Antechinus swainsonii (Waterhouse, 1840)
|
publication ID |
https://doi.org/ 10.5281/zenodo.6608102 |
|
DOI |
https://doi.org/10.5281/zenodo.6602821 |
|
persistent identifier |
https://treatment.plazi.org/id/EA7087C1-FF88-2466-FA0A-F7F606EA0C44 |
|
treatment provided by |
Felipe |
|
scientific name |
Antechinus swainsonii |
| status |
|
38. View On
Dusky Antechinus
Antechinus swainsonii View in CoL
French: Antéchinus de Swainson / German: Swainson-BreitfuRbeutelmaus / Spanish: Antequino oscuro
Other common names: Swainson's Antechinus; Dusky Marsupial Mouse
Taxonomy. Phascogale swainsonii Waterhouse, 1840 ,
“ Van Diemen’s Land ” (= Tasmania), Australia.
This species was described by G. R. Waterhouse in 1840 from a Tasmanian specimen of unknown specific locality, simply inscribed “Van Diemen’s Land,” held in the private collection of W. J. Swainson and later acquired by the British Museum. About three years after the description, Waterhouse, deferring to the wisdom of J. Gould, placed swainsonii under minimus ; it was another three years before Waterhouse was able to examine the A. minimus holotype; he thereafter re-established swainsonii . In 1924, O. Thomas described the subspecies mimetes. In their checklist ofAustralian mammals of 1934, T. Iredale and E. Le G. Troughton attached the distribution data “Northern New South Wales” to the subspecies mimetes and “Tasmania, Victoria” to the nominate swainsonii . In response to this, N. A. Wakefield and R. M. Warneke noted the absence of a geographical break in the mainland distribution of A. swainsonii , a lack of morphological distinction between populations from northern New South Wales and Victoria, and only “slight” morphological variation between Victorian and Tasmanian populations. They therefore proposed that the trinomial A. s. swainsonii be applied only to representatives of the species from Tasmania. They maintained that Thomas's trinomial A. s. mimetes should apply to the entire mainland population. Forty-six years before Iredale and Troughton published their checklist, Thomas also made reference to occurrence of the species in Victoria. In 1924, Thomas again noted the Victorian distribution of the nominate form in his description of mimetes. Clearly, therefore, establishment of mimetes was intended to contrast with the Victorian population, not contain it. Wakefield and Warneke were obliged to demonstrate sufficient division between Tasmanian and Victorian populations to justify retention of the trinomial A. s. mimetes; they could not do so but, in any case, chose not to sink the subspecies. In 1991, M. J. Davison raised the subspecies insulanus in recognition of the generally larger individuals from that region compared with other mainland A. swainsonii . Most recently, genetic studies have shown A. swainsonii to be markedly different from all congeners, consistently placed as sister to either A. arktos or A. minimus . Three subspecies recognized.
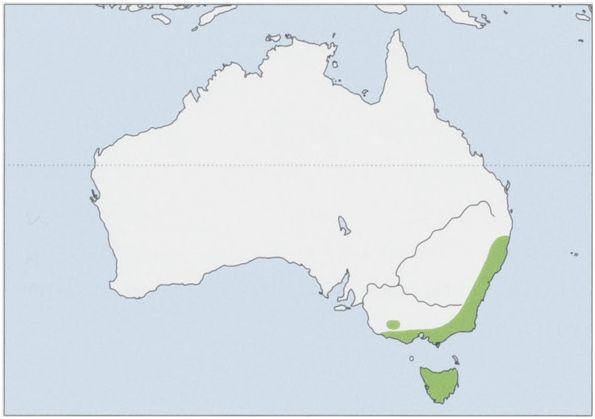
Subspecies and Distribution.
A.s.swainsoniitWaterhouse,1840—Tasmania.
A.s.insulanusDavison,1991—SEAustralia,restrictedtotheGrampiansNationalPark,WVictoria.
A. s. mimetes Thomas, 1924 — SE Australia (excluding the Grampians National Park), from SW Victoria (Wannon district) to NE New South Wales up to just N of Guy Fawkes, Ebor, in New England National Park. View Figure
Descriptive notes. Head-body 8.9-18.8 cm (males) and 8.5-14 cm (females), tail 8-13.1 cm (males) and 7.5-10 cm (females); weight 42-178 g (males) and 35-100 g (females). There is marked sexual dimorphism for size. The Dusky Antechinusis characterized by deep brown-black or grizzled gray-brown fur that is fairly uniform in color from head to rump, sometimes with a slight warming brownish toward rump. Claws are long, particularly noticeable on hands; they are about as long as those on feet. These features and large overall size are distinctive; indeed, the Dusky Antechinusis the largest species in the genus, with a large and very narrow snout and very large holes in front of palate. The Black-tailed Antechinus (A. arktos ) and the Swamp Antechinus (A. minimus ) are probably most similar to the Dusky Antechinus in terms of external and skull morphology, but they are distinctively different. The Swamp Antechinus has a marked change in color from lead-grayish head to brownish-yellow rump, and its tail is shorter, relative to head-body length, than that of the Dusky Antechinus . The Swamp Antechinus is also smaller, on average, with less pronounced narrowness of rostrum and usually smaller holes in palate. The subspecies mimetes is more uniformly deep brown-black to grizzled gray-brown from head to rump, with brownish (clove brown-raw umber) hair on upper surface of hindfoot and tail, whereas the Black-tailed Antechinus is more vibrantly colored, with a marked change from brownish head to orange-toned rump, fuscous black hair on upper surface of hindfoot, and dense short fur on black tail. Furthermore, the Black-tailed Antechinus has marked orange fur on lower eyelids, cheeks, and in front of ears and very long guard hairs all over body, giving it a distinctively shaggy appearance; all these features,if present, are subtler in the Dusky Antechinus .
Habitat. Variety of rainforest habitats in Tasmania, mainly in the west, with 2500 mm of rain/year (nominate subspecies swainsonii ) and dense vegetation in damp environments (more than 800 mm rainfall/year) from sea level to elevations of more than 1800 m, where vegetation types include alpine heath, woodland, high and low elevation rainforest, woodlands of Banksia (Proteaceae) , and wet heath (subspecies mimetes in Victoria). In Tasmania, dominant species include Nothofagus cunninghamii ( Nothofagaceae ), Atherosperma moschatum ( Atherospermataceae ), FEucryphia lucida ( Cunoniaceae ), Phyllocladus rhomboidalis ( Phyllocladaceae ), and Anodopetalum biglandulosum ( Cunoniaceae ). Fire-affected areas may be dominated by Eucalyptus spp. (Myrtaceae) , understory plants including Olearia argophylla ( Asteraceae ), Pittosporum bicolor ( Pittosporaceae ), Tasmania lanceolata (= Drimys lanceolata, Winteraceae ), Persoonia gunnii (Proteaceae) , Anopterus glandulosus ( Escalloniaceae ), and