Eudocima afrikana, Borth & Kons, 2022
|
publication ID |
https://doi.org/ 10.11646/zootaxa.5148.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:20BC627E-56A1-4674-A6B2-96F9B8DB15F7 |
|
DOI |
https://doi.org/10.5281/zenodo.7635985 |
|
persistent identifier |
https://treatment.plazi.org/id/ED434E3C-FFEE-FFDB-FF71-FF45FBF1FD07 |
|
treatment provided by |
Plazi |
|
scientific name |
Eudocima afrikana |
| status |
sp. nov. |
Eudocima afrikana sp. n.
Type material. The type series is restricted to specimens with DNA sequences and/or genitalic dissections.
Holotype male ( Figure 15 View FIGURE 15 :G): Togo, Forest of Missahoe , Kpalime-Kloto, 1–25.ix.2018, (DNA Sample ID No. 24834-150918-TO, Dissection No. HLK: 2633), leg. Chmielowiec ( YPM).
Paratypes: TANZANIA: 1 male ( Figure 27 View FIGURE 27 :H) Tanga, Nguu mtns., Kilindi village, Tamota Forest , 10.ii.2007, (DNA No. 9346-100207- TA, Dissection No. HLK: 2501), ( RJB) .
TOGO: 1 female ( Figure 27 View FIGURE 27 :K) Forest of Missahoe , Kpalime-Kloto, 1–25.ix.2018, (DNA No. 24816-130918- TO, Dissection No. HLK: 2645), leg. Chmielowiec ( RJB) .
MADAGASCAR: Fianarantsoa, Ranomafana, N -21.26 ˚ E47.42˚, 850m: 1 male ( Figs 1 View FIGURE 1 :C, 15:I): 15.xi.2018, (DNA No. 24833-151118-MA, Dissection No. HLK: 2632), leg. Golovizin ( RJB) . 1 female ( Figures 1 View FIGURE 1 :B, 15:J), 15.xi.2018, (DNA No. 24831-131118-MA), leg. Golovizin, ( RJB) ; 1 male ( Figure 27 View FIGURE 27 :F), 2.iii.2019, (DNA No. 24832-171118-MA, Dissection No. HLK: 2503), leg. Golovizin, ( RJB) ; 1 male ( Fig. 27 View FIGURE 27 :G, 21:I), 2.iii.2019, (DNA No. 24889-090319-MA), leg. Golovizin, ( RJB) ;
UGANDA: 1 male ( Fig. 27 View FIGURE 27 :E), Karbole Fort Portal , Lake Nkuruba Nature Reserve, N0.518 ˚ E30.302 ˚, 1519m (DNA No. 25027-211119- UG), leg. Golovizin, ( RJB) GoogleMaps .
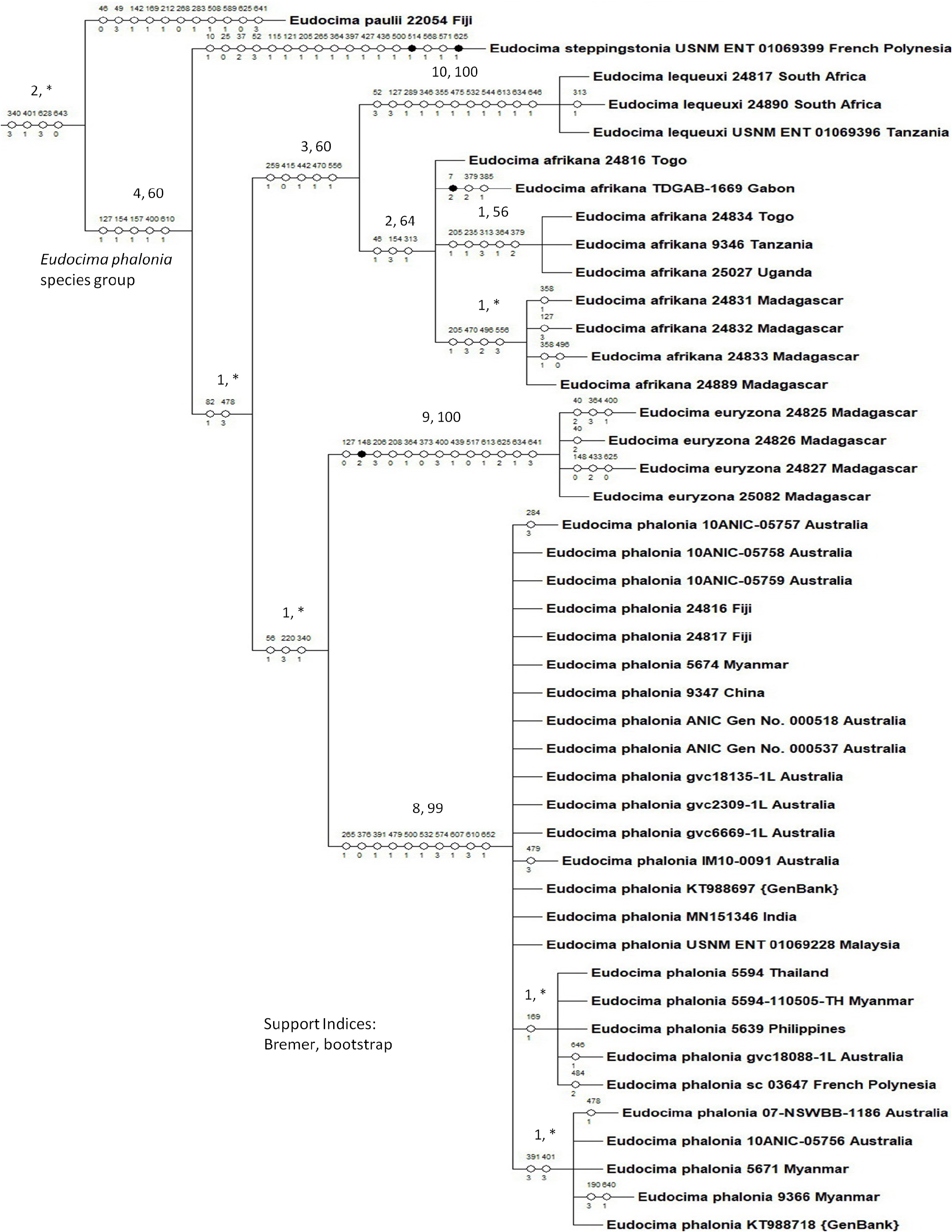
Diagnosis. The Sub-Saharan African distribution separates E. afrikana from its more eastern relatives including E. phalonia (found from India eastward to Hawaii), E. oliveri (found over 11,000 km to the east in the Vanuatu Archipeligo), and E. steppingstonia (found in the Marquesas Islands another 4,600 km further east). However, E. afrikana could be confused with the sympatric E. lequeuxi and E. euryzona . At least five species in the Eudocima phalonia group are diagnosable both by unique combinations of male genitalic and COI 5’ mitochondrial DNA characters ( E. oliveri has not been sequenced), and all six species are diagnosable by discrete differences in male genitalia. Male genitalic and abdominal structures are compared for E. afrikana , E. phalonia , E. euryzona , and E. lequeuxi in Figures 33–58 View FIGURE 33 View FIGURE 34 View FIGURE 35 View FIGURE 36 View FIGURE 37 View FIGURE 38 View FIGURE 39 View FIGURE 40 View FIGURE 41 View FIGURE 42 View FIGURE 43 View FIGURE 44 View FIGURE 45 View FIGURE 46 View FIGURE 47 View FIGURE 48 View FIGURE 49 View FIGURE 50 View FIGURE 51 View FIGURE 52 View FIGURE 53 View FIGURE 54 View FIGURE 55 View FIGURE 56 View FIGURE 57 View FIGURE 58 , and female genitalic and abdominal characters are compared for E. afrikana and E. phalonia in Figures 59–69 View FIGURE 59 View FIGURE 60 View FIGURE 61 View FIGURE 62 View FIGURE 63 View FIGURE 64 View FIGURE 65 View FIGURE 66 View FIGURE 67 View FIGURE 68 View FIGURE 69 . The male genitalia of E. afrikana is most similar to E. lequeuxi whereas E. phalonia is most similar to E. euryzona .
Versus Eudocima phalonia . Wings: Eudocima afrikana cannot be reliably separated from E. phalonia by wing pattern or external characteristics.
