fir-tree forest; 62°72'54''N, 40°28'38''E, 5 August 2003, coll. P. E. Chetverikov.
Type material. Protogyne female holotype (slide # 61-03), 16 protogyne female paratypes and 5 males (on same slide) deposited in Saint-Petersburg State University, Department of Invertebrate Zoology (Universitetskaya naberejnaya, 7/9, Saint-Petersburg, 199034, Russia). Paratypes (slides #70-03 and #65-04) are also deposited in the Acarological Collection of Zoological Institute of Russian Academy of Sciences ( ZIN RAS).
Additional material. 3 protogyne females, 5 deutogyne females, 2 males and 7 nymphs (on microscope slides: #17-05, #18-05 #19-05) collected from Carex atherodes Spreng. (Cyperaceae) [inside leaf sheaths; no apparent damage was observed] RUSSIA: Chelyabinsk Prov., Miass area, on right bank of river Maliy Syrostan; 55°11'73''N, 59°83'78''E; 27 July 2005, coll. P. E. Chetverikov; 11 diapausing protogyne females and 28 diapausing deutogyne females (slide #96-03), same host [inside leaf sheaths; no damage was observed], type locality, 10 November 2003, coll. T. G. Chetverikova; 24 protogyne females, 7 deutogyne females and 7 males in slide #70-03 (12 August 2003) plus 19 protogyne females, 14 deutogyne females and 4 males in slide #65-04 (20 August 2004) plus 27 protogyne females, 13 deutogyne females and 2 males in slide #a1-05 (29 August 2005), same host, type locality, coll. P. E. Chetverikov.
Distribution. Up to the present, P. atherodes sp. n. has been recorded only from North-West Russia (Arkhangelsk Prov.) and the Ural region of Russia (Chelyabinsk Prov.), Fig. 3 View FIGURE 3 . However, C. atherodes , the host-plant of P. atherodes , also occurs in China, North America, Mongolia and Far East of Russia ( Egorova, 1999), so future surveys could reveal a wider distribution for this new mite species.
Etymology. The species name is derived from the name of the host-plant ( Carex atherodes ) and is an adjective, gender masculine, Singulāris. According to Gledhill (2008), the adjective “ atherodes ” is derived from the Greek word αθερος (bristle, beard) + ωδης (-resembling, -similar to) and refers to the botanical character, “bristle-eared”.
Differential diagnosis. The new species, P. atherodes , is closest to P. hirtae Roivainen 1950 and Phytoptus swalesi Keifer, 1966 ( Table 6 View TABLE 6 ). In comparison to the other species of Phytoptus from sedges, these three species have very short setae sc (1–3 μm).
1) Differences between P. atherodes and P. h i r t a e: Mites of P. atherodes sp. n. have the entire median line extending from the anterior to the rear shield margin ( Fig. 1 View FIGURE 1 B) whereas specimens of P. h i r t a e have the median line with a gap at the anterior one third ( Fig. 2 View FIGURE 2 A). Summer females of P. atherodes and P. hirtae are almost indistinguishable from each other by measurements ( Table 3 View TABLE 3 & 4 View TABLE 4 ), although they differ in the form of the median line (complete in the protogyne and deutogyne of P. atherodes and with a gap in the protogyne and deutogyne of P. hirtae , ( Fig. 1 View FIGURE 1 A, B, C & Fig. 2 View FIGURE 2 A, C) in the length of the empodia [14 (13–15) and 10 (10–11), respectively] and in the empodial ray number (9/8 and 7/6, respectively). In addition, these mite species live on different host-plants, namely C. atherodes and C. hirta .
2) Differences between P. atherodes and P. s w a l e s i. These two species differ from each other in the design of the prodorsal shield and the length of setae c1 (s.sd.), d (s. v.1) and f (s. v.3) ( Table 6 View TABLE 6 ). The median and admedian lines on the prodorsal shield of P. atherodes extend from the anterior to rear shield margin ( Fig. 1 View FIGURE 1 B) whereas in P. swalesi , the se lines are present in the rear 2/3 of the prodorsal shield ( Keifer 1966, plate 10). Moreover, P. atherodes has two short lines near the posterior part of the median line near the rear shield margin and granulations before setae ve ( Fig. 1 View FIGURE 1 B). These elements are absent on the prodorsal shield of P. s w a l e s i.
