Sarcophilus harris (Boitard, 1841)
|
publication ID |
https://doi.org/ 10.5281/zenodo.6608102 |
|
DOI |
https://doi.org/10.5281/zenodo.6602787 |
|
persistent identifier |
https://treatment.plazi.org/id/EA7087C1-FFBD-2452-FAC3-F2EC087405CE |
|
treatment provided by |
Felipe |
|
scientific name |
Sarcophilus harris |
| status |
|
25. View On
Tasmanian Devil
French: Diable de Tasmanie / German: Beutelteufel / Spanish: Diablo de Tasmania
Other common names: Devil, Native Devil
Taxonomy. Ursinus harrisic Boitard, 1841,
Tasmania, Australia.
Liutenant G. Harris, General Surveyorfor the Van Diemen’s Land Colony, was also an amateur naturalist. In 1808, he described a new species of didelphid (then Didelphis ursina), the Tasmanian Devil, to the Linnean Society of London. In his description, Harris noted that colonists knew the animal colloquially as the “native devil.” F. G. Cuvier erected the genus Sarcophilus (“flesh-lover”) in 1837, and then in 1841, P. Boitard, the French naturalist, named the Tasmanian Devil ( Ursinus harrisii ), in honor of Harris who had originally made its discovery. In 1903, prolific British taxonomist O. Thomas noted that the original scientific name, Didelphis ursina, had already been ascribed to the Common Wombat (by G. Shaw in 1800) and, following Cuvier’s generic placement, suggested as an alternative, S. satanicus (“Satan’s flesh-lover”). Then in 1912, Thomas noted that Boitard had aptly named the “devil” using the specific epithet harrisii much earlier and promoted the retention of Cuvier’s generic and Boitard’s specific nomenclature, Sarcophilus harrisii . In 2004, the name S. laniarius , the specific moniker originally coined by renowned British biologist and paleontologist R. Owen in 1838 for a mainland fossil (then Dasyurus laniarius ), was promoted as nominal replacement to S. harrisii . Nevertheless,it is unclearif the larger S. laniarius coexisted as a separate species with S. harrisui, or if S. laniarius over time suffered a size reduction to be represented today by S. harrisiiin Tasmania. In any case, the prevailing scientific community has retained S. harrisii to represent modern Tasmanian Devils. Recent genetic (mtDNA and nDNA) phylogenies indicate that S. harrisii is sister to the quoll group ( Dasyurus albopunctatus , D. geoffrou, D. hallucatus , D. maculatus , D. spartacus , and D. viverrinus ). There are at least two genetically distinct subpopulations of S. harrisii in Tasmania—one in the eastern half of the state and a smaller population in the north-west, separated by a band of less suitable habitat; however, these subpopulations are not formally recognized in the taxonomy. Monotypic.
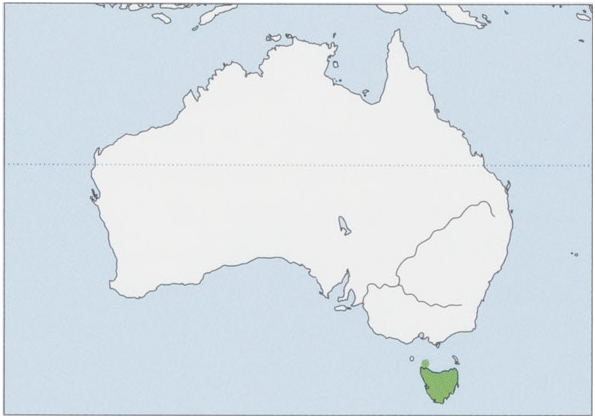
Distribution. Now restricted to Tasmania, including Robbins I. View Figure
Descriptive notes. Head-body 65-2 cm (males) and 57 cm (females), tail 25-8 cm (males) and 24-4 cm (females); weight 8-14 kg (males) and 5-9 kg (females). There is marked sexual dimorphism for size, and the largest male Tasmanian Devils may reach a total length of 131 cm. After the demise of the Tasmanian Tiger or Thylacine ( Thylacinus cynocephalus ), the Tasmanian Devil became the world’s largest surviving carnivorous marsupial. It has highly distinctive black fur that sometimes has a reddish tinge. There are typically white marks on chest and sometimes on shoulders and rump. The Tasmanian Devilis the size of a small-to-medium domestic dog.
