Melitaea timandra timandra Coutsis & van Oorschot, 2014
|
publication ID |
https://doi.org/ 10.5852/ejt.2022.830.1865 |
|
publication LSID |
lsid:zoobank.org:pub:C1F47AD9-ECF6-4F0F-9928-55A45332FF4B |
|
DOI |
https://doi.org/10.5281/zenodo.6855856 |
|
persistent identifier |
https://treatment.plazi.org/id/EB42F330-FFD8-B334-92A3-FE8AFC4EFA38 |
|
treatment provided by |
Felipe |
|
scientific name |
Melitaea timandra timandra Coutsis & van Oorschot, 2014 |
| status |
|
Melitaea timandra timandra Coutsis & van Oorschot, 2014
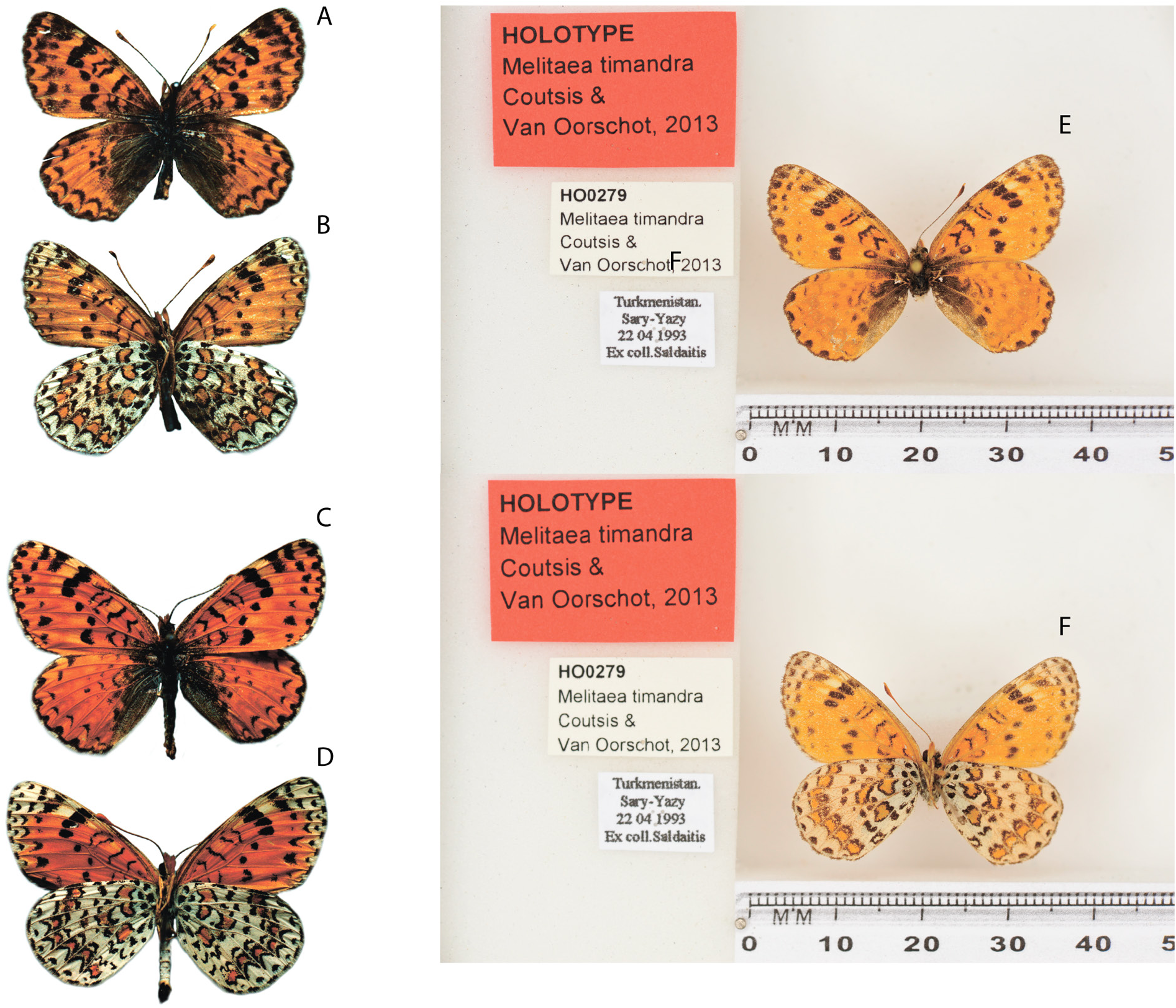
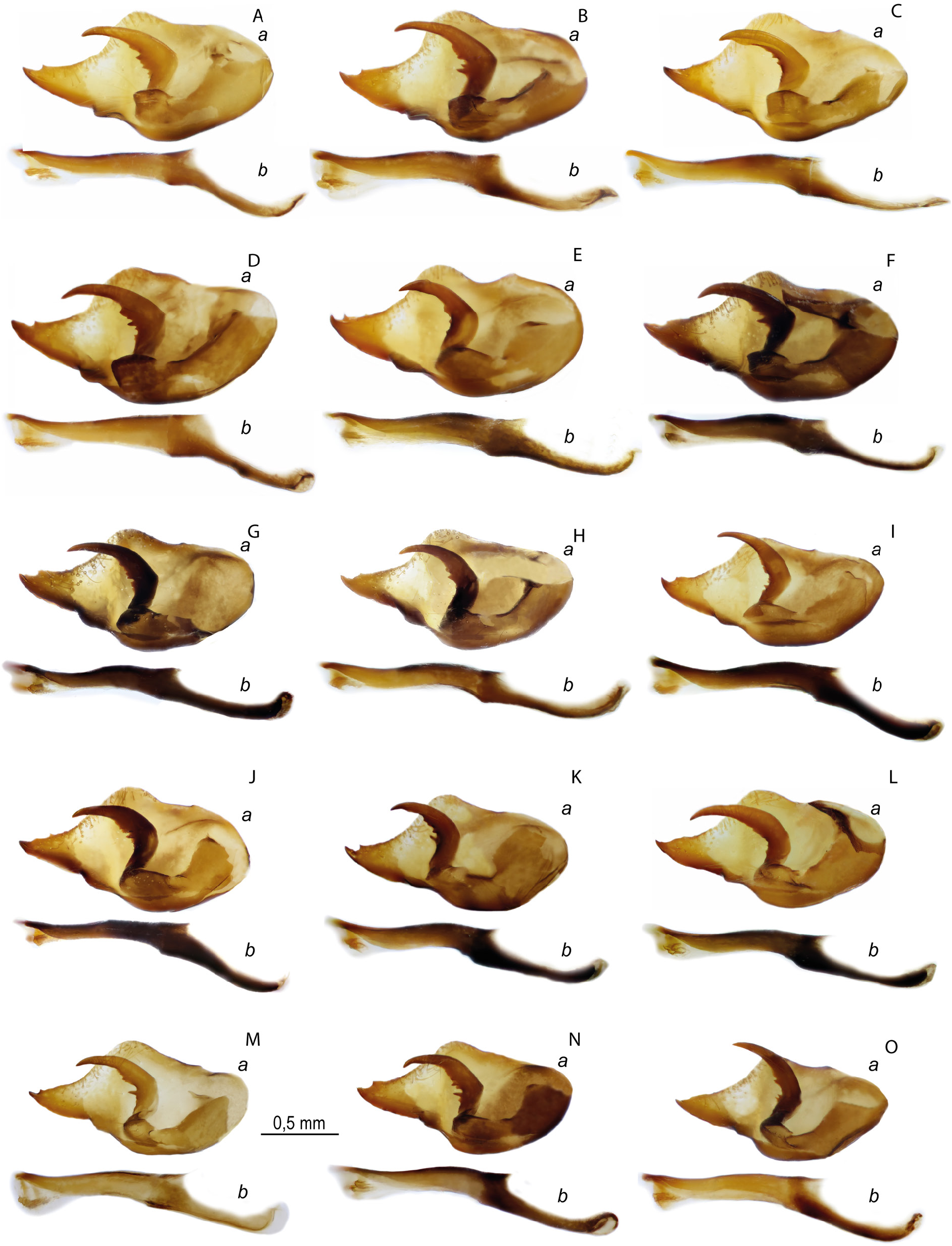
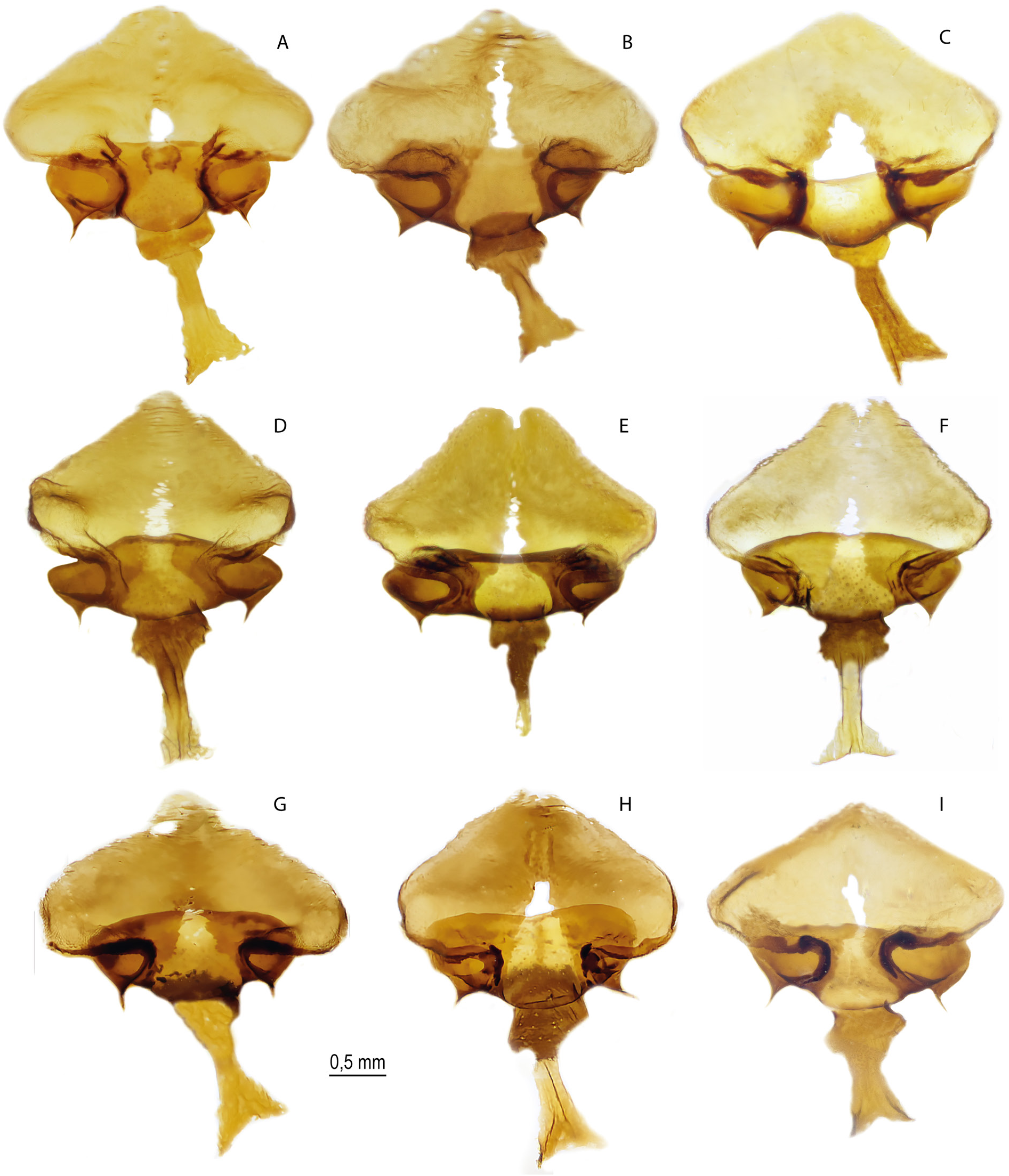
Figs 1E–F View Fig , 9 View Fig , 10A – F View Fig , 11A – C View Fig , 12–13 View Fig View Fig , 17A – C View Fig , 19 A – C View Fig , 29G View Fig , 30 View Fig ; Table 2 View Table 2
“ Melitaea timandra Coutsis & van Oorschot , sp. nov. ” van Oorshot & Coutsis, 2014: 72, pl. 14 figs 23, 25, pl. 15 fig. 1, genitalia: pl. 44 fig. 16, pls 186–187.