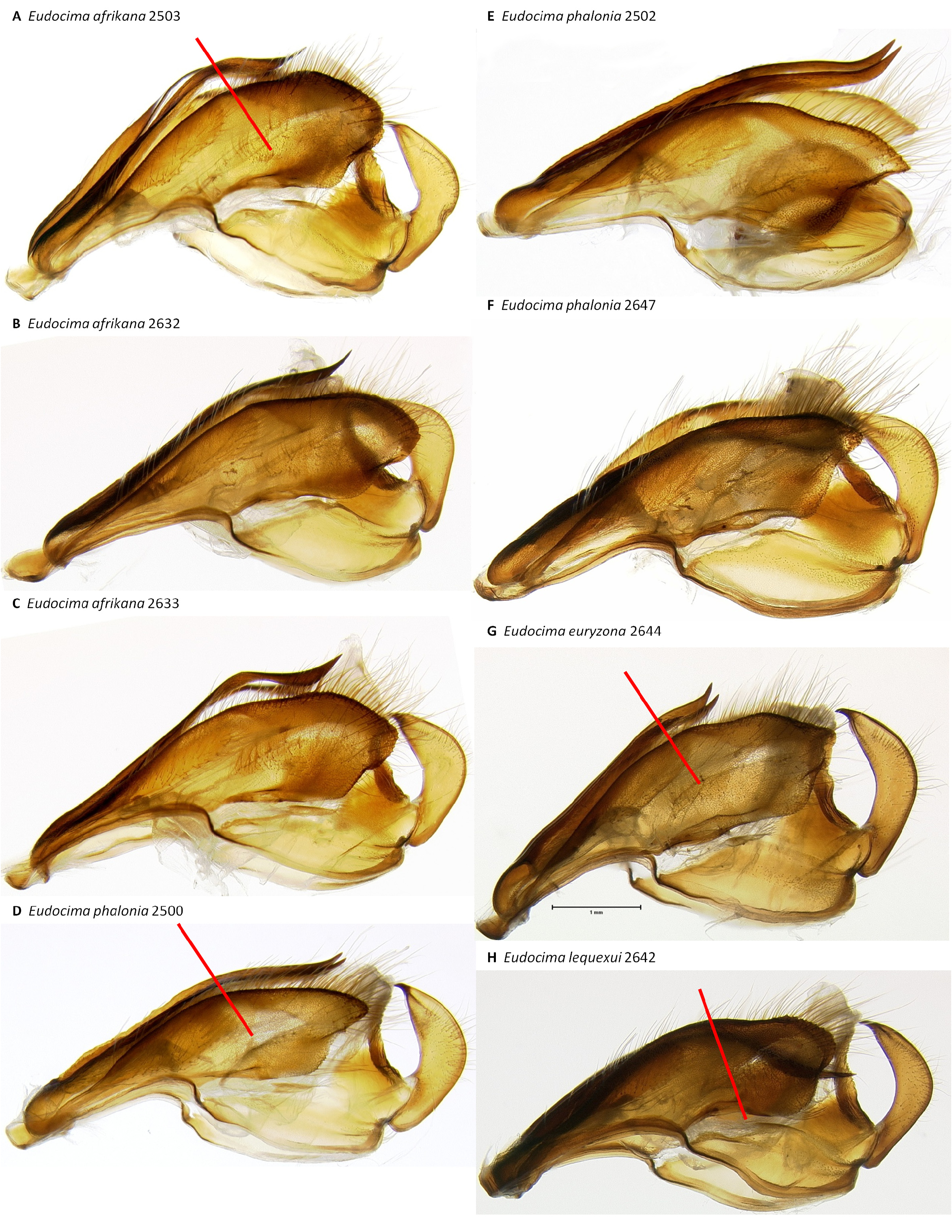
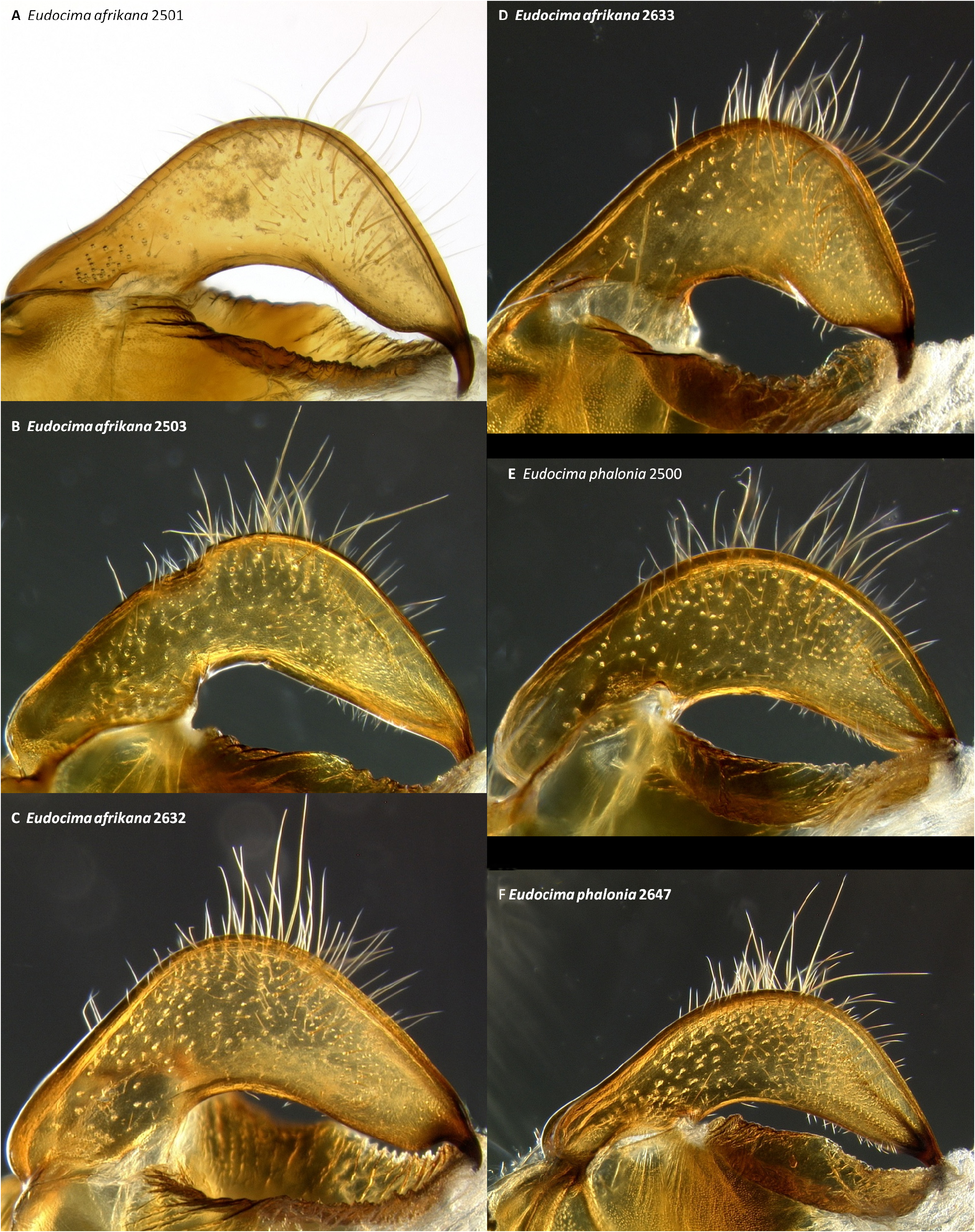
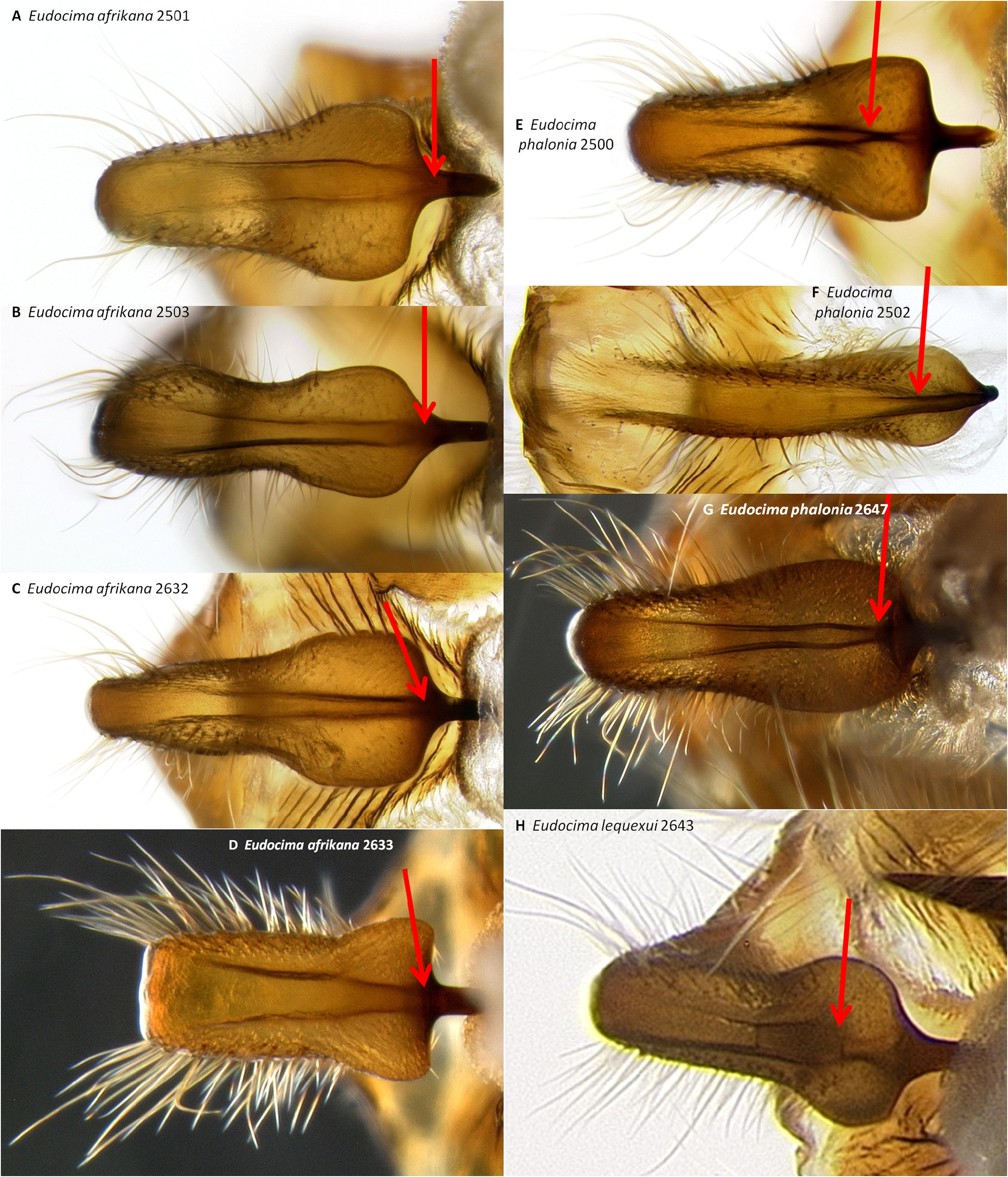
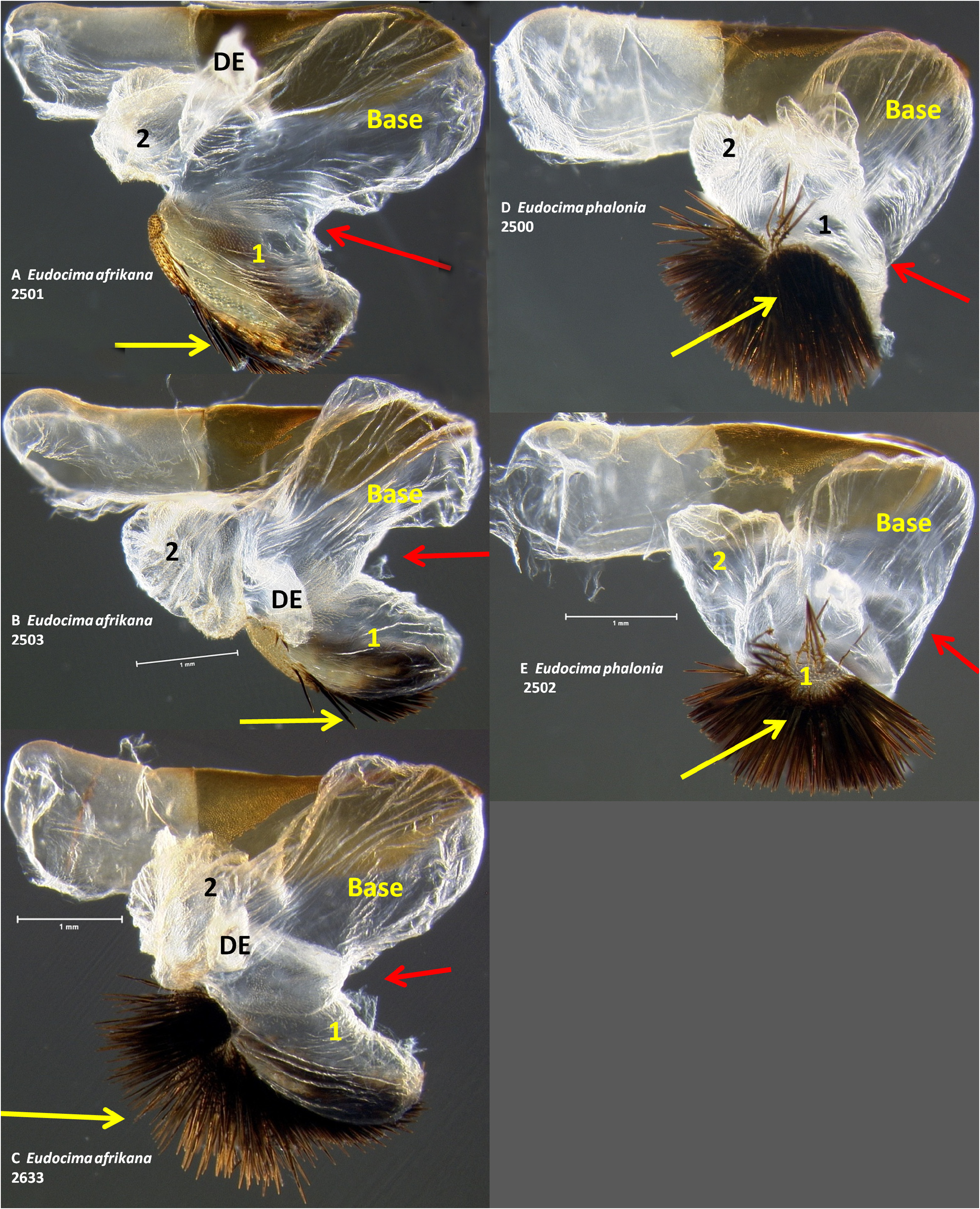
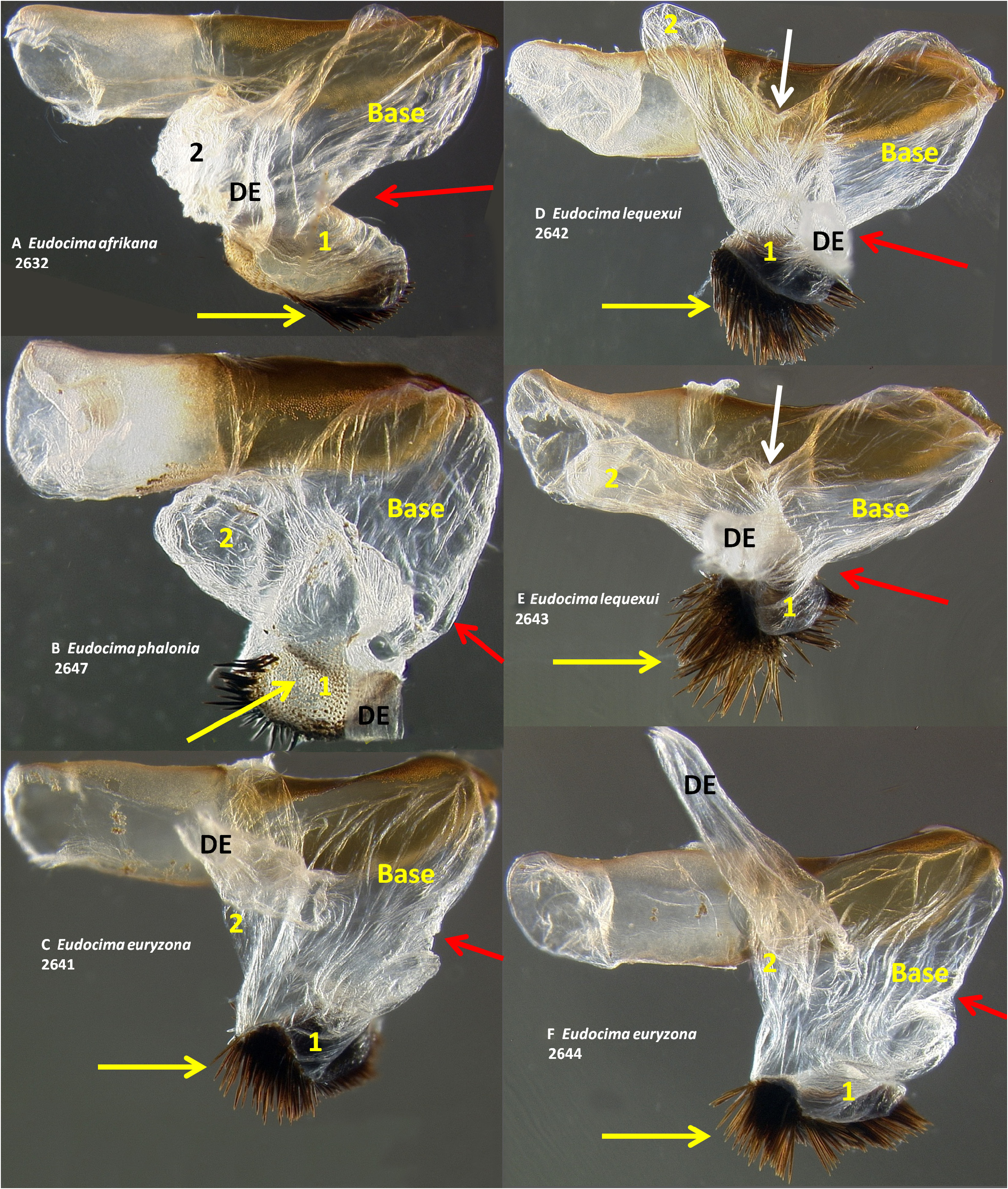
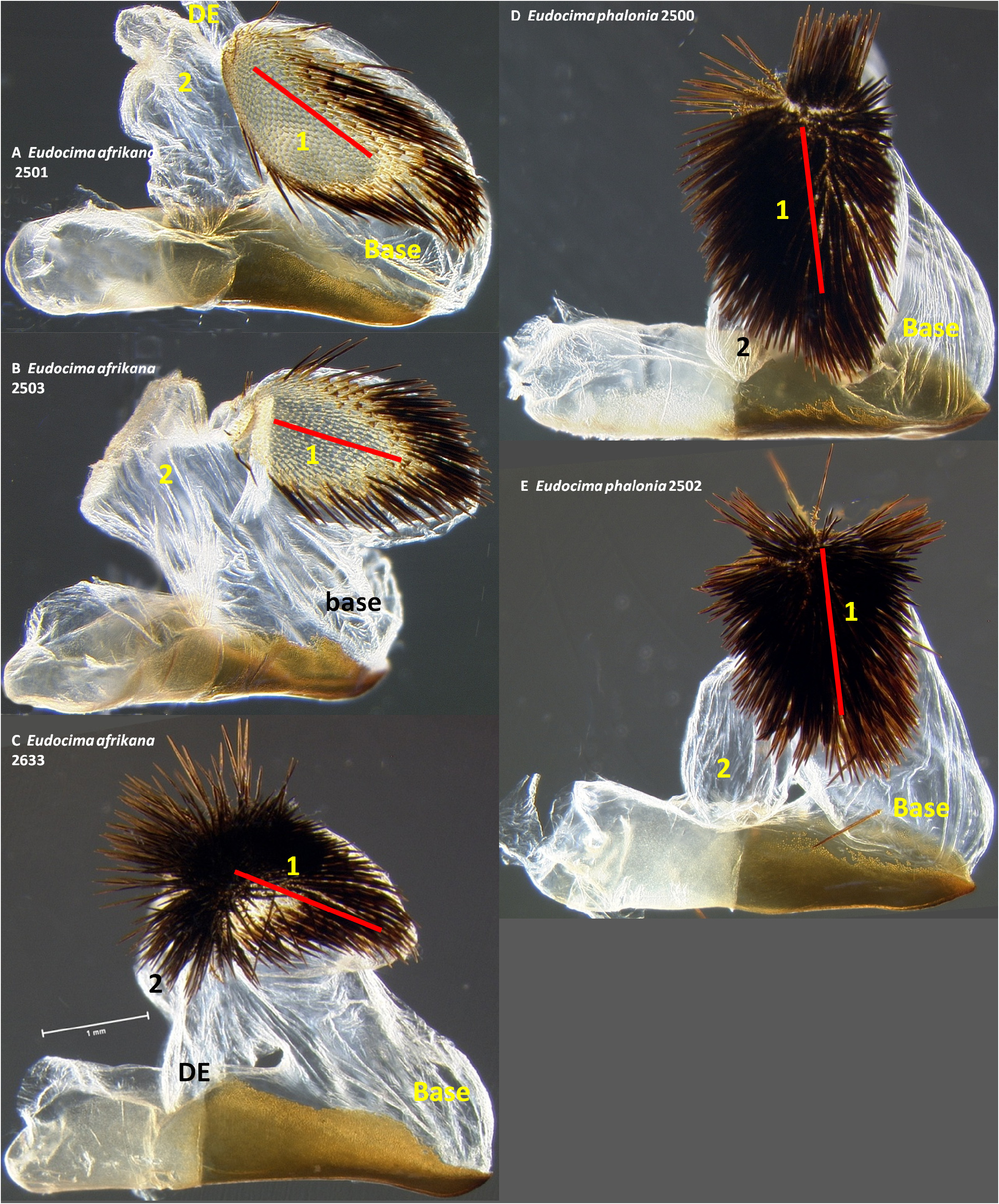
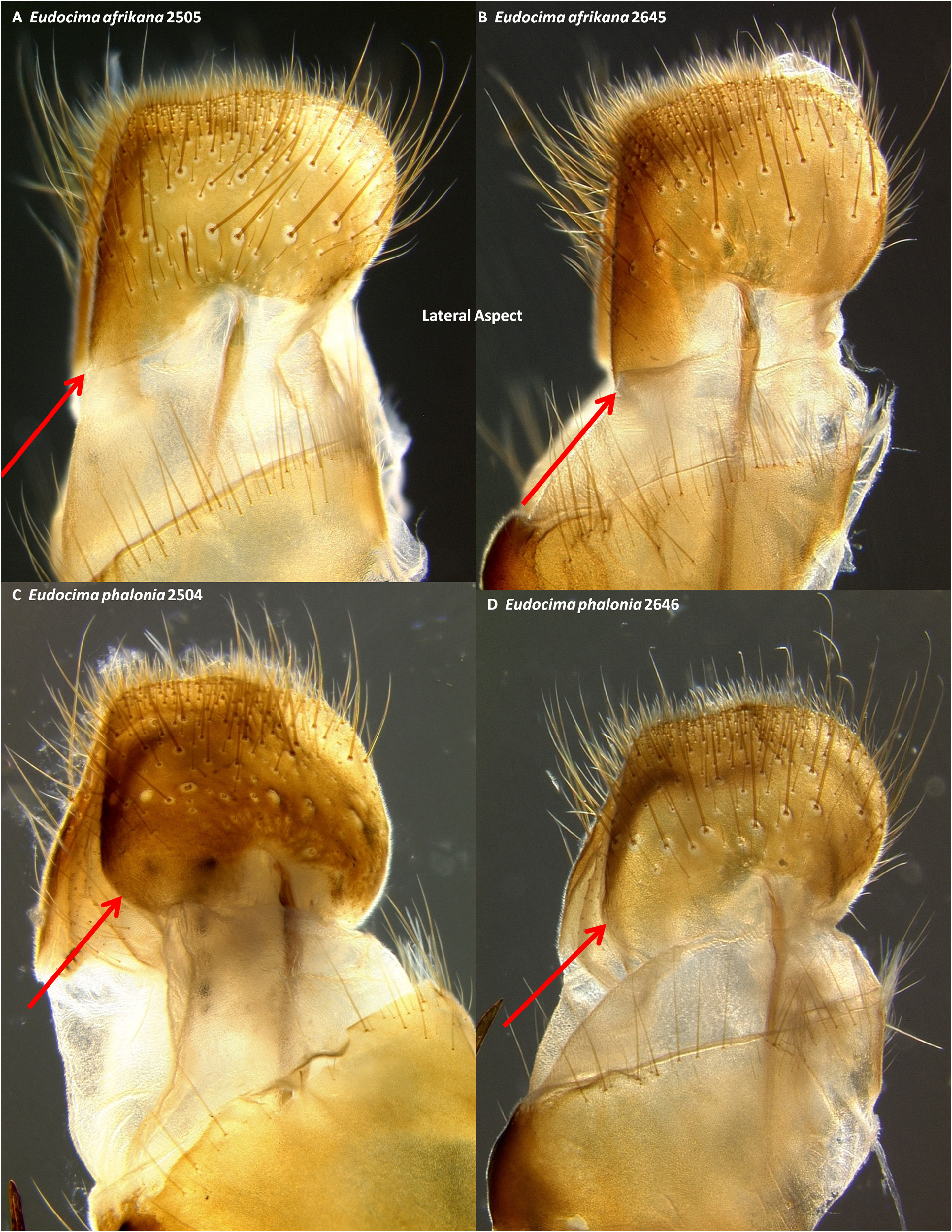
Male genitalia. We found multiple consistent differences in the male genitalia. The posterior terminal process of the valvae are relatively broader in E. afrikana ( Figs 39 View FIGURE 39 : A–D) than in E. phalonia ( Figs 39 View FIGURE 39 : E–G, black arrows). The distance from the anterior median of the juxta to the point where the posterior processes diverge is 0.47–0.58 mm in E. phalonia versus 0.8–1.02 mm in E. afrikana ( Fig. 38 View FIGURE 38 ). The paired posterior processes of the juxta are wider in E. phalonia ; the widest point measured 0.68 to 0.70 mm in E. phalonia versus 0.44–0.52 in E. afrikana ( Fig. 38 View FIGURE 38 ). The point where the posterior juxta processes diverge is broader and U-shaped in E. phalonia ( Figs 38 View FIGURE 38 : E–G) versus Vshaped with a sharp point in E. afrikana ( Figs 38 View FIGURE 38 : A–D). In dorsal or ventral aspects there are a pair of conspicuous dark pigmented bands near the inner margin of the anterior portion of the juxta processes in E. afrikana ( Figs 34 View FIGURE 34 : A–C, red arrows) but not in E. phalonia ( Figs 34 View FIGURE 34 : D–F). In lateral aspect the paired juxta processes bend posteriorly farther distally in E. afrikana ( Figs 36 View FIGURE 36 : A–C) than in E. phalonia ( Figs 36 View FIGURE 36 : D–F) (red lines in these figures cross the inflection point). In ventral aspect, with the natural three dimensional structure intact, the apices of the paired juxta processes cross in E. phalonia ( Figs 34 View FIGURE 34 : D–F, 37: B), whereas they are widely separated in E. afrikana ( Figs 34 View FIGURE 34 : A–C, 37: A). In ventral aspect the paired dark pigmented bands on the uncus