Variability of empodial ray number of P. atherodes . Mites of the genus Phytoptus from the group “ caricis ” have asymmetrical empodia with the number of rays on the inner side exceeding by 1 to 2, the number of rays on the outer side (Chetverikov et al. 2009). Similar empodia can be found within the genera Oziella Amrine, Stasny and Flechtmann 2003 , Acathrix Keifer 1962 and Trisetacus Keifer 1952 ( Smith 1984; Chetverikov et al. 2009; Walter et al. 2009, p. 345, Fig. 13.19 E,J*). The number of empodial rays is one morphological character that distinguishes closely related species of the genus Phytoptus occurring on sedges. Variability of this character among Phytoptus species has rarely been reported. The only rare discussion concerning such variation was included in the descriptions of Phytoptus oculatus Smith 1977 and Phytoptus rigidus Roivainen 1950 as well as in the supplementary description of Phytoptus liroi Roivainen 1947 ( Roivainen 1950; 1951) where this was 6–8, 8–10 and 6–7, respectively.
* Walter et al. (2009) wrongly designated Fig. 13.19E for the empodium of Acathrix sp. and Fig.13.19J for the empodium of Trisetacus sp. However, Fig. 13.19E actually illustrates an empodium that is not of a phytoptoid mite and Fig.13.19J is a copy of the empodium figure of Acathrix trimatus Keifer, 1962 as it appears on page 17, plate 9. Fig. F.
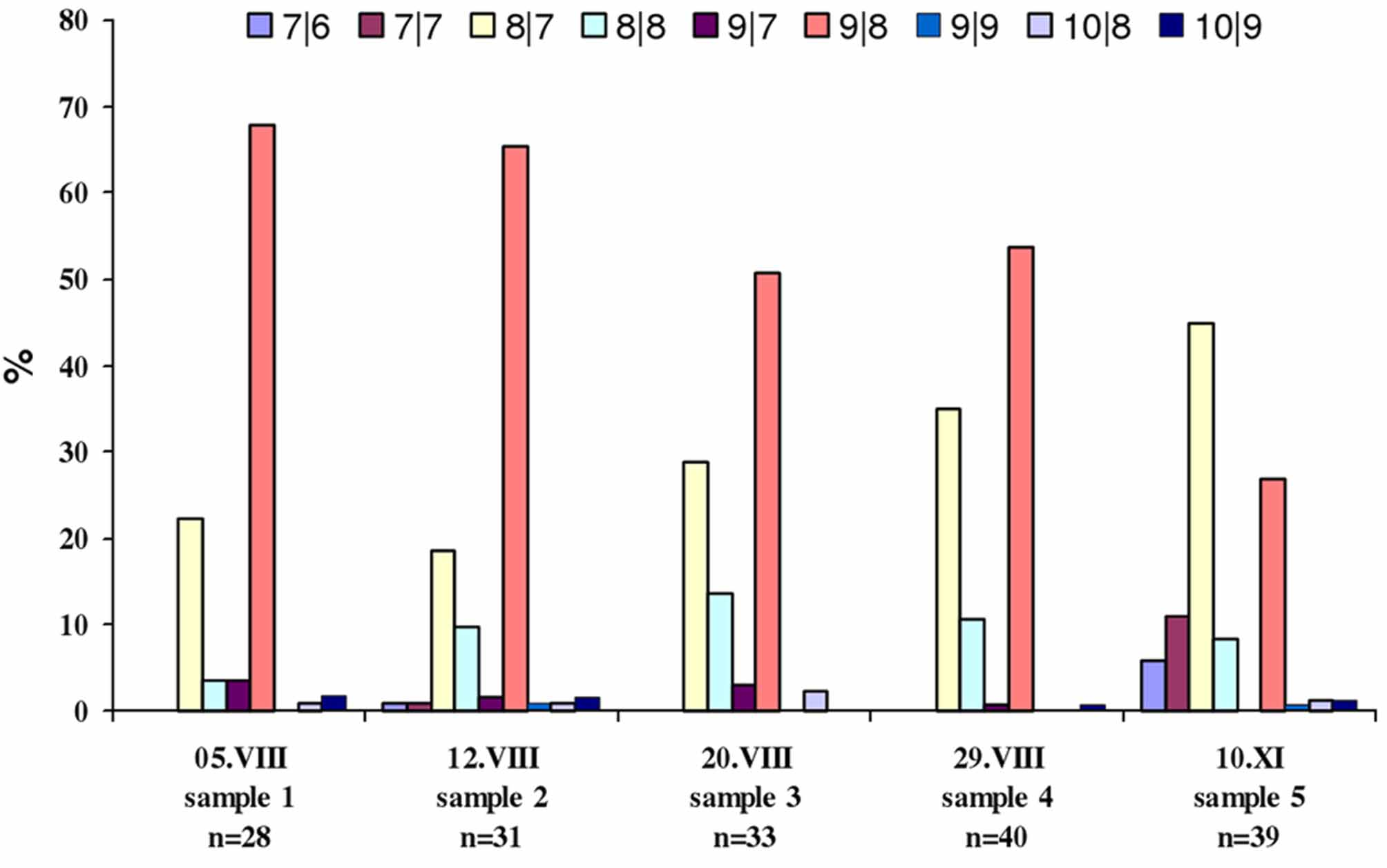
I studied the variability of the number of empodial rays in specimens of P. atherodes . The number of rays on the inner and outer sides of the empodia on legs I and II of 171 females from samples ## 1 to 5 ( Table 2 View TABLE 2 ), were counted. The frequency of different variants of the empodial number was tabulated and a graph was plotted ( Fig. 4 View FIGURE 4 ). In the course of analysis of the data, I concluded that:
1) within the same sample, mites were found with different numbers of empodial rays (7/6, 7/7, 8/7, 8/8, 9/7, 9/8, 9/9, 10 /8, 10/9);
2) one and the same mite may have different empodia which differ in number of rays, for example, [9/8 - 10 /9 - 10/8 - 9/8];