Type locality: “ Turkmenistan, Sary-Yazy” [ Turkmenistan, SE Kara-Kum, Mary velayat, vicinities of Sary-Yazy village].
Type material
Holotype ( Fig. 1E–F View Fig ) TURKMENISTAN • “Turkmenistan, Sary-Yasy; 22.04.1993; ex coll. Soldatis ”; NBC HO0279 .
Paratypes TURKMENISTAN • 1 ♂, 1 ♀; Badkhyz Reserve , Kepeli cordon; 20–23 Apr. 1986; A. Devyatkin leg.; EDMSU. Designated as paratypes by van Oorschot & Coutsis (2014) .
Remarks
It is necessary to elucidate upon the issues of identification of the holotype, the type locality and the origin of the paratypes of M. timandra .
Unfortunately, van Oorschot & Coutsis (2014) made many inaccuracies when describing the taxon timandra . In the text of the description, “ Turkmenistan, Kopet Dagh, Sary-Yazi” is indicated as the type locality of the taxon timandra . This indication of the type locality leads to uncertainty, since the village of Sary-Yazy is located 200 km from the nearest spurs of the Kopet Dag Ridge. As a holotype, they indicate a specimen from the de Heer collection while not providing data on either the collector or the date of collection and without reference to the image of the type specimen. From the caption to the drawing of the genitalia of the holotype ( van Oorschot & Coutsis 2014: 264, table 186), it already follows that it comes from the locality “ Turkmenistan, Kopet Dagh, Kara-Kala” and is in the ZMA collection. This information contradicts the original designation of the holotype and the type locality (the village of Kara-Kala is located at more than 600 km northwest of the village of Sary-Yazy). Some inaccuracies in the comments made by one of the authors ( van Oorschot & Coutsis 2014) have been corrected by Coutsis (2016). In the table of preparations of the genitalia, the non-existent locality “Mary-Yazi” was corrected to “Sary-Yazi” and the confusion in the attribution of the depicted genitalia to one or another species was eliminated. It is obvious that there was confusion when labeling the specimens collected, and the two names were combined – the city of Mary, the center of the velayat of the same name, and the village of Sary-Yazy, located about 150 km south of it. The curator of the collection in Leiden, Ms Eulália Gassó Miracle, where the type specimens of M. timandra are currently stored, kindly provided us with photos of the holotype and its labels, from which it follows ( Fig. 1E–F View Fig ) that the type locality of the holotype is the village of Sary-Yazy.
In addition, all indications of the findings of M. timandra in the Western Kopet Dag, given by the authors of the description of the taxon timandra , as well as available in the scientific literature, raise doubts about the reliability and need additional verification and confirmation. It is highly likely that they are based on erroneous labeling of specimens. Tuzov & Churkin (2000) provide photos of M. lutko , corresponding in external morphology to the taxon timandra , allegedly caught by the second author from Kara-Kala.
A more complicated story takes place with a specimen from the vicinity of Kara-Kala, which appears as a paratype of the taxon timandra ( van Oorschot & Coutsis 2014) . The male from the Monjukly Ridge is mentioned on page 348 in the general list of specimens whose genitalia have been studied by these authors. The copy number and the number of the genital preparation are, respectively, NO 1102 and JC 5197. The information on the label is as follows: “Kopet Dagh, SW of Monjukly Mt. range, Saf Kara-Kala, 10. iv. 1992 ” without the name of the collector. There is a note to this specimen: “ paratype, mtDNA sequencing by Wahlberg (NW 15–3 & 16–1)”. Images of the specimen itself and genitalia are not given, it is also completely unclear how many specimens with similar labels besides the one mentioned are included in the type series. In the text of the description of the subspecies timandra ( van Oorschot & Coutsis 2014: 70) in the enumeration of paratypes there is the following entry: “ 2 ♂, 2 ♀, Kara-Kale (sic!), 20. iii. 1991; 1 ♂, same locality but 10. iv. 1992 ”. Since the date of the capture of the last male coincides with the above label, it can be assumed that the mentioned specimen from the Monjukly Ridge was meant. This specimen was used in the work on the assessment of molecular evolution of the genus Melitaea ( Leneveu et al. 2009) as a voucher under the number NW15–3, but already with the label “West China ” (sic!). A photo of a sequenced voucher specimen with a label is contained in the database of electronic resources ( Anonymous 2016). A special investigation undertaken by the authors of the present publication showed that none of the collectors indicated on the labels caught M. timandra on the Monjukly Ridge or in the Kara-Kala area. It is likely that there was a substitution of labels as a result of negligence in the subsequent processing and transfer of the collected specimens to the collections. We believe that all references to M. timandra from the Kara-Kala area are the result of erroneous identification of the collection site, and all specimens with similar labels originate from the vicinity of the village of Sary-Yazy.