converge distinctly proximal of the apical spine in E. phalonia ( Figs 44 View FIGURE 44 : E–G), whereas in E. afrikana these bands remain separated where they meet the spine ( Figs 44 View FIGURE 44 : A–D). The apex of the scoop-shaped region of the ductus ejaculatorius has a large terminal fold that completely covers the tip in E. phalonia ( Figs 46 View FIGURE 46 : E–G), whereas E. afrikana has only a small fold that does not overlap with the tip ( Figs 46 View FIGURE 46 : A–D). With the ventral phallus hood orientated behind the vesica opening and tilted to the left ( Figs 48–49 View FIGURE 48 View FIGURE 49 ), there is a deep U–shape on the posterior side of the vesica between the base and diverticulum 1 in E. afrikana ( Figs 48 View FIGURE 48 : A–C, 49: A, red arrows), whereas this U–shape is not visible in the same orientation in E. phalonia ( Figs 48 View FIGURE 48 : D–E, 49: B, red arrows). With the ventral phallus hood orientated behind the vesica opening ( Figs 48–49 View FIGURE 48 View FIGURE 49 ), the sclerotized plate of cornuti on diverticulum 1 is partially on top in E. phalonia ( Figs 48 View FIGURE 48 : D–E, 49: B, yellow arrows), whereas it is entirely underneath in E. afrikana ( Figures 48 View FIGURE 48 : A–C, 49: A, yellow arrows). With the ventral phallus hood orientated above ( Figs 50–51 View FIGURE 50 View FIGURE 51 ), vesica diverticulum 1 projects to the right in E. phalonia ( Figs 50 View FIGURE 50 : D–E, 51: B), whereas it projects to the left in E. afrikana ( Figs 50 View FIGURE 50 : A–C, 51: A). In this same orientation, the sclerotized curved cornuti plate on diverticulum 1 is entirely underneath in E. phalonia , whereas it occupies lateral and ventral planes in E. afrikana (same figures as preceding character, red arrows). With the ventral phallus hood orientated lateral and down ( Figs 52–53 View FIGURE 52 View FIGURE 53 ) the plate with deciduous spines is nearly perpendicular to the phallus in E. phalonia ( Figs 52 View FIGURE 52 : D–E, 53: B, red lines), whereas it is roughly parallel to the phallus in E. afrikana ( Figs 52 View FIGURE 52 : A–C, 53: A, red lines).