Habitat. Preferred habitats of open, dry eucalypt forests, grassy woodlands, coastal scrub, and pasture/dry-bush mosaics. In Tasmania, the Tasmanian Devil is most likely found in the medium-to-drier rainfall zone of the east, north, and north-west. It can reach very high densities in scrub/pasture areas where food supply is enhanced through activities associated with livestock industries, such as carcasses of domestic sheep and cattle and increased numbers of wallabies and wallaby culling. The Tasmanian Devil is found state-wide but at low density in rainforests and buttongrass moorlands of the south-west. Surprisingly, it is common in urban fringes.
Food and Feeding. Tasmanian Devils are predominantly ground dwelling, but they are capable climbers, especially juveniles, that include birds in their diet. Hunting strategy of the Tasmanian Devil involves covering large distances (averaging an impressive 9 km/night), seeking carrion, and ambushing, or persistently pursuing and running down, prey. Primary prey species of the Tasmanian Devil are macropods, possums, and wombats. They feed socially at carcasses of larger prey, with the dominance hierarchy being determined by large size, older age, and degree of hunger. Up to 22 Tasmanian Devils have been recorded squabbling over remains at a cow carcass; more typically 1-3 individuals are involved, and even fewer in farmland where there are abundant remainsto scavenge. Tasmanian Devils are noisy feeders: snorting, barking, and growling may quickly escalate to furious screaming. This ruckus, combined with loud crunching of bones, are reportedly enough to “raise the dead,” or at very least attract passing Tasmanian Devils to partake in a carcass meal.
Breeding Tasmanian Devils typically den in underground burrows concealed in wooded terrain; however, they sometimes rest in logs, underneath buildings, or in thick patches of scrub. Tasmanian Devils maintain a primary den but may use several dens in their home range. Dens in rocky substrate are coveted as prime sites for females to raise young. Mating is a to-and-fro of sexual conflict: blood-curdling screams, genuine affection, and brutal encounters. Males are 30% larger than females but dominate them for just a few days each year when the female is in estrus. Females are far from passive at these times, aggressively asserting preferences for older and larger males. Different males may sire litters of up to four offspring; males ferociously guard females to prevent other suitors. Females are seasonally polyestrous and can cycle a second time (but nota third) if they lose a litter orfail to conceive. Estrous cycle is 35 days long, but females are receptive only up to five days, during which their pouches become red and oily and their necks swell to protect them from inevitable bites they receive during mating bouts. Most mating occurs in February-March. After gestation of 21 days, 15-20 young are born from c.40 ovulated eggs, but only four young can attach to teats; after teats are fully occupied, the rest of the young quickly perish. Newly born Tasmanian Devils are 6 mm long. Young spend c.16 weeks in the pouch. Afterthis period, they will roam independently, farther and farther from the den, from October until January, when they are weaned and males disperse. Most females breed three times, some four times, usually starting at two years of age but occasionally starting at 18 months. Males may not mate until they are 3-4 years of age. Tasmanian Devils live for c.6 years in the wild. Recent studies have discovered that number of wild Tasmanian Devils is declining as a result of the fatal, transmissible Devil Facial Tumor Disease (DFTD). A captive insurance population program has been initiated, but current captive breeding rates are far from optimal. Research has therefore been conducted to better understand their estrous cycle and discover potential causes of failed male-female pairings. Female Tasmanian Devils were found to be more likely to produce pouch young if pairing with the male extended into late proestrus, thereby decreasing the time between pairing and presumed ovulation. Unsuccessful females had 1-3 estrous cycles within a single year. Successful females were predominantly wild-caught (17/19, 90%), and most produced young following the first estrous cycle of the season (18/20, 90%). Unsuccessful females were predominantly captive born (20/27, 74%). Plausibly, a proportion of female Tasmanian Devils that do not produce pouch young achieve conception, but information about timing of reproductive failure continues to be elusive.