Material examined
TURKMENISTAN • 5 ♂♂ (2 dissected), 10 ♀♀ (3 dissected); Badkhyz Reserve, Kyzyl-Jar Gorge ; 27 Apr. 1986; A. Devyatkin leg.; EDMSU • 2 ♂♂ (all dissected), 1 ♀ (dissected); same collection data as for paratypes; EDMSU • 5 ♂♂ (2 dissected), 2 ♀♀; Dushak ; 30 Apr. 1987; A. Devyatkin leg.; EDMSU • 1 ♀ (dissected); Bakharden ; 4 May 1987; A. Devyatkin leg.; EDMSU • 2 ♀♀ (all dissected); Chaacha ; 1 May. 1987; A. Devyatkin leg.; EDMSU • 28 ♂♂ (10 dissected), 33 ♀♀ (5 dissected); Sary-Yazy ; 25 Apr. 1992; I. Pljusch leg.; EDMSU • 18 ♂♂ (6 dissected), 21 ♀♀ (3 dissected); same collection data as for preceding; S. Churkin leg.; EDMSU • 1 ♂; Murgab river, Sary-Yazy ; 23 Apr. 1992; S. Churkin leg.; EDMSU • 2 ♂♂, 1 ♀; 30 km E of Bairam-Ali, Zahmet station ; 22 Apr. 1991; I. Pljusch leg.; EDMSU • 1 ♂ (dissected); Kara-Kum Des., 30 km of Mary-Tedjen , 30 km SW of Mary; 6 Apr. 1979; B. Sokolov leg.; EDMSU • 1 ♀; Kushka ; 24 Apr. 1992; EDMSU • 1 ♀; Bairam-Ali vic.; Apr. 1977; V. Potopolskiy leg.; ZMMSU • 2 ♂♂; Repetek ; ZIN • 11 ♂♂, 12 ♀♀; Sary-Yazy ; 24–25 Apr. 1992; S. Churkin leg.; coll. S. Churkin • 2 ♂♂, 2 ♀♀; Sary-Yazy ; 25 Apr. 1993; S. Churkin leg.; coll. S. Churkin • 1 ♂; same collection data as for preceding; 18 Apr. 1987; A. Kotlobay leg.; coll. A. Kotlobay • 2 ♂♂, 2 ♀♀, same collection data as for preceding; 18 Apr. 1993; A. Kotlobay leg.; coll. A. Kotlobay • 1 ♀; Mary Reg. , v. Bairam-Ali vic.; 15 Apr. 1987; A. Kotlobay leg; coll. A. Kotlobay.
Redescription
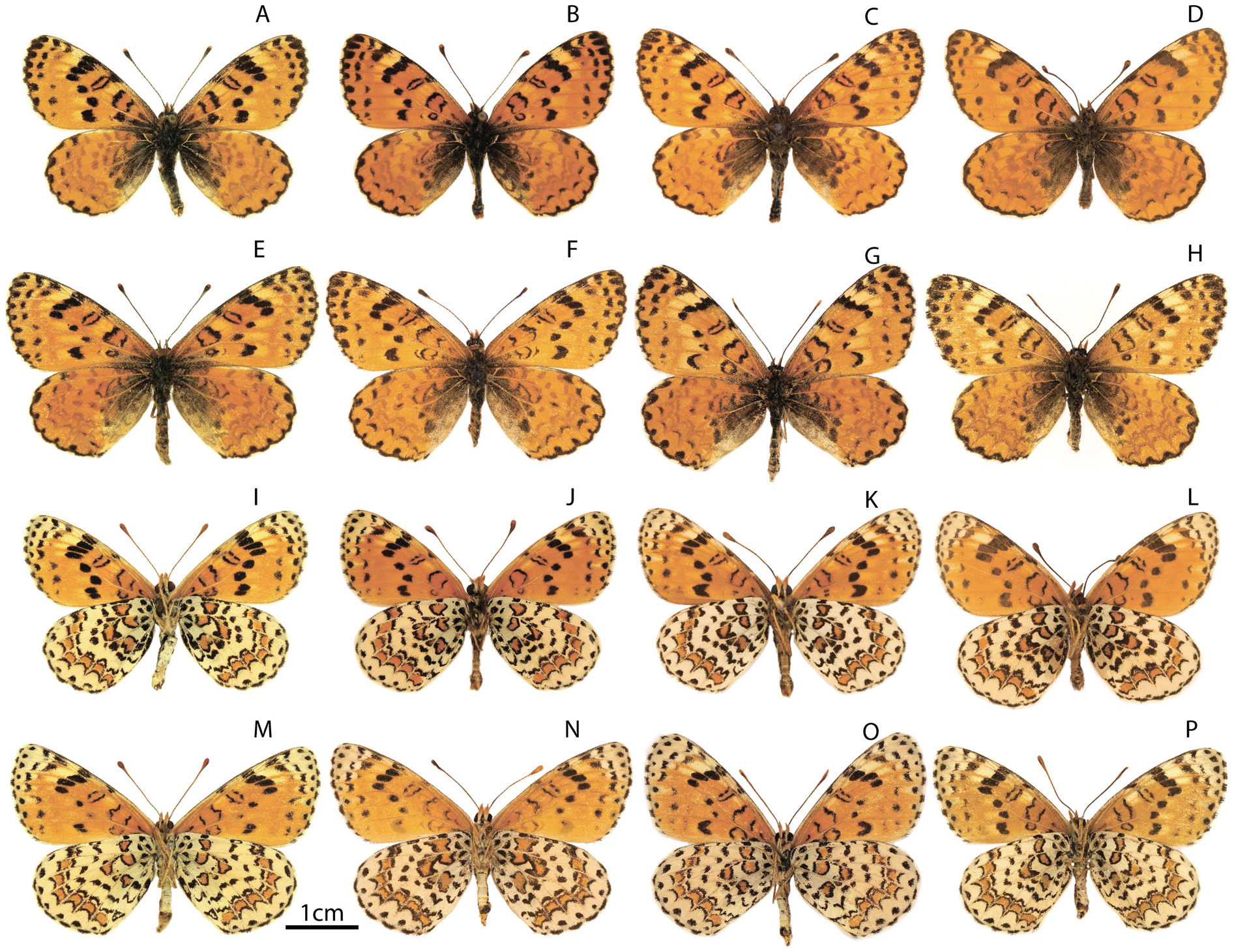
Male ( Fig. 9A–D, I–L View Fig )
WINGS. FW length is 18–21.5 mm, in the holotype 19.5 mm, in the paratype 21 mm. UPS ground color is bright yellow-orange. UPS black pattern is partly reduced; thin UPS black marginal border with well-defined marginal spots along the outer edge of the wings; UPS submarginal row formed by fine black spots or strokes; UPF discal row represented by rather small black spots usually fused near the costa; UPF postdiscal pale area, located behind the black discal spots, represented by disconnected pale-yellowish macules located along the entire length. There is a weak pale-yellowish macule in the distal part of the discoidal cell; UPH discal row is absent; UPH basal suffusion is poorly developed and covers no more than ¼ of the wing surface. UNF is yellow-orange with a well-defined whitish postdiscal macules and pale area of the outer edge of the wing between the veins Sc and M2; and along the outer edge of the wing between the veins Sc and M2. UNH ground color is white, without admixture of dark scales. UNH lunules forming the proximal edge of submarginal orange fascia are usually not sharply pointed.