Female genitalia. In comparing female genitalic structures between two specimens each of E. afrikana and E. phalonia , only one consistent difference was found. The ventral anterior edge of the ovipositor lobe is convex and smoothly curved in E. phalonia ( Figs 61 View FIGURE 61 : C–D, red arrows), whereas it is more triangular and protracted anteriorly in E. afrikana ( Figs 61 View FIGURE 61 : A–B, red arrows).
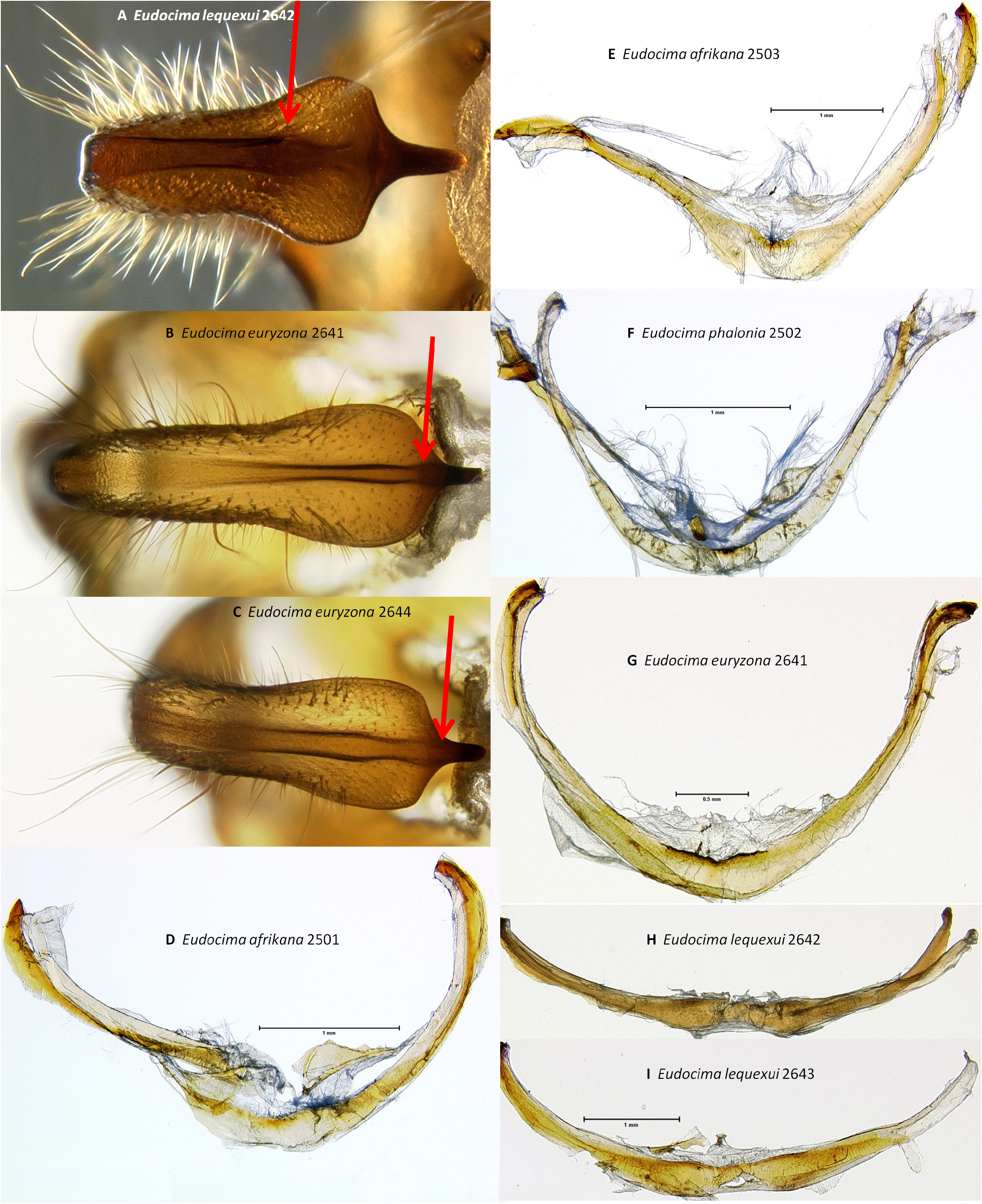
Versus Eudocima lequeuxi . Wings: Brou & Zilli (2016) compared E. lequeuxi and E. afrikana (called E. phalonia ) and noted differences in wing shape, genitalia, and the abdominal coremata. Some of the following characters were previously noted in Brou & Zilli (2016), whereas others are newly reported. In E. lequeuxi there is a concave indentation in the forewing outer margin between veins M3 and R5 ( Fig. 15 View FIGURE 15 : E–F, Brou & Zilli (2016)), whereas this area is fairly straight in E. afrikana ( Figs 1 View FIGURE 1 : A, 15: G). The female forewing has less contrast between lighter and darker areas in E. afrikana ( Figs 1 View FIGURE 1 : B, 15: H, J) than in E. lequeuxi ( Fig. 15 View FIGURE 15 : F). The distance between the dorsal hindwing black marginal band and medial patch is wider in E. lequeuxi ( Figs 15 View FIGURE 15 : E–F, Brou & Zilli (2016)) than E. afrikana ( Figs 15 View FIGURE 15 : G–J). The basal side of the dorsal hindwing medial patch is convex in E. afrikana whereas there is a concave indentation in E. lequeuxi (same figures as preceding character, Brou & Zilli (2016)). The ventral hindwing black marginal band of E. lequeuxi is more tapered posterior of vein Cu2, whereas the posterior apex of this band is abruptly squared off in E. afrikana (same figures as two preceding characters).