3) 9/8- and 8/7-rayed empodia are the most common form
4) the frequency of 9/8-rayed empodia decreases from the middle of summer until the end of autumn: 70% at the beginning of August, 50% at the end of August and 30% in the middle of November (all refer to the northern hemisphere);
5) the frequency of 8/7-rayed empodia increased from the middle of summer until the end of autumn: 22% at the beginning of August, 35% at the end of August and 45% in the middle of November;
6) the frequency of 9/7-, 9/9-, 10/8- and 10/9-rayed empodia is consistently low (1–3%); 7/6- and 7/7-rayed empodia are rare in summer (<1%), and their frequency increases in autumn (5% and 10% respectively); the frequency of 8/8-rayed empodia is about 10% during summer and autumn. In opposition to 9/8- and 8/7-rayed empodia, I consider 9/7-, 9/9-, 10/8-, 10/9-, 7/6- and 7/7-rayed empodia to be aberrant or transitive forms appearing in the second part of summer between distinct protogyne and deutogyne females. The appearance of transitive-morphological females during transformation from protogyne to deutogyne females within populations have also been revealed in other eriophyoid mites ( Eriophyinae and Pyllocoptinae) (R.Petanoviċ, personal communication July 2011).
So, distinct seasonal variability of the empodial ray number can be observed among females of P. atherodes . I consider this to be a result of deuterogeny. In the course of the morphological investigation of winter females, it was revealed that they differ from summer females in the length of the body, prodorsal shield, length of v, φ and с 1, number of dorsal annuli and number of empodial rays ( Table 3 View TABLE 3 ).
Deutogyne female form of Phytoptus atherodes (n=10). Body vermiform, yellowish, 242–269 long. Prodorsal shield 32–36 long; dorsal pedipalp genual setae d 9–11 long. Design of prodorsal shield similar to protogyne (summer) female. Tibial solenidion 6–8, empodia 12–13 long, 8/7-rayed ( Fig. 1 View FIGURE 1 C, I). Opisthosoma with 80–84 microtuberculate annuli, subdorsal seta c1 70–100 long.