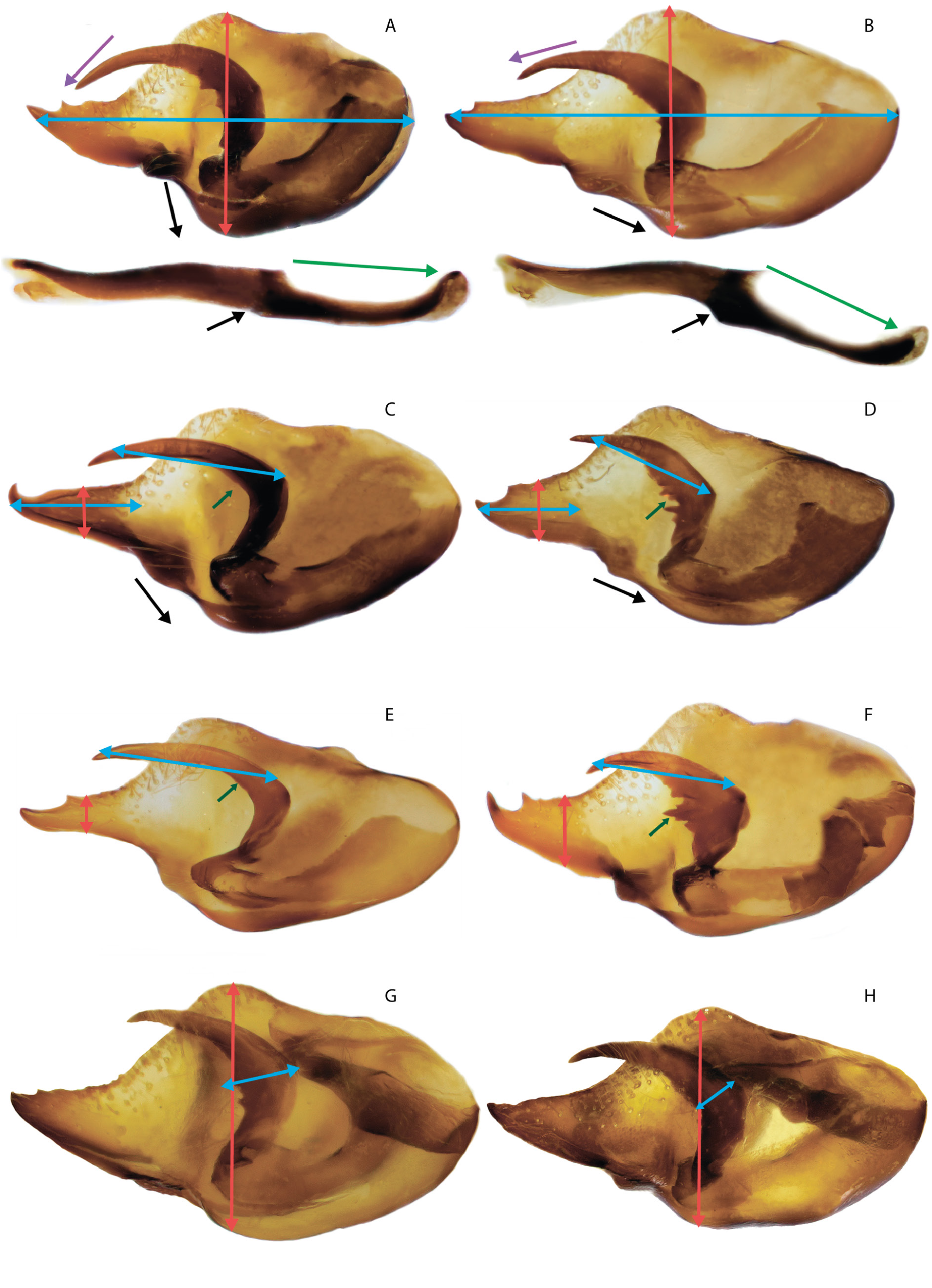
MALE GENITALIA ( FigS 10A–F View Fig , 17A–C View Fig , 19A–C View Fig , 29G View Fig ). Valva is elongated, its length is 2 times greater than the width with a relatively short and wide caudal process, with a spine on the dorsal side of its distal part. Harpe is thickened in the central part due to the presence of teeth on the inner surface. Aedeagus with a slightly convex dorsal edge, its posterior part is mostly located at an angle to the anterior part and is directed downward. When both parts of the aedeagus are joined, there is a well-marked protrusion on the ventral side. Thin saccus is pointed distally, its length is 2 times greater than the width. In some specimens saccus is deeply divided into relatively wide rounded lobes.
Female ( Fig. 9E–H, M–P View Fig )
WINGS. FW length is 20–25 mm, the paratype is 22 mm. UPS ground color is pale orange-red. Externally the female is similar to the male, but UPF postdiscal pale-yellowish area is well expressed. There is a well-defined pale macule in the discoidal cell. UPF submarginal row is represented by thin black strokes. UPH black discal row is usually absent. UNS pattern is similar to that of males.
FEMALE GENITALIA ( Fig. 11A – C View Fig ). The postvaginal plate is rounded-triangular in shape. The antevaginal plate is narrow in the dorsoventral direction, its outer edge does not go beyond the boundaries of the bend of the postvaginal plate (auricules).