Activity patterns. One study used radio-telemetry to monitor body temperatures of Tasmanian Devils, ranging freely in their natural habitat throughout winter. They did not enter torpor, even under prolonged, severe weather conditions; number of hours spent active did not differ between summer and winter or between moderate and severe winter weather conditions. Body temperatures averaged 35-7°C (range 31-3— 37-5°C) for the four (male and female) individuals under study. A diel cycle in body temperature was observed; temperatures rose each evening when they became active, remained high throughout the night despite ambient temperaturesfalling to diel minima, and were lower during the day when they were inactive in dens. The Tasmanian Devil spent a mean of 7-9 active hours /night, of which 5-2 hours were spent active away from the den (out of receiver range). Interestingly, number of hours of activity at night and number of hours away from the den did not differ for one female when she had large young (seven-month-old) in a den compared with all other records when she was not burdened with young in the den.
Movements, Home range and Social organization. Tasmanian Devils are wide-ranging. Home ranges, which are larger in males, are 5-27 km?. Long-distance movements of individuals have been recorded: an astonishing 50 km in 48 hours for an adult male, including a regular 40km return trip to visit a rubbish tip. Dispersal of juveniles is male-biased, although some females also disperse. Dispersal movements of 10-30 km have been recorded directly, but average dispersal distance is most likely much greater than that. Tasmanian Devils evidently forage ¢.8 hours/night, starting at dusk. Juveniles are occasionally sighted during the day, as are adults, in deep snow conditions that tend to restrict movements and evasiveness. In a recent study, structure of the contact network among individuals had a profound effect on transmission of infectious diseases such as DFTD. Using proximity-sensing radio collars, the study found that because of their sociality, all Tasmanian Devils were connected in a single giant network that permitted disease to spread from any single infected individual. Contact networks differed during mating and non-mating seasons, with more extended male-female associations during the mating season and a greater frequency of female—female associations outside the mating season. Results suggest that the Tasmanian Devil is highly contact-connected and there is limited potential to control DFTD by targeting various age or sex classes.
Status and Conservation. Classified as Endangered on The IUCN Red List. Listed as Endangered in Australia. Early diarists note Tasmanian Devils around camps during the Hobart settlement (early 19" century) and that they were likely widespread in Tasmania. It is now clear that Tasmanian Devils once occurred over most, if not all, of continental Australia (possibly not central Australia). Toward the end of last century, numbers of Tasmanian Devils were high compared with their reported scarcity around 1900. Stock-station records rarely mention Tasmanian Devils in north-western Tasmania, with only one lamb being reported killed by them at Surrey Hills (1838), while many lambs and sheep were apparently killed by domestic dogs, Tasmanian Tigers, vagabonds, and Aborigines. The first evidence on numbers of Tasmanian Devils is from the mid-1800s, when reports claimed they were very numerous on the eastern coast, an astonishing 143 being caught at Apsley during one winter. They were evidently scarce in cultivated areas but were abundant in wilder places. Fossil and subfossil remains of Tasmanian Devils have been found widely in deposits, mainly caves and dunes, on the continent of Australia. Victorian records indicate that the Tasmanian Devil formerly lived there; one report commenting that it was once widely distributed over the Victorian plains. The southern range of the Tasmanian Devil apparently extended into South Australia and across the Nullabor into Western Australia. They were first reported from New South Wales in 1877; the Tasmanian Devil may have been confined to damper coastal regions and mountain ranges where moisture and shade could be found. Nevertheless, the species is now restricted to Tasmania; increasing aridity and competitive killing by Dingoes (Canis lupus dingo) and Aborigines probably caused their extirpation on mainland Australia 430-5000 years ago. The Tasmanian population may have become insulated from some of these pressures after being isolated since the sea level rose at the end of the last ice age and flooded the Bassinian Plain—the Bass Straight land bridge that historically connected the island of Tasmania to the main Australian land mass. In