Some notes on the biology of Phytoptus atherodes . These mites form large colonies inside the leaf sheaths of Carex atherodes (up to 100 females, males and nymphs per sheath). In the middle of summer, about 80% of females are protogynes and 20% deutogynes ( Fig. 5 View FIGURE 5 ). By the beginning of autumn, the quantity of males, nymphs and protogyne females decreases, whereas number of deutogyne females increases. Among overwintering mites, neither males nor nymphs were found. In sample #5 ( Table 2 View TABLE 2 ), 28 (72%) deutogyne and 11 (28%) protogyne females were counted.
Supplementary description of Phytoptus hirtae Roivainen 1950 . The only data concerning the morphology and variability within P.h i r t a e is found within the original description by Roivainen 1950, which was considered inadequate ( Table 1 View TABLE 1 ). Below, I present a supplementary description of a solitary protogyne female of P. hirtae from sample #9 ( Table 2 View TABLE 2 ). The measurements of both summer and winter females as well as males of this species ( Table 4 View TABLE 4 & 5) are also included and the morphological variability within P. hirtae and P. atherodes is also discussed.
* Characteristics of the samples are given in the Table 2 View TABLE 2 .
TABLE 3. Measurements of Phytoptus atherodes sp. n. (the most contrasting differences are marked in bold).
| CHARACTER Length of body | SUMMER FEMALE sample 1* (n=10) Min–max Mean ± SD 270–350 323 ± 26.5 | WINTER FEMALE sample 5* (n=10) Min–max Mean ± SD 242–269 255 ± 10.1 | MALE sample 1* (n=5) Min–max Mean ± SD 255–290 273 ± 14.0 |
|---|---|---|---|
| Width of body Length of prodorsal shield | 55–66 62 ± 3.9 35–41 39 ± 1.9 | 52–57 55 ± 2.3 32–36 35 ± 1.7 | 59–64 62 ± 2.5 36–39 38 ± 1.3 |
| Width of prodorsal shield | 34–46 41 ± 3.6 | 36–40 37 ± 1.7 | 37–42 39 ± 1.9 |
| Length of gnathosoma | 24–26 25 ± 0.7 | 22–26 24 ± 1.8 | 25–27 26 ± 0.8 |
| Length of v (s. apic.) | 14–17 16 ± 1.0 | 9–11 10 ± 0.8 | 13–16 14 ± 1.1 |
| Length of ve (s.d.1) | 15–20 18 ± 1.5 | 15–19 17 ± 1.6 | 12–16 14 ± 1.7 |
| Distance between ve | 21–25 23 ± 1.6 | 20–24 22 ± 1.6 | 23–27 24 ± 1.6 |
| Length of sc (s.d.2) | 1–3 2 ± 0.8 | 1–2 2 ± 0.5 | 2–3 2 ± 0.4 |
| Distance between sc | 20–24 21 ± 1.6 | 19–21 20 ± 0.8 | 21–24 23 ± 1.3 |
| Distance between ve and sc | 22–23 23 ± 0.7 | 18–22 19 ± 1.6 | 19–21 20 ± 0.8 |
| Length of leg I | 32–36 34 ± 1.5 | 31–33 32 ± 1.1 | 23–28 26 ± 1.9 |