Preimaginal stages: egg ( Fig. 12 View Fig , Table 2 View Table 2 )
Material studied: 1 ♀, 3 eggs, Turkmenistan, Badhyz Res., Kyzyl-Dzhar. 2 ♀♀, 18 eggs, S Turkmenistan, Sary-Yazy.
The egg is oval. The height of the egg varies from 686.0 µm to 691.6 µm, the width is from 547.0 µm to 555.6 µm ( Table 2 View Table 2 ). The sculpture of the micropilar area is formed from four to five rows of pentahexagonal cells of various lengths and widths. The diameter of the micropile rosette in the widest part varies from 49.5 µm to 61.0 µm. The micropilar rosette is formed by 7–10 primary quatro-pentahedral cells with a width from 5.0 µm to 17.0 µm and a length from 9.0 µm to 37.0 µm. The micropile is rounded in shape, with an average diameter of about 8 µm. 26–28 lateral longitudinal ribs limit the micropilar area and drop to ⅓ of the egg surface. The transverse ribs are pronounced in most of the eggs studied. Below the lateral ribs, the chorion is relatively smooth.
The eggs of the nominate subspecies are similar in shape to M. timandra binaludica subsp. nov. ( Kolesnichenko & Kotlobay 2020). A distinctive feature of the morphology of the eggs of M. timandra timandra is their smaller size. In M. timandra binaludica the egg height is more than 750.0 µm, and the width is just over 600.0 µm. The micropilar rosette of M. timandra timandra is formed by 7–9 primary cells, while the micropile of the egg of M. shahvarica sp. nov. is surrounded by 7–8 primary cells.
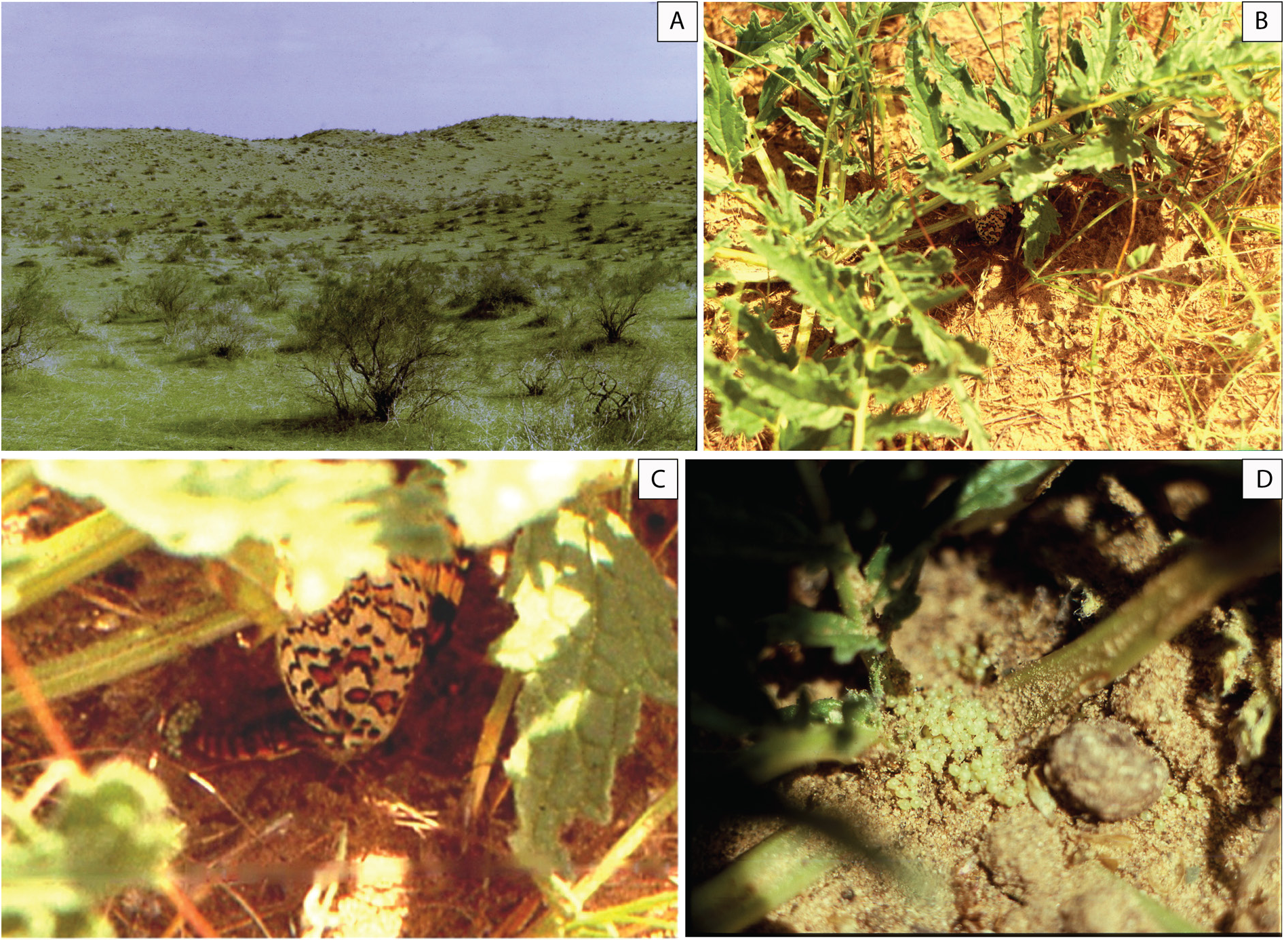
Biology ( Fig. 13 View Fig )
Observations in nature on the behavior and biology of M. timandra timandra were carried out in 1991– 1993 on the left bank of the Murgab River in the area of the Sary-Yazy reservoir in the vicinity of the village of Sary-Yazy in the Mary velayat of Turkmenistan. The flight of butterflies usually occurs in the first days of April and lasts for three to four weeks. The beginning and end of the flight period may shift by a week depending on weather conditions. The habitat of butterflies is typical of the southern zone of the Kara-Kum desert: ridge-bumpy and bumpy-cellular overgrown sands covered with psammophilic shrub and herbaceous vegetation ( Fig. 13A View Fig ). The absolute altitude of the area above sea level is 320– 330 m. The relative height of the sand ridges and bumps is 10– 15 m. The shrubs are represented by juzguns ( Calligonum spp. ) and sand acacia ( Ammodendron conollyi Bunge ex Boiss. ). The space between them is occupied by ephemeroid-ephemeral grass communities, in which the background species are swollen sedge – ilak ( Carex physodes M. Bieb. ), celine ( Aristidia spp. ), eastern wheatgrass ( Eremopyrum orientale Jaub. & Spach , bulbous bluegrass ( Poa bulbosa L.), cheat grass ( Bromus tectorum (L.) Kuntze). In spring, on the slopes of hills and ridges, small thickets of ferula (Ferula spp.) and single flowering plants of iris (Iris spp.) are occasionally found. Poppies ( Papaver pavoninum Boiss. & Buhse ) are common in inter-ridge depressions. Observations have established that the host plant of M. timandra in the studied region is Phlomoides regeliana (Aitch. & Hemsl.) Adylov, Kamelin & Makhm. ( Fig. 13B View Fig ), which forms sparse curtains with an area of up to several hundred square meters in the inter-ridge depressions. Together with M. timandra in this biotope fly blue Neolycaena tengstroemi (Erschoff, 1874) , steppe clouded yellow ( Colias erate (Esper, [1805]) , painted lady ( Vanessa cardui (Linnaeus, 1758)) . Melitaea timandra butterflies are active in sunny weather in the first half of the day. Males are more active than females, moving throughout the territory occupied by the host plant. Females are restricted to short flights within a few, adjacent specimens of Ph. regeliana . For laying eggs, the female chooses a plant with a shaded basal leaf rosette. Laying is carried out directly on the soil near the leaf rosette ( Fig. 13C View Fig ). Before laying, the female samples the soil with the ovipositor for a few minutes, apparently choosing the optimal consistency, temperature, and humidity. Under one host plant, the female produces one fairly compact clutch ( Fig. 13D View Fig ). The number of eggs in the five studied clutches ranged from 28 to 152 (28, 67, 113, 150, 152 respectively). In two cases, it was possible to directly observe the process of egg laying in nature. Laying of 113 eggs