Male genitalia. Eudocima lequeuxi is the only one of these four species with a serrate inner margin of the posterior processes of the juxta ( Figs 38 View FIGURE 38 : J–L, Brou & Zilli (2016, Figs 9–12 View FIGURE 9 View FIGURE 10 View FIGURE 11 View FIGURE 12 )). When the saccus and arms of the vinculum are flattened out on a slide, the arms of the vinculum are distinctly less strongly curved posteriorly in E. lequeuxi ( Figs 45 View FIGURE 45 : H–I) than in the other three species ( Figs 45 View FIGURE 45 : D–G). The paired dark bands on the ventral side of the uncus are strongly concave on the outer sides subapically in E. lequeuxi ( Figs 44 View FIGURE 44 : H, 45: A, distal to the red arrows) whereas they are straighter in the other three species ( Figs 44 View FIGURE 44 : A–G, 45: B–C). The apex of the scoop-shaped region of the ductus ejaculatorius is strongly folded over ( Fig. 46 View FIGURE 46 : I), similar to E. phalonia and E. euryzona ( Figs 46 View FIGURE 46 : E–H), but unlike the smaller fold of E. afrikana ( Figs 46 View FIGURE 46 : A–D, red arrows). With the phallus hood orientated lateral and down ( Figs 52–53 View FIGURE 52 View FIGURE 53 ) diverticulum 2 appears narrower and more elongate in E. lequeuxi ( Figs 53 View FIGURE 53 : D–E) than in E. afrikana ( Figs 52 View FIGURE 52 : A–C, 53: A). The same is true with the phallus hood orientated lateral and up ( Figs 54–55 View FIGURE 54 View FIGURE 55 ).
Male abdomen. The coremata on sternite 8 is more elongate in E. lequeuxi ( Figs 57 View FIGURE 57 : C–E, Brou & Zilli (2016, Fig. 19 View FIGURE 19 )) than in the other three species ( Figs 56 View FIGURE 56 , 57 View FIGURE 57 : A–B).
Versus Eudocima euryzona . Wings: The forewing of E. euryzona has a more pronounced falcate apical tip ( Figs 16 View FIGURE 16 : A–B). The abdomen of E. euryzona is dorsally covered by orange hairs and scales throughout ( Figs 16 View FIGURE 16 : A–B), whereas the anterior half of the abdomen in E. afrikana has extensive brown hairs and scales along the dorsal midline ( Figs 15 View FIGURE 15 : H–J). The space between the medial patch and marginal hindwing band is narrower in E. afrikana ( Figs 15 View FIGURE 15 : G–J) than in E. euryzona ( Figs 16 View FIGURE 16 : A–B). Females of both species are variable but the background color of the forewings of E. euryzona is generally lighter ( Fig. 16 View FIGURE 16 : B). The ventral hindwing black marginal band of E. euryzona tapers to a triangular point posterior of vein Cu2 ( Fig. 21 View FIGURE 21 : J), whereas the posterior apex of this band is abruptly squared off in E. afrikana ( Fig. 21 View FIGURE 21 : I). The ventral hindwing apical area of E. euryzona is a pale cream color ( Fig. 21 View FIGURE 21 : J) versus the orange-yellow coloration of E. afrikana ( Fig. 21 View FIGURE 21 : I).
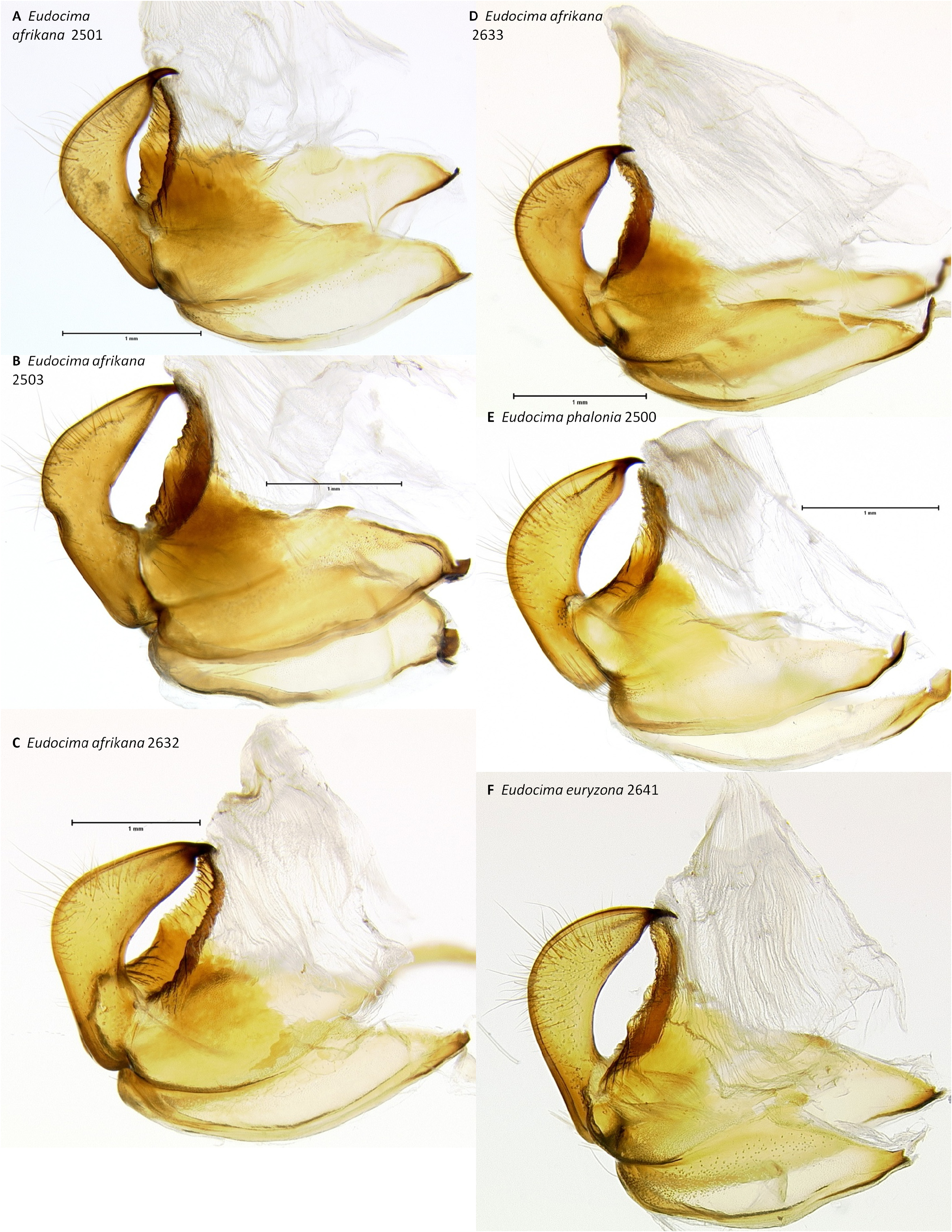
Male genitalia. In ventral aspect, with the natural three dimensional structure intact, the apices of the paired juxta processes cross in E. euryzona ( Figs 34 View FIGURE 34 : G–H) whereas they are widely separated in E. afrikana ( Figs 34 View FIGURE 34 : A–C, 37: A). In dorsal or ventral aspects there are a pair of conspicuous dark pigmented bands near the inner margin of the anterior portion of the juxta processes in E. afrikana ( Figs 34 View FIGURE 34 : A–C, red arrows) but not in E. euryzona ( Figs 34 View FIGURE 34 : G–H). With the ventral phallus hood in lateral aspect, the top of the hood is more strongly convex in E. euryzona ( Figs 47 View FIGURE 47 : I (bottom image), 53: C, F, 55: C, F) than in E. afrikana (Figs: 47: C–D (bottom images), 52: A–C, 53: A, 54: A–C, 55: A). With the ventral phallus hood orientated behind the vesica opening and tilted to the left ( Figs 48–49 View FIGURE 48 View FIGURE 49 ), there is a deep U–shape on the posterior side of the vesica between the base and diverticulum 1 in E. afrikana ( Figs 48 View FIGURE 48 : A–C, 49: A, red arrows), whereas this U-shape is much shallower in the same orientation in E. euryzona ( Figs 49 View FIGURE 49 : C, F, red arrows). With the ventral phallus hood orientated behind the vesica opening ( Figs 48–49 View FIGURE 48 View FIGURE 49 ), the sclerotized plate of cornuti on diverticulum 1 is partially on top in E. euryzona ( Figs 49 View FIGURE 49 : C, F yellow arrows), whereas it is entirely underneath in E. afrikana ( Figs 48 View FIGURE 48 : A–C, 49: A yellow arrows).
Versus Eudocima oliveri . This species is known from only two males and one female from the Vanuatu Archipelago ( Zilli et al. 2017), and no specimens were examined by us; however, a diagnosis is provided by Zilli et al. (2017). E. oliveri has shorter, broader forewings with a noticeably darker and more evenly convex PM line, and a narrower medial patch on the hindwing ( Figs 16 View FIGURE 16 : G–H). Zilli et al. (2017) also noted E. oliveri has an outwardly projecting apex of the valvae. This character is unique among all species in the E. phalonia group.
Versus Eudocima steppingstonia . This species is known from only one male and two females from the Marquesas Islands ( Zilli et al. 2017), and no dissections were examined by us. Eudocima steppingstonia has paler, yellowish hindwings with an indistinct outer margin and medial patch ( Figs 15 View FIGURE 15 : C–D). Zilli et al. (2017) show this species has an elongated narrow uncus, which differs from the much wider uncus of E. afrikana , E. phalonia , E. euryzona , and E. lequeuxi (Figs: 42: D–F, 43).
COI 5’: This species is diagnosable by the following unique combination of COI 5’ character states: 46(C), 154(T), 313(C or T). There are 13 consistent character state differences between E. afrikana and E. lequeuxi , 21 between E. afrikana and E. phalonia , and 24 between E. afrikana and E. euryzona ( Fig. 86 View FIGURE 86 ).
Description. Head (Male). Vertex and frons with predominately pale purple hairs and scales, except ventral margin of frons with light tan hairs and scales. Labial palp basal segment predominately brown but mottled with lighter tan scales and hairs, narrow bands of iridescent pale purple scales along apical margin and dorso-lateral sides; middle segment with predominately dark purple scales and hairs on dorso-lateral side, predominately bluish grey scales and hairs on ventro-lateral side except proximally where dark brown scales dominate; terminal segment with tan and dark brown scales for roughly proximal 2/3 of length, distal 1/3 appearing wider with a lateral patch of blue rimmed with black, extreme apex light tan. Terminal segment appearing much narrower than proximal two segments ( Fig. 32 View FIGURE 32 : K).
Proboscis ( Figs 71 View FIGURE 71 : A, 72: A, H). Approximately basal 2/3 simple ( Fig. 71 View FIGURE 71 : A). Subapical portion with lightly sclerotized rasping spines. Apex with glossy dark, smooth sclerotization, contrasting with the ribbing of the more lightly sclerotized remainder of the proboscis ( Figs 72 View FIGURE 72 : A, H). Unsclerotized ovoid areas present around heavily sclerotized tearing hooks ( Figs 72 View FIGURE 72 : A, H). Extreme apex with heavily sclerotized hooks lacking the unsclerotized ovoid areas ( Fig. 72 View FIGURE 72 : H). Dorsal galeal ligulae extend for most of length ( Fig. 71 View FIGURE 71 : A), except for extreme apex ( Fig. 72 View FIGURE 72 : H).
Thorax (Male) ( Fig. 1 View FIGURE 1 : A). Patagia mottled with purple, brown, and lighter tan scales. Tegulae pattern similar to patagia except at apex and along distal outer margin, where pattern sharply changes to mottled blue, black, and sparser white scales. Elsewhere dorsally a mix of brown, lighter tan, and purple scales and hairs. Ventrally with dense brown and tan hairs and scales.
Wings ( Figs 1 View FIGURE 1 , 15 View FIGURE 15 : G–J, 21: I, 27: E–L). Length of anterior forewing base to apex: 37–45 mm, mean= 42 mm males (n=8); 40–46 mm, mean= 43.4 mm females (n=7); ratio of (anterior forewing base to apex)/ (anterior forewing base to tornal angle): 1.8–2.1, mean=1.9 (males); 1.8–2.1, mean=1.9 (females). Anal flap prominent, sharply pointed with convex sides. Tornal hook prominent, outer side convex, inner side concave, apex bluntly pointed to narrowly rounded. Shape of wings similar in both genders, but forewing fringe scalloped in female.
Forewing upperside ( Figs 1 View FIGURE 1 : A–B, 15: G–J, 27: E–L). Sexually dimorphic.
Males. Background color predominantly brown with most pattern elements diffused, some individuals with distinctly lighter brown distal to the postmedial line. Veins accented by black scaling, often broken, creating the appearance of dashed-lines along the veins, especially distal to the postmedial line. Basal dash and basal line absent. Antemedial line diffuse, fairly straight and slanted proximally anterior to posterior, bent slightly basally at costa, terminating basal of anal flap along inner margin. Paired terminal lines present but often faint, barely discernible in some individuals, slightly paler than background color, distal terminal line extending from costa to postmedial line, basal terminal line extending from costa to posterior margin of discal cell. Medial line diffuse, convex, slightly darker brown than background color when discernible. Postmedian line diffuse, convex, curving strongly basally posterior of vein M3 and straightening between vein Cu1 and inner margin, darker brown than background color, tending to be more conspicuous than aforementioned lines, thinner and sharper than medial line. When discernible, reniform spot a simple band of lighter tan scaling spanning the width of the distal edge of the discal cell. Small, inconspicuous darker brown dot in discal cell basal to reniform. When discernible, subterminal line broad and diffuse with indistinct margins, darker brown than background color, convex, primarily spanning between veins Cu2 and M1. Apical line conspicuous, contrasting light tan to pale greenish tan, extends basally from subapical area at costa to vein M1 as a slightly convex arc, then bent distally between veins M1 and M2, sometimes weakly chevronshaped between veins M1 and M2. Small white apical patch sometimes present distal of apical line and anterior of vein R4, with diffuse white scaling between veins R4 and R5. Margin with a thin band slightly darker brown than background color, fringe concolorous with margin. Forewing covered with a glossy sheen.
Females. Background color variable, mottled with grey, brown, olive-grey, and violet-grey. Pattern elements generally better defined than in males. Veins accented by black scaling, often broken, creating the appearance of dashed-lines along the veins, especially distal to the postmedial line. Basal dash and basal line absent. Antemedial line sharp, edged with white on distal side, fairly straight, bent slightly basally at costa, terminating basal of anal flap along inner margin. Paired terminal lines absent. Medial line a variable, wide, diagonal black band posterior of veinlet between veins Cu2 and Cu1, broken between this veinlet and vein M3, a much thinner, more diffused arc distal to the reniform between veins M3 and the costa. Postmedian line similar to male except contrasting more strongly against background color and variably edged with white on the distal side posterior of vein Cu1. Postmedian line transversed by a chevron-shaped whitish mark between veins Cu1 and M3. Reniform spot strongly contrasting against background, partially edged with black and white and filled with dark brown to blackish scales, shape an inverted chevron, sometimes surrounding area with extensive whitish diffusion. Small, sharply contrasting black dot in discal cell basal to reniform. When discernible, subterminal line narrow and diffuse with indistinct margins, lighter tan than background color, bulging distally between veins Cu1 and M1. Margin with extensive whitish suffusion, especially posterior to vein Cu1. Fringe distinctly scalloped, dark brown-grey anterior of veinlet between veins Cu1 and Cu2, lighter tan to whitish posterior of this veinlet.
Hindwing upperside ( Figs 1 View FIGURE 1 : A–B, 15: G–J, 27: E–L). Not sexually dimorphic except in males the medial patch averages larger. Background color orange, variable amount of brown suffusion in basal area. Medial patch large and black, basal side simple and convex, distal side convex on each end and strongly concave in the middle. Marginal black band wide, widest anterior of vein M1, abruptly narrows across vein M1, then gradually narrows until abruptly squared off slightly posterior of vein Cu2, in some individuals extending slightly farther along wing margin with a small disjunct tornal spot sometimes present at vein 2A. Fringe chequered black and creamish-white, black patches at vein apices from veins Cu2 to Rs, creamish-white patches between the veins from Cu2 to Sc+R1, orange at anal angle proximal of vein Cu2 except for a small black patch contiguous with the tornal spot (when present).
Forewing underside ( Figs 1 View FIGURE 1 : C, 21: I). Background color yellow-orange. Marginal band thick and black, basal margin sharp, distal margin less distinct. Apical area between marginal band and outer margin with lighter grayishblack, diffused with pale orange scaling. Postmedial band yellow-orange and completely surrounded with black, extends from vein parallel to costa to cell between veins Cu2 and 2A. Medial band sharp and black, fused with marginal band posterior of vein Cu2. Anterior of discal cell, antemedial band darker orange than postmedial band; posterior of discal cell largely concolorous with postmedial band. Basal band comprised of two small black patches separated by orange along vein Cu2. Basal area dull orange with a small black patch parallel to the costa. Area between costa and parallel vein mottled with black and yellow-orange. Fringe sexually dimorphic, solid dark brownish black in male, chequered with black patches at ends of veins and pale tan between in female.
Hindwing underside ( Figs 1 View FIGURE 1 : C, 21: I). Background color varies from orange basally to yellow-orange distally. Medial patch like upperside. Marginal band similar to upperside posterior of vein M1, but partially replaced by pale cream suffused with black between veins M1 and R2 (on the distal side), and nearly completely replaced with pale cream suffused with black anterior of vein Rs. Tornal spot (when present) like upperside. Fringe similar to upperside.
Legs (Male) ( Fig. 70 View FIGURE 70 ).
Legs covered with tan scales and hairs, male metatibia with more elongate ventral hairs than female.
Foreleg ( Figs 70 View FIGURE 70 : A–C): Protibia ( Fig. 70 View FIGURE 70 : C) and profemur ( Figs 70 View FIGURE 70 : A–B) unspined. Protibia with small convex sulcus with radiating spines near basal extremity on the inner side ( Fig. 70 View FIGURE 70 : C). Protibial flange in shallow ovoid pit, margins of flange smooth ( Fig. 70 View FIGURE 70 : C). Foreleg sclerotized throughout except for inner side of protibia distal of the flange ( Fig. 70 View FIGURE 70 : A–C).
Midleg ( Figs 70 View FIGURE 70 : A–B, D). Mesotibia and femur unspined with no hair pencil groove on mesotibia ( Figs 70 View FIGURE 70 : A–B). Midleg sclerotized throughout except for a narrow translucent area at apex of mesotibia ( Figs 70 View FIGURE 70 : A–B).
Hindleg ( Figs 70 View FIGURE 70 : A–B, E–F). Metafemur and metatibia unspined ( Figs 70 View FIGURE 70 : A–B). Sclerotization pattern as observed for Catocala and other Erebinae, with femur sclerotized throughout, metatibia translucent white except for proximal edge ( Figs 70 View FIGURE 70 : A–B), metatarsomere 1 translucent white except at distal apex ( Fig. 70 View FIGURE 70 : E), remaining tarsomeres sclerotized throughout ( Figs 70 View FIGURE 70 : E–F).
Tarsi ( Figs 70 View FIGURE 70 : D–F). Spination similar on all legs. Tarsomeres 1–4 with three ventral rows of large triangular spines, with one to three extra spines between the middle and right outer rows of spines at tarsomere apices (ventral aspect with claw on left) ( Fig. 70 View FIGURE 70 : E). Tarsomere 5 with the same two outer rows of triangular spines but spines reduced in size, dense smaller spines between them not arranged in rows ( Fig. 70 View FIGURE 70 : F). Minute translucent hair-like spines present on lateral sides of tarsomeres ( Fig. 70 View FIGURE 70 : F) and along dorsal midline. Tarsomere 5 with two pairs of elongate, narrow, tubular spines dorsally at apex ( Figs 70 View FIGURE 70 : D, F). Tarsal claws strongly bifid ( Fig. 70 View FIGURE 70 : D, F), arolium translucent on edges, with two dark lateral bands and a somewhat opaque whitish center, distal margin strongly emarginate ( Fig. 70 View FIGURE 70 : F).