the last decade, however, DFTD, an infectious neuroendocrine cancer, has caused major population decline across twothirds of the Tasmanian Devil's distribution. DFTD is consistently fatal; restricted to the Tasmanian Devil; transmissible between individuals by intimate, injurious contact; and kills individuals within a year of their attaining adulthood (importantly, before they would breed under normal conditions). This results in very young age-structured populations. Interestingly, genetic diversity was evidently low in Tasmanian Devils prior to emergence of DFTD for functional major histocompatibility complex MHC genes and mitochondrial and nuclear loci. Reduced MHC diversity evidently preceded isolation of Tasmania because of sea level rise (up to) 13,000 years ago. Nevertheless, although low genetic diversity may have played a role in the evolution of transmissibility, DFTD has evolved a sophisticated mechanism to evade the immune system of the host, involving down-regulation of MHC expression in the tumor. Currently, spread of DFTD is reducing population size of the Tasmanian Devil but with unknown effects on its already low genetic diversity. Indeed, since DFTD was first detected in 1996 at Mount William National Park in north-eastern Tasmania, it has spread to the majority of the distribution of the Tasmanian Devil, causing more than 85% overall population decline, with local declines in excess of 95%. Transmission of DFTD is strongly frequency-dependent; this creates a serious risk of disease-driven extinction because transmission is sustained even at very low population densities through the requirement of contact for reproduction. Nevertheless, geographical spread of DFTD will most likely be slower than gene flow because adults (predominant infectious hosts) are restricted to a home range, but juveniles (which are seldom infected with DFTD) move greater distances away from their natalsite to establish their own territory. Far north-western Tasmania now holds the last remaining disease-free wild populations of Tasmanian Devils. Recent discovery of unique MHC genotypes in north-western Tasmania has raised the possibility that some individuals may be naturally resistant to DFTD. One study examined differences in epidemiology and population effects of DFTD at three well-studied, affected sites in eastern Tasmania and one site (West Pencil Pine) in western Tasmania. In contrast to the three eastern locales, there has been no rapid increase in prevalence of DFID or evidence of population decline at West Pencil Pine. This is also the only site at which population age structure has remained unaltered four years after first detection of disease. A recent study examined MHC diversity in historical and ancient Tasmanian Devils to determine if loss of diversity is recent or predates European settlement in Australia. Results indicated no additional diversity in historical Tasmanian samples. Therefore, low MHC diversity has apparently been a feature of populations since at least the mid-Holocene and could explain their tumultuous population history. Road mortalities, persecution, and habitat loss from land clearing and poisoning also threaten populations of Tasmanian Devils. The recent introduction of the Red Fox (Vulpes vulpes) has prompted an eradication program using “Foxoffl,” a bait containing poison sodium monofluoroacetate (commonly called 1080). One study of poison uptake found that captive Tasmanian Devils had varying interest in the bait, but unfortunately, all wild individuals appeared to find it palatable. In the captive study, males and younger, captive-born individuals were more likely to excavate and remove bait. Subterranean burial at 15 cm was the most effective deterrent to bait excavation; effectiveness decreased at shallower depths and with surface-level bait buried beneath soil mounds. Results suggest that the current fox-baiting campaign may negatively impact individual Tasmanian Devils.
Bibliography. Boitard (1841), Briiniche-Olsen et al. (2013), Cuvier (1837), Ewer (1969), Firestone (2000), Guiler (1970a, 1970b, 1982), Hamede, Bashford et al. (2009), Hamede, Lachish et al. (2012), Hamede, McCallum & Jones (2008), Harris (1808), Hawkins et al. (2008), Hughes et al. (2011), Jones (2008b), Jones & Barmuta (1998), Jones, Cockburn et al. (2008), Jones, Grigg & Beard (1997), Jones, Paetkou et al. (2004), Keeley et al. (2012), Lachish et al. (2009), Miller, W. et al. (2011), Morris et al. (2012), Owen (1838), Pemberton & Renouf (1993), Shaw (1800), Thomas (1903, 1912b).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
SubClass |
Metatheria |
|
Order |
|
|
Family |
|
|
Genus |
Sarcophilus harris
| Russell A. Mittermeier & Don E. Wilson 2015 |
Ursinus harrisic
| Boitard 1841 |