lasted continuously for 45 minutes, 150 eggs – a little more than an hour. Three females were placed in a cage, where after a fairly short time they also began to lay eggs on the bottom of the cage next to the host plant leaf placed there. The butterflies laid their eggs in three or four batches, several dozen in each clutch. The eggs are light, almost white, with a light yellowish-greenish color, with a diameter of about 0.5 mm. The further development of the eggs was monitored in the cage. The release of single caterpillars was noted on the third day, the mass emerging of caterpillars in the cage was observed 8–9 days after egg laying. The emerged caterpillars are slightly more than a millimeter long, they partially eat the chorion then switch to feeding on the host plant mining the leaf. The color of the body and the head capsule of newborn caterpillars of the first instar is a solid light green with a yellowish tinge. One of the caterpillars, which emerged from the egg three days after laying, went to molt in the second instar three days later. Unfortunately, for several reasons, further observations of the development of the caterpillars could not be carried out. The question at what stage happened the estivation and subsequent hibernation of M. timandra – the caterpillar of senior instars, or pupa – remains open.
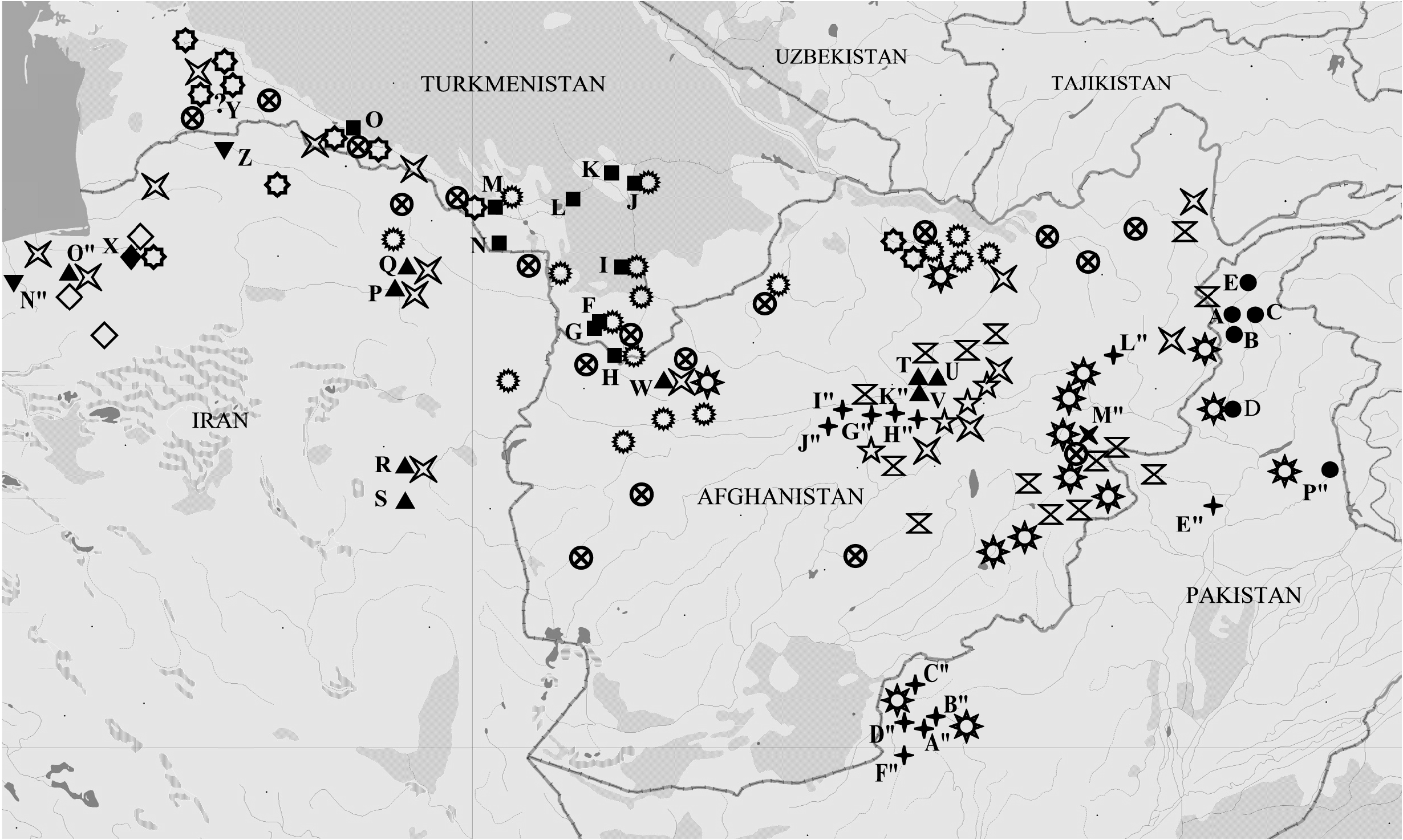
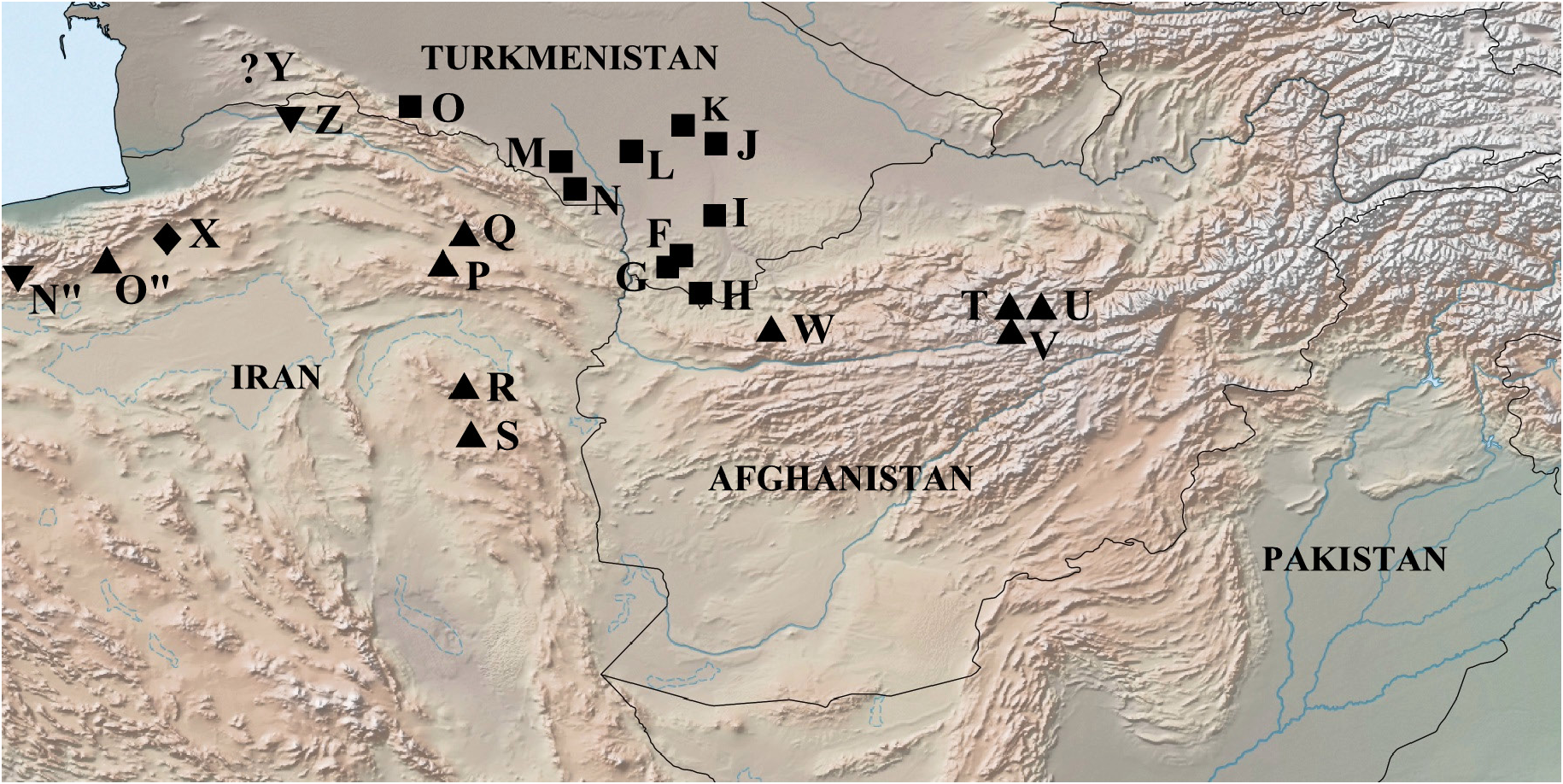
Distribution ( Fig. 14 View Fig )
Turkmenistan: Badkhyz (Kyzyl-Jar Gorge, Kepeli), SE Kara Kum, Kopet Dag foothill plain (Bakharden). The main places of capture are located along the valley of the Murghab River (Sary-Yazy), up to the delta (Bairam Ali, Mary) and in the Tejen River basin (Dushak, Chaacha). To the north, it reaches Repetek. The village of Bakharden is the westernmost, and the vicinity of the village Repetek is the northernmost reliably known habitat of M. timandra timandra .
| ZIN |
Russian Academy of Sciences, Zoological Institute, Zoological Museum |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Melitaea timandra timandra Coutsis & van Oorschot, 2014
| Kolesnichenko, Kirill A. & Kotlobay, Anatoly A. 2022 |
Melitaea timandra
| Coutsis & van Oorschot 2022 |