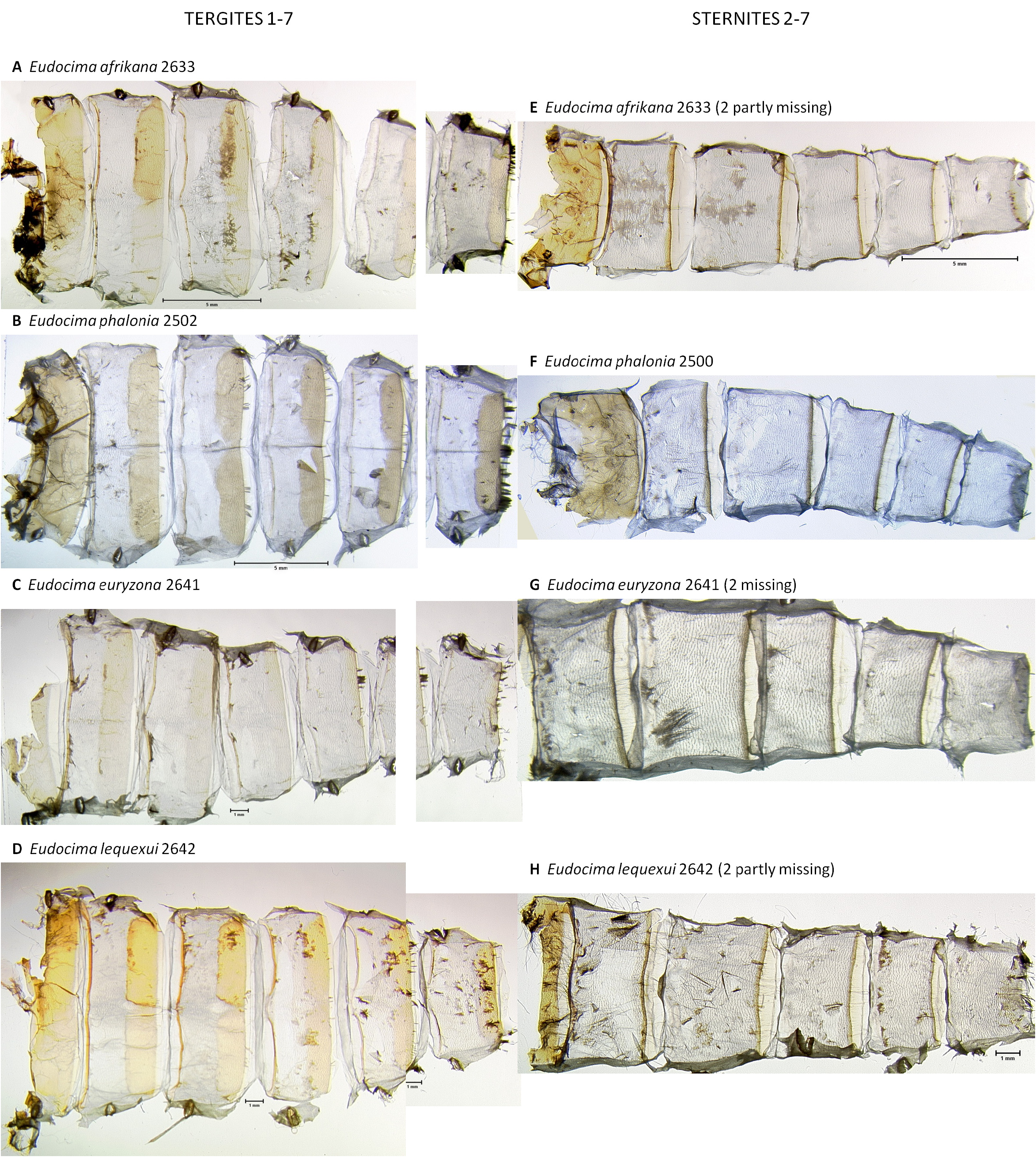
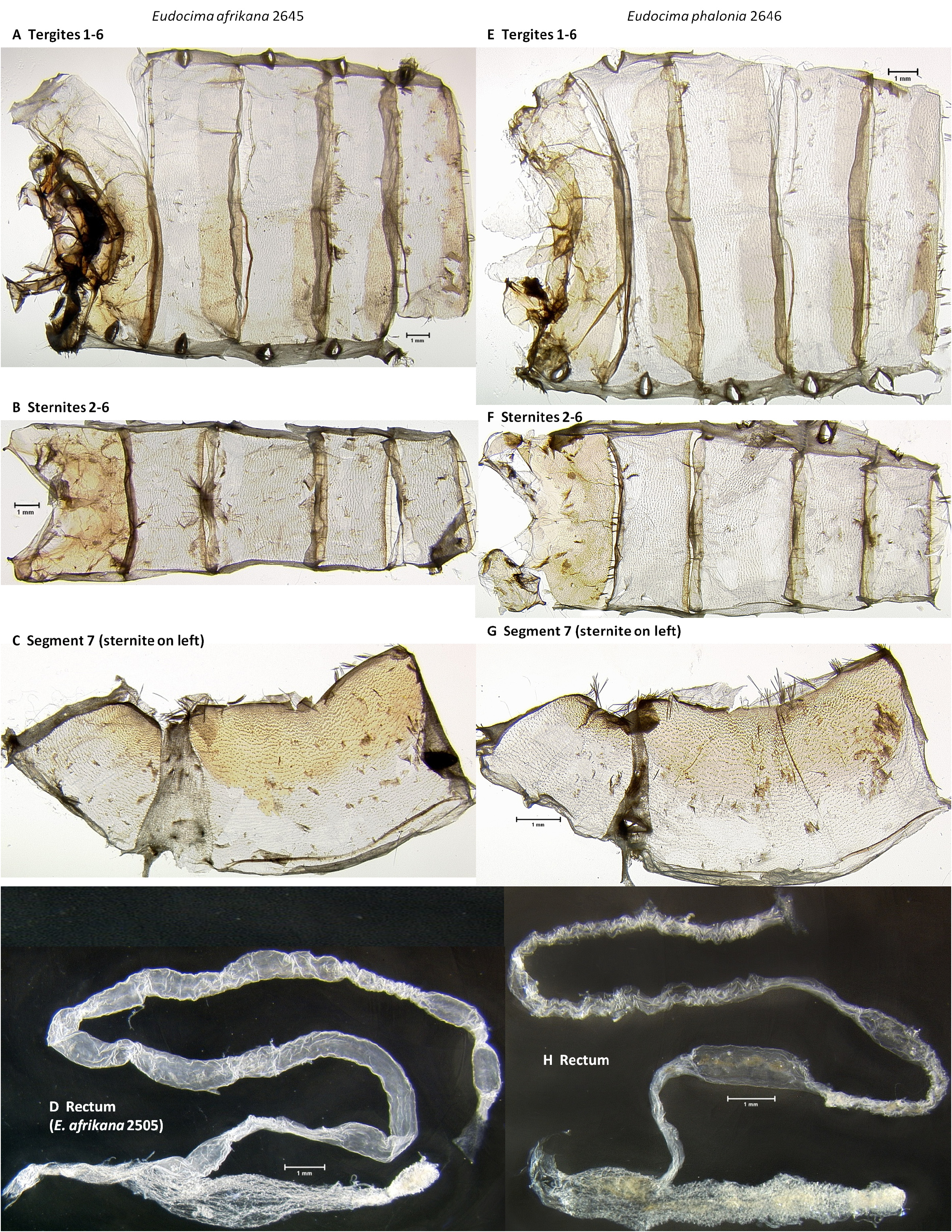
Abdominal Scale Pattern ( Fig. 1 View FIGURE 1 ). Dorsally predominately orange scales and hairs except for anterior two segments and apex where brownish-tan hairs dominate. Male with apical brown hairs protruding from the genital capsule. Brownish-tan hairs decrease anterior to posterior except at apex. Ventrally with brownish-tan hairs throughout.
Abdominal cuticle ( Figs 56 View FIGURE 56 : A–D, G, 58: A, E, 69: A–C). Male as shown in Figures 56 View FIGURE 56 & 57 View FIGURE 57 , segment 8 highly modified, corema on sternite 8 comprised of deep pocket densely filled with hair pencils, tergite 8 sclerotization deltoid with deeply concave sides ( Figs 56 View FIGURE 56 : A–D, G). Female as shown in Fig. 69 View FIGURE 69 : A–C.
Male genitalia ( Figs 33–55 View FIGURE 33 View FIGURE 34 View FIGURE 35 View FIGURE 36 View FIGURE 37 View FIGURE 38 View FIGURE 39 View FIGURE 40 View FIGURE 41 View FIGURE 42 View FIGURE 43 View FIGURE 44 View FIGURE 45 View FIGURE 46 View FIGURE 47 View FIGURE 48 View FIGURE 49 View FIGURE 50 View FIGURE 51 View FIGURE 52 View FIGURE 53 View FIGURE 54 View FIGURE 55 ).
Capsule ( Figs 33 View FIGURE 33 : A–B, I–J, 34: A–C, 35: A–C, 36: A–C). Juxta and vinculum weakly fused with valvae, vinculum weakly fused with tegumen, vinculum arms expanded midventrally to a contiguous saccus without separation at the midpoint ( Figs 34 View FIGURE 34 , 35 View FIGURE 35 , 45 View FIGURE 45 : D–E). Diaphragma membranous except for juxta ( Fig. 34 View FIGURE 34 ).
Valvae ( Figs 33 View FIGURE 33 : A–B, I–J, 39: A–D). Outer surfaces densely covered with elongate brown and tan hairs and scales except for anterior and dorsal portions of sacculus ( Figs 33 View FIGURE 33 : A–B, I–J); we could not get these hairs and scales to reflect iridescent green, although we observed this in two preparations of E. phalonia ( Fig. 33 View FIGURE 33 : C). Dorsal edge of sacculus strongly concave ( Figs 39 View FIGURE 39 : A–D). Posterior margin with broad convex process extending around to both dorsal and ventral sides; dorsal margin with blunt triangular subapical process. Sclerotized throughout except for a translucent subapical band arching over the dorsal triangular process. Ventral fold with fang-shaped medial gap. No claspers ( Figs 39 View FIGURE 39 : A–D).
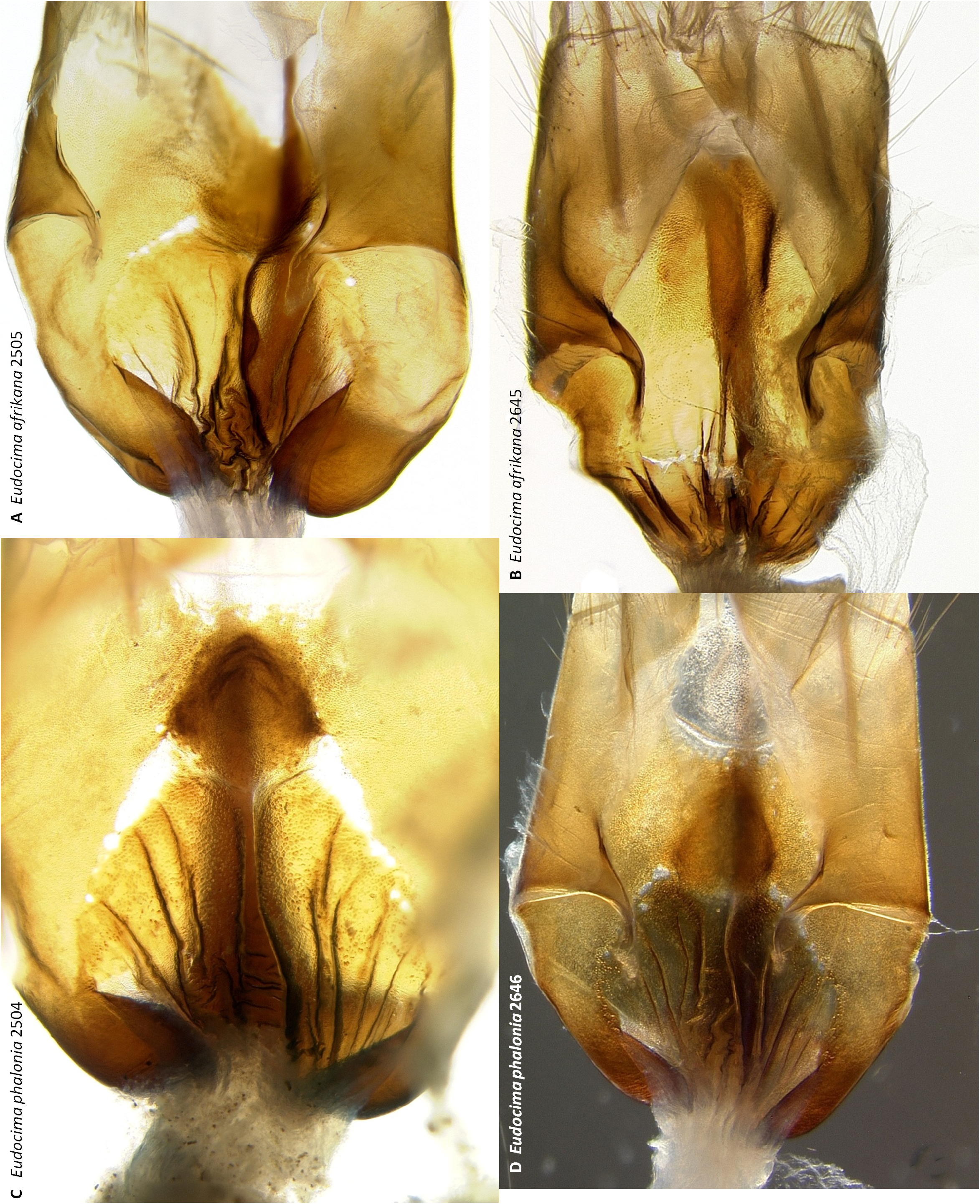
Juxta ( Figs 34 View FIGURE 34 : A–C, 36: A–C, 37: A, 38: A–D). No distinct separation into a juxta and anellus, thus we refer to the entire structure as the juxta. Anterior margin U-shaped with a triangular median notch ( Fig. 38 View FIGURE 38 : A–D). Diverges into two elongate processes with sharply pointed apices, anterior point of divergence triangular and sharply pointed ( Figs 37 View FIGURE 37 : A, 38: A–D); distance from the anterior median to the point where the processes diverge 0.80–1.02 mm, processes 0.44–0.52 mm at widest point. Shape as shown in Figs 37 View FIGURE 37 : A (three dimensional) and 38: A–D (flattened out between two slides). In lateral aspect fairly straight in roughly anterior 2/3 but sinusoidal in posterior 1/3 ( Figs 36 View FIGURE 36 : A–C). In dorsoventral aspect paired dark pigmented bands along inner margin anterior in roughly anterior fourth ( Figs 34 View FIGURE 34 : A–C, 37: A).