| Length of em I | 13–15 14 ± 0.7 | 12–13 12 ± 0.5 | 10–12 11 ± 0.9 |
| Length of ω I | 11–15 12 ± 1.2 | 11–12 11 ± 0.4 | 11–13 12 ± 1.0 |
| Length of tibial solenidion φ | 8–10 9 ± 0.9 | 6–8 7 ± 0.8 | 6–9 7 ± 1.1 |
| Length of tibia I | 6–8 7 ± 0.8 | 6–7 6 ± 0.5 | 5–6 5 ± 0.4 |
| Length of l’ (s.tib.I) | 2–3 3 ± 0.5 | 2–3 3 ± 0.4 | 2–3 2 ± 0.5 |
| Length of tarsus I | 6–7 7 ± 0.4 | 6–6 6 ± 0.0 | 4–5 5 ± 0.4 |
| Length of leg II | 29–34 31 ± 1.5 | 28–30 29 ± 0.8 | 22–26 24 ± 1.5 |
| Length of em II | 12–17 15 ± 1.5 | 12–13 12 ± 0.5 | 11–12 11 ± 0.5 |
| Length of ω II | 12–15 13 ± 1.1 | 11–12 11 ± 0.4 | 11–13 12 ± 0.7 |
| Length of tibia II | 6–7 7 ± 0.5 | 5–6 6 ± 0.5 | 4–6 5 ± 0.7 |
| Length of tarsus II | 6–7 6 ± 0.3 | 5–6 6 ± 0.5 | 4–5 4 ± 0.3 |
| Length of 1b (s.cox.1) Distance between 1b | 18–24 22 ± 2.6 14–17 15 ± 1.1 | 15–19 17 ± 1.5 12–15 14 ± 1.1 | 12–21 17 ± 3.3 15–17 16 ± 0.8 |
| Length of 1a (s.cox.2) Distance between 1a | 15–40 26 ± 11.0 10–12 11 ± 0.8 | 22–28 26 ± 2.5 9–10 10 ± 0.4 | 22–30 26 ± 3.0 12–13 13 ± 0.4 |
| Length of 2a (s.cox.3) Distance between 2a | 45–63 53 ± 7.9 20–32 28 ± 4.8 | 40–51 44 ± 4.8 24–27 25 ± 1.1 | 38–46 42 ± 3.2 28–31 30 ± 1.1 |
| Length of epigynium /epiandrium | 11–15 12 ± 1.4 | 10–12 11 ± 0.8 | 10–13 12 ± 1.3 |
| Width of epigynium /epiandrium | 19–23 21 ± 1.4 | 18–20 19 ± 1.0 | 15–20 18 ± 1.8 |
| Length of 3a (s.gen.) Length of c1 (s.sd.) | 9–13 11 ± 1.3 100–125 113 ± 8.7 | 10–12 11 ± 0.7 70–100 88 ± 11.4 | 7–11 10 ± 1.5 28–40 33 ± 4.6 |
| Length of c2 (s.l.) | 45–66 56 ± 7.5 | 44–53 48 ± 3.5 | 28–35 31 ± 2.7 |
| Length of d (s.v.1) | 30–38 34 ± 2.7 | 24–28 26 ± 1.6 | 18–24 20 ± 2.3 continued next page |
TABLE 4. Measurements of Phytoptus hirtae Roivainen 1950 (the most contrasting differences are marked in bold).
| CHARACTER Length of body | SUMMER FEMALE sample 10* (n=10) Min–max Mean ± SD 332–413 379 ± 23.2 | WINTER FEMALE sample 10* (n=10) Min–max Mean ± SD 383–416 400 ± 15.1 | MALE sample 9* (n=5) Min–max Mean ± SD 281–356 324 ± 33.6 |
|---|---|---|---|
| Width of body Length of prodorsal shield | 60–73 69 ± 4.6 35–40 37 ± 1.4 | 63–74 69 ± 4.4 36–40 38 ± 1.5 | 59–67 64 ± 3.6 34–38 36 ± 1.7 |
| Length of gnathosoma | 22–27 25 ± 1.6 | 20–25 23 ±1.9 | 23–25 24 ± 0.8 |
| Length of ve (s.d.1) | 11–15 13 ± 1.3 | 15–19 17 ± 1.5 | 10–13 12 ± 1.3 |
| Distance between ve | 24–30 28 ± 1.5 | 24–28 25 ± 1.7 | 25–28 27 ± 1.3 |
| Length of sc (s.d.2) | 2–3 2 ± 0.4 | 3–3 3 ± 0.0 | 2–3 2 ± 0.5 |
| Distance between sc | 19–25 22 ± 1.4 | 23–25 24 ± 0.9 | 21–24 22 ± 1.3 |
| Distance between ve and sc | 20–25 22 ± 1.3 | 21–25 23 ± 1.6 | 20–21 20 ± 0.5 |
| Length of leg I | 32–38 35 ± 1.8 | 34–38 36 ± 1.6 | 31–32 32 ± 0.6 |
| Length of em I | 10–11 10 ± 0.5 | 11–13 12 ± 0.7 | 7–9 8 ± 0.8 |
| Number of rays of inner side of em I | 5–7 7 ± 0.6 | 7–7 7 ± 0.0 | 5–6 6 ± 0.5 |
| Number of rays of outer side of em I | 6–6 6 ± 0.0 | 6–7 6 ± 0.4 | 5–6 5 ± 0.5 |
| Length of ω I | 10–11 10 ± 0.5 | 10–12 11 ± 0.7 | 7–9 8 ± 1.0 |
| Length of tibial solenidion | 7–9 8 ± 0.7 | 7–10 9 ± 1.2 | 6–7 7 ± 0.5 |
| Length of tibia I | 7–8 7 ± 0.4 | 7–9 8 ± 0.8 | 5–6 6 ± 0.5 |
| Length of tarsus I | 6–8 7 ± 0.8 | 7–8 7 ± 0.5 | 5–6 6 ± 0.5 |
| Length of leg II | 31–34 32 ± 1.2 | 34–36 35 ± 1.0 | 25–27 26 ± 1.0 |
| Length of em II | 10–11 10 ± 0.5 | 11–13 11 ± 0.9 | 7–9 8 ± 0.8 |
| Number of rays of inner side of em II | 6–8 7 ± 0.6 | 7–7 7 ± 0.0 | 6–6 6 ± 0.0 |
| Number of rays of outer side of em II | 5–7 6 ± 0.6 | 5–6 6 ± 0.6 | 5–5 5 ± 0.0 |
| Length of ω II | 10–11 10 ± 0.5 | 10–12 11 ± 0.9 | 9–10 10 ± 0.5 |
| Length of tibia II | 6–8 7 ± 0.6 | 7–8 7 ± 0.5 | 5–6 6 ± 0.5 |
| Length of tarsus II | 6–7 6 ± 0.6 | 6–7 7 ± 0.5 | 5–6 6 ± 0.6 |
| Length of 1b (s.cox.1) Distance between 1b | 17–20 19 ± 1.1 17–23 18 ± 1.8 | 10–16 14 ± 2.3 16–20 18 ± 1.5 | 14–18 16 ± 1.8 16–18 17 ± 0.8 |
| Length of 1a (s.cox.2) Distance between 1a | 23–28 26 ± 2.2 12–15 14 ± 1.0 | 21–33 27 ± 4.3 12–15 14 ± 1.1 | 17–25 20 ± 3.6 13–15 14 ± 1.0 |
| Length of 2a (s.cox.3) Distance between 2a | 50–60 55 ± 3.0 31–36 33 ± 1.8 | 45–55 49 ± 4.6 34–35 34 ± 0.4 | 40–42 41 ± 1.0 29–34 32 ± 2.2 |
| Length of epigynium/epiandrium | 12–14 13 ± 0.6 | 14–16 15 ± 0.9 | 8–10 9 ± 0.8 |
| Width of epigynium/epiandrium | 20–23 21 ± 0.8 | 23–24 23 ± 0.4 | 20–23 22 ± 1.3 |
| Length of 3a (s.gen.) Length of c1 (s.sd.) | 10–17 12 ± 2.0 108–128 117 ± 7.2 | 12–14 13 ± 0.8 115–130 120 ± 5.8 | 7–9 8 ± 1.0 25–29 27 ± 2.1 |
| Length of c2 (s.l.) | 36–45 42 ± 2.7 | 45–54 49 ± 3.4 | 20–30 27 ± 4.7 |
| Length of d (s.v.1) Length of e (s.v.2) Length of f (s.v.3) | 23–26 25 ± 1.0 9–13 11 ± 1.6 45–54 50 ± 3.4 | 27–32 30 ± 2.2 11–16 13 ± 2.1 56–64 60 ± 2.9 | 12–19 16 ± 3.4 7–11 8 ± 1.9 31–40 35 ± 3.9 continued next page |
| ZIN |
Russian Academy of Sciences, Zoological Institute, Zoological Museum |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |