Dorotea papuana, Corbari & Frutos & Sorbe, 2019
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4568.1.4 |
|
publication LSID |
lsid:zoobank.org:pub:70574616-46B2-4A53-A2FF-715485DB8D74 |
|
DOI |
https://doi.org/10.5281/zenodo.5929653 |
|
persistent identifier |
https://treatment.plazi.org/id/1DA18C19-D7A4-460F-8906-B8AC5A3933A4 |
|
taxon LSID |
lsid:zoobank.org:act:1DA18C19-D7A4-460F-8906-B8AC5A3933A4 |
|
treatment provided by |
Plazi |
|
scientific name |
Dorotea papuana |
| status |
sp. nov. |
Dorotea papuana View in CoL sp. nov.
Figs 2–5 View FIGURE 2 View FIGURE 3 View FIGURE 4 View FIGURE 5
Type material. Holotype (MNHN-IU-2015-745), mature female, 14.8 mm BL; MADEEP cruise, RV Alis ; station DW4290 (off Budibudi Island , north of Laughlan Archipelago, Solomon Sea), 30/04/2014, 09°13'S 153°54'E, 593 m; detritic sediment (coral rubbles) and rocky slab (https://expeditions.mnhn.fr/campaign/madeep/event/ DW4290). GoogleMaps
Type locality: Off Budibudi Island , north of Laughlan Archipelago, Solomon Sea .
Etymology. The species name refers to the geographical area in which the specimen was collected.
Diagnosis. Same as for new genus.
Description. Body ( Fig. 2 View FIGURE 2 ): laterally compressed, dorsally smooth.
Head ( Fig. 3 View FIGURE 3 ): slightly smaller than pereonites 1+2. Rostrum short. Lateral lobes weakly produced. Anteroventral corner acute. Eyes absent.
Pleonites 1–3 ( Fig. 2 View FIGURE 2 ): posterodistal corner subquadrate in epimeron 1, rounded in epimeron 2, with tiny blunt cusp in epimeron 3. All epimera with convex posterior margin, epimera 2 and 3 with convex distal margin.
Antenna 1 ( Fig. 3 View FIGURE 3 ): larger than antenna 2. Peduncle shorter than main flagellum, articles without distal projections; article 1 sub-equal to article 2, with 2 short inferodistal stout setae; article 3 the smallest, 0.3x article 2 length. Main flagellum (probably broken) with 97 articles and many calceoli of type 5 [see Lincoln & Hurley (1981)], with cup-shaped proximal element well separated from the distal element; proximal article elongate. Accessory flagellum 1-articulated, shorter than flagellum article 1 and tapering distally, with one distal seta.
Antenna 2 ( Fig. 3 View FIGURE 3 ): peduncular article 3 with 2 short infero-distal stout setae; article 5 0.6x article 4 length. Flagellum (probably broken) with 68 articles and many calceoli (same type as for antenna 1), first proximal article elongate.
Upper lip ( Fig. 3 View FIGURE 3 ): entire, distally rounded, crescent-shaped submarginal row of setules. Epistome bluntly pointed.
Mandibles ( Fig. 3 View FIGURE 3 ): right mandible broken (not figured). Left incisor with long incurved cutting edge, poorly dentate (2+), ending in a strong blunt tooth. Right incisor with at least a strong blunt tooth. Left lacinia mobilis 5- toothed (all apexes blunt), ending in a strong tooth, the other ones much smaller. Left and right molars medium and triturative, grinding surface ringed by medium contiguous blades, higher than wide. Left and right setal rows with 6 setae. Left and right palp article 3 longer than article 2, slightly falciform, tapering distally; article 2 thicker than article 3.
Maxilla 1 ( Fig. 3 View FIGURE 3 ): inner plate with 3 subapical setae; outer plate with 10 apical stout setae (8 cuspidate and 2 simple); palp with distal article the longest, 2 rows of simple setae on distal half inner margin, 3 simple setae on distal half outer margin.
Maxilla 2 ( Fig. 3 View FIGURE 3 ): inner plate broader and shorter than outer plate, submarginal row of simple setae on distal half inner margin, bunch of setules on proximal inner margin; outer plate with apical simple setae.
Lower lip ( Fig. 3 View FIGURE 3 ): broad, inner lobes weak; mandibular lobe bluntly pointed.
Maxilliped ( Fig. 3 View FIGURE 3 ): heavily setose; palp, articles ordinary; inner plate with 3 short, blunt and stout setae and 2– 3 simple setae on apical margin; outer plate large reaching less than half length of palp article 2, with 4 stout setae on mesial basal part, inner margin of article 4 with 4 stout setae.
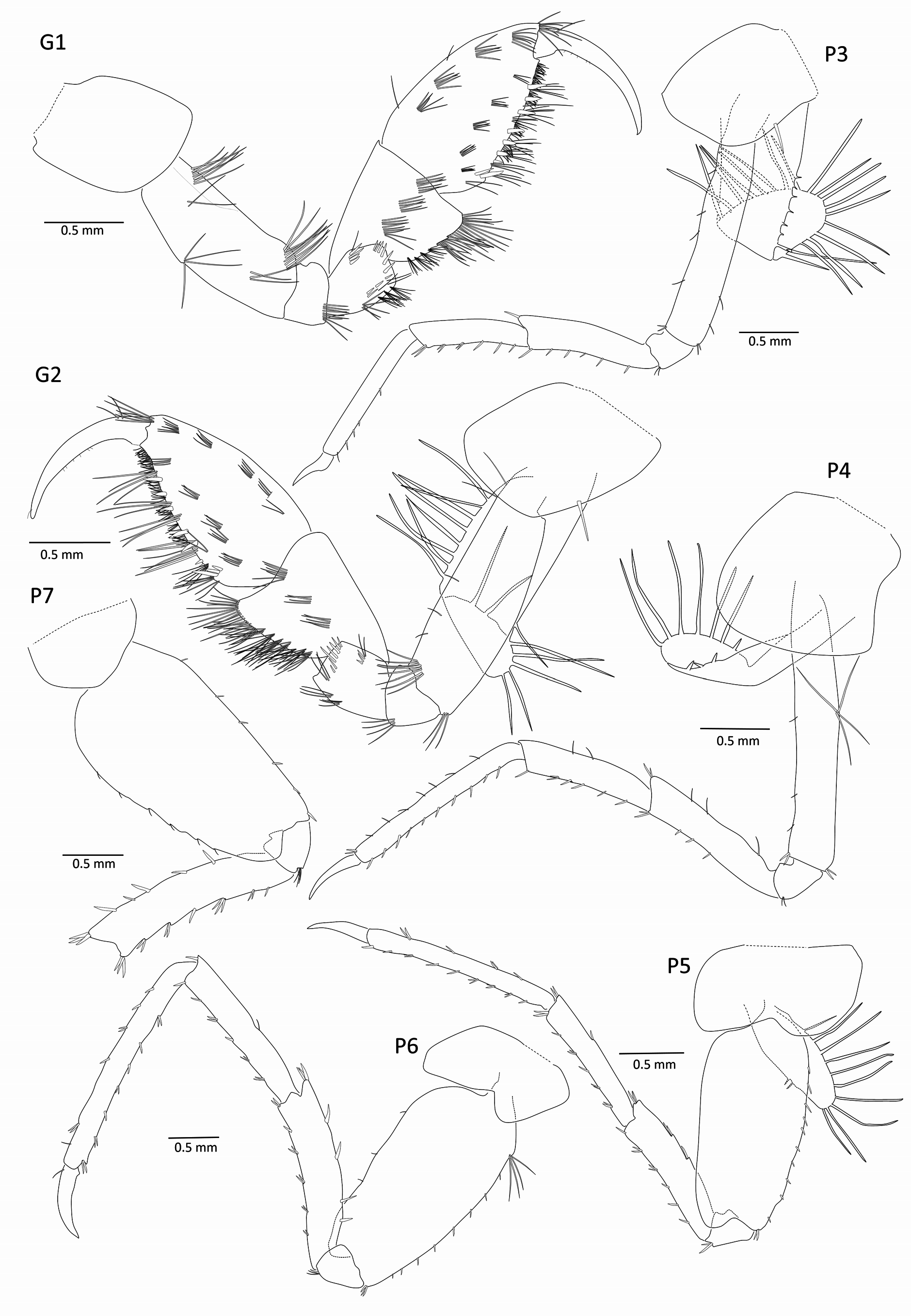
Coxae ( Figs 2 View FIGURE 2 , 4 View FIGURE 4 ): coxae 1–4 medium, about as deep as broad, broadly rounded, not produced; coxae 2–3 bearing one long stout seta on posterior margin; coxa 4 slightly excavate posteriorly; coxae 5–6 bilobate; coxa 7 the smallest, unilobate.
Gnathopods 1–2 ( Fig. 4 View FIGURE 4 ): similar and subequal, both strongly subchelate; merus strongly spinose; carpal lobes broad; palm strongly oblique, with 6 – 7 stout setae, proximally limited by 2 (G1) or 3 (G2) stout setae for dactylus insertion; dactylus curved, with one (G1) or 2 (G2) long subproximal setae on anterior margin, posterior margin sparsely setulated.
Pereopods 3–7 ( Fig. 4 View FIGURE 4 ): elongate, simple; dactylus simple, not styliform and slightly curved, 0.3x of propodus length. Pereopods 3 – 4 ordinary, merus slightly longer than carpus. Pereopods 5 – 7 homopodous, basis with posterodistal lobe. Pereopod 7, posterior margin of basis slightly denticulate.
Uropod 1 ( Fig. 5 View FIGURE 5 ): peduncle longer than rami, with a row of 6 short stout setae on left and right margins and a blunt distoventral process bearing a distal stout seta; apex of both rami broken; inner ramus longer than outer, bearing 2 proximal stout setae on outer margin and 2 ones on inner margin; outer ramus more spinose on both margins than inner one.
Uropod 2 ( Fig. 5 View FIGURE 5 ): peduncle with stout setae on distal outer and inner margins; inner ramus longer than outer; inner and outer margins of both rami with stout setae, except at apex.
Uropod 3 ( Fig. 5 View FIGURE 5 ): peduncle with stout setae on distal outer and inner margins; inner ramus longer than outer one; inner and outer margins of both rami with stout setae, except at apex.
Telson ( Fig. 5 View FIGURE 5 ): elongated (1.8x as long as broad), cleft 21.2%, lobes apically divergent with blunt apices, without distal armament.
Brood plates ( Fig. 4 View FIGURE 4 ): on coxae 2 – 5; coxa 3 oostegite shorter than basis, 3.7x as long as broad; coxa 4 oostegite slightly shorter than basis, 5.2x as long as broad; coxa 5 oostegite shorter than basis, 2.8x as long as broad; margins with long setae.
Gills: on pereopods 2 – 7, simple and subquadrate.
Molecular identification. A mtCOI sequence (a 657 base pair fragment) was obtained for the type of D. papuana gen. nov., sp. nov. examined in the present study. These sequences are available in GenBank under the following accession number: MK 260193 View Materials . Following the definition given by Pleijel et al. 2008, the holotype of this new species (MNHN-IU-2015-745) is designed as the hologenophore of this sequence.
Remarks. Within the family Eusiridae , our specimen shares strong morphological similarities with the genus Cleonardo . Dorotea papuana gen. nov., sp. nov. can be distinguished from the other known eusirid genera by the presence of a telson distally cleft and distinctly bilobate, of a distal spiniform process on uropods 1 and simple stout and medium length dactylus on pereopods 5–7. Eusirioid families were often defined by morphological characters presenting continuous variation so that their limits were highly debatable ( Verheye et al. 2016). The shape of the telson could be considered as one of the most important state of characters defining the families within Eusiroidea ( Bousfield & Hendrycks 1995). The cleft state could have a phylogenetic significance at some lower taxonomic levels, but as it is highly homoplasious it should be used with caution to characterize taxonomic groupings ( Verheye et al. 2016). Considered as important functional traits in Eusiridae , carnivory and predation in deep-water Eusiridae are characterized by the presence of slender, long-dactylate pereopods allowing to stand on soft bottoms, awaiting prey ( Bousfield & Hendrycks 1995). Such a character is not observed in our specimen whose pereopod dactylii are rather short by comparison with Cleonardo species, suggesting that its habitat is rather different from that eusirid species.
Bellan-Santini & Ledoyer (1987) described the new sub-Antarctic amphipod Eusiroides aberrantis from a male specimen (BL = 12 mm) collected near Marion and Prince Edward Islands. This bathyal species (180–527 m according to De Broyer et al., 2007) was initially assigned to family Eusiridae sensu lato and to genus Eusiroides , due to a mix of morphological characters between Eusiroides and Cleonardo (its species name suggesting such an ambiguity). Later on, this genus has been transferred to Pontogeneiidae ( Lowry & Myers, 2013) . Based the original description, it is clear that E. aberrantis is quite different from all other known Eusiroides species (eyes absent, pereopods 3–7 relatively slender, telson 19.2% cleft with dehiscent lobes) as underlined by the authors and must therefore be excluded from Pontogeneiidae due to the absence of apical stout setae on uropod 1 rami (uropod 2 unknown). Furthermore, it shows very close morphological affinities with the new genus Dorotea (head and antennae, buccal appendages, ornamentation of coxae 1–2, shape of coxa 4, shape of pereopod 5–7 basis, posterodistal corner of epimeron 3, shape of telson cleft), suggesting its transfer to this genus. However, some distinctive morphological characters by comparison with D. papuana (Table 1) show that it is a different species. In conclusion to this comparative morphological analysis, we consider that E. aberrantis should be transferred to genus Dorotea (Eusiridae) and we propose the following new nomenclatural combination: Dorotea aberrantis ( Bellan-Santini & Ledoyer, 1987) .
| RV |
Collection of Leptospira Strains |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
ParvOrder |
Eusiridira |
|
Family |
|
|
Genus |