Uncus ( Figs 43 View FIGURE 43 : A–D, 44: A–D). Broad in lateral aspect, widest medially, posterior side strongly convex, anterior side strongly concave ( Figs 43 View FIGURE 43 : A–D). Narrower in posterior aspect, shape variable but always distinctly flared and dorsoventrally flattened subapically ( Figs 44 View FIGURE 44 : A–D). Apical spine heavily sclerotized, dorsoventrally flattened with a blunt tip ( Figs 44 View FIGURE 44 : A–D), appearing fang shaped in lateral aspect ( Fig. 43 View FIGURE 43 : A–D), in lateral aspect transition from uncus to apical spine nearly entire on posterior side, with a strongly convex bulge on anterior side ( Figs 43 View FIGURE 43 : A–D). Setae dense on sides throughout length ( Figs 44 View FIGURE 44 : A–D), projecting posteriorly to laterally ( Figs 44 View FIGURE 44 : A–D), longest medially, longest setae of comparable length to maximum width of uncus in lateral aspect ( Figs 43 View FIGURE 43 : A–D), row of short setae projecting anteriorly along medial to subapical anterior edge ( Figs 43 View FIGURE 43 : B, D).
Tuba analis ( Figs 41 View FIGURE 41 : A–D). Membranous except for scaphium and posterior–lateral corners. Scaphium an invaginated, concave plate tapering to a point slightly dorsal of the apical spine.
Phallus ( Figs 47 View FIGURE 47 : A–D). Translucent throughout, coecum distinctly less sclerotized than remainder. Width and shape variable, fairly straight, apical triangular process present on ventral phallus hood highly variable in size, shape, and length. Coecum opening and posterior opening both on dorsal side.
Ductus ejaculatorius ( Figs 46 View FIGURE 46 : A–D). Slender region much longer than scoop-shaped region. Scoop slender with small fold at distal end of inner side. Portion inside the phallus readily everts with the vesica (labeled “DE” in Figs 48–55 View FIGURE 48 View FIGURE 49 View FIGURE 50 View FIGURE 51 View FIGURE 52 View FIGURE 53 View FIGURE 54 View FIGURE 55 ), adjacent to and posterior of diverticulum 2 ( Figs 48 View FIGURE 48 : A–C, 49: A).
Vesica ( Figs 48 View FIGURE 48 : A–C, 49: A, 50: A–C, 51: A, 52: A–C, 53: A, 54: A–C, 55: A). Two simple unilobal diverticula present and a basal bulge. Diverticulum 1 with a sclerotized ovoid plate with dense deciduous heavily sclerotized spine-like cornuti. Orientation of diverticula as described in the diagnosis.
Female genitalia ( Figs 59–68 View FIGURE 59 View FIGURE 60 View FIGURE 61 View FIGURE 62 View FIGURE 63 View FIGURE 64 View FIGURE 65 View FIGURE 66 View FIGURE 67 View FIGURE 68 ).
Papillae anales ( Figs 61 View FIGURE 61 : A–B, 62: A–B, 63: A–B). Sclerotized throughout, shape as shown in Figs 61 View FIGURE 61 : A–B, setae project primarily posteriorly, dense short setae along posterior edge, more widely separated and elongate setae elsewhere.
Apophyses ( Figs 60 View FIGURE 60 , 66 View FIGURE 66 : A–B). Posterior apophyses conspicuous, sclerotized, elongated rods ( Fig. 60 View FIGURE 60 ). Anterior apophyses short and unpigmented distally ( Figs 66 View FIGURE 66 : A–B).
Intersegmental membrane between papillae and segment 8 ( Figs 59 View FIGURE 59 : A–B, 60: A). Gradually widening posteriorly to anteriorly. Ratio of length to width at anterior end about 0.8.
Segment A8 ( Figs 64 View FIGURE 64 : A–B, 66: A–B). Shape as shown in Figs 64 View FIGURE 64 : A (ventral) and 66: A–B (lateral). Elongate, posteriorly projecting setae arranged irregularly along posterior edge ( Figs 64 View FIGURE 64 : A–B, 66: A–B).
Intersegmental membrane between lamella and segment 8 on ventral side ( Fig. 64 View FIGURE 64 : A). Heavily sclerotized sinus vaginalis with two tones of sclerotization, including a more darkly sclerotized interior ovoid pattern; only two narrow bands of unscleriotized tissue (not present in Fig. 64 View FIGURE 64 : B, where this area appears to be deformed). Sclerotization pattern posterior of antrum ornate with radiating bands of darker sclerotization as shown in Figs 64 View FIGURE 64 : A–B.
Lamella antevaginalis (LAV)/Antrum ( Figs 64 View FIGURE 64 : A–B (ventral), 66: A–B (lateral)). A large amount of variation is present between our two preparations as one specimen (HLK: 2645) appears to have a deformed antrum and lamella antevaginalis. Specimen HLK: 2505 is typical of other dissections we have prepared or examined in the literature from the E. phalonia species group. Both preparations have two asymmetrical pockets with a long, tapering triangular medial spine extending posterior of the sinus vaginalis, with the left pocket larger than the right (ventral aspect). The spine is fairly straight in HLK: 2505 ( Fig. 64 View FIGURE 64 : A) whereas it is strongly curved to the right and much longer in HLK: 2645 ( Fig. 64 View FIGURE 64 : B).
Ductus bursae ( Fig. 59 View FIGURE 59 : A). Membranous, dorsoventrally flattened with broad vertical striations, forks posteriorly into asymmetrical diverging, pointed extensions where fused with antrum ( Fig. 65 View FIGURE 65 : A).
Corpus bursae ( Figs 59 View FIGURE 59 : A–B, 60). Longitudinal raised striations throughout. Shape irregular, posteriorly bulging, anteriorly gradually tapering and curving ventrally. Note the degree of inflation varies among preparations in Fig. 59 View FIGURE 59 . While we fully inflated the corpus bursae with 99% IsOH, it quickly retracted and lost its shape before it could be photographed.
Ductus seminalis ( Fig. 67 View FIGURE 67 : B). Total length approximately 3.8 mm. Simple, uncoiled, bulla begins about 0.9 mm distal of base. Note the bulla is not inflated in Fig. 67 View FIGURE 67 : B, but is inflated in Fig. 67 View FIGURE 67 : C of E. phalonia .
Colleterial gland complex ( Figs 67 View FIGURE 67 : D–E, 68: A, C, E). Terminology follows Mitter (1988). Adjoining differentiated canals of spermathecal duct with 1.5 coils basal to the vesicle; abrupt transition to undifferentiated section at base of vesicle; vesicle unsclerotized, elongate and weakly curved ( Fig. 68 View FIGURE 68 : C). Utriculus elongate, terminus somewhat crescent-shaped, heavily ribbed ( Fig. 68 View FIGURE 68 : E). Lagena globular with short triangular stalk ( Fig. 68 View FIGURE 68 : E). Colleterial gland (separated from the vagina in Fig. 67 View FIGURE 67 : E) tubular for most of length, narrowest at base, widening medially, then constricting basal to a distal asymmetric expansion from which the paired glands arise at its apex ( Fig. 67 View FIGURE 67 : E). Note the colleterial gland is not inflated in Fig. 67 View FIGURE 67 : E of E. afrikana but is inflated in Fig. 67 View FIGURE 67 : F of E. phalonia . Oviductus communalis narrow with the paired branches much longer than basal stalk ( Fig. 67 View FIGURE 67 : D). Vagina ovoid ( Fig. 67 View FIGURE 67 : B).
Rectum/Intestine ( Fig. 69 View FIGURE 69 : D). Rectum sculptured throughout with small ovuloid shapes with slightly raised walls. Intestine robust, as shown in Fig. 69 View FIGURE 69 : D.
COI 5’ Mitochondrial DNA. The holotype with a DNA Sample ID Number of 24834-150918-TO has a complete 658 base pair COI 5’ sequence as follows:
AACATTATATTTTATTTTTGGTATTTGAGCAGGTATAGTAGGAACCTCACTCAGTTTATTAATTCGAGCTGAATTAGGAAACCCAGGATCACTAATTGGAGATGATCAAATTTATAATACTATTGTCACAGCTCATGCTTTTATTATAATTTTTTTCATAGTAATACCTATTATAATTGGAGGATTTGGAAATTGATTAGTACCCCTTATATTAGGAGCCCCTGATATAGCTTTCCCCCGAATAAATAATATAAGTTTCTGACTTCTTCCCCCTTCTTTAACTCTTCTTATTTCAAGAAGAATTGTAGAAAATGGAGCAGGAACTGGATGAACAGTTTAT C C C C C A C T T T C AT C TA ATAT T G C C C ATA G A G G TA G T T C G G TA G AT T TA G C TAT T T T T T C C CTTCATTTAGCTGGAATTTCATCAATTTTAGGAGCTATTAACTTTATTACAACAATTATTAATATACGACTAAATAATTTATCATTTGATCAAATACCATTATTTATTTGAGCTGTTGGAATTACTGCATTTTTATTACTTCTTTCTTTACCTGTCTTAGCAGGTGCTATTACAATACTTTTAACAGATCGAAATTTAAATACATCTTTCTTTGACCCCGCTGGTGGTGGAGATCCTATTCTATATCAACATTTATTT
Infraspecific variation is shown in Table 1.
Taxonomic notes. Eudocima phalonia ( Linnaeus, 1763) (= fullonia Clerck, 1764 ) includes the following synonyms, along with their type countries in parentheses:
Phalaena (= Noctua ) phalonia Linnaeus, 1763 ( India) ,
Othreis fullonia Clerck, 1764 ( India) ,
Ophideres fullonica Linnaeus 1767 (incorrect spelling of fullonia ),
Noctua dioscoreae Fabricius, 1775 (Oriental India),
Phalaena pomona Cramer, 1776 ( India) ,
Ophideres obliterans Walker [1858] ( Samoa) .
A thorough nomenclatural review of E. phalonia was undertaken by Brou & Zilli (2016), who concluded that no names were based on material of African origin. Terrell (2020) found no overall genitalic variance in dissections of 14 female and 16 male specimens of E. phalonia from Indomalya and Australasia, but did not include any African material. Thus, all material included in that study constitutes nominate E. phalonia .
Etymology. The new species is named after the Swahili spelling of Africa.
| YPM |
Peabody Museum of Natural History |
| TA |
Timescale Adventures Research and Interpretive Center |
| UG |
Museo del Departamento de Estratigrafia y Paleontologia |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
SubFamily |
Calpinae |
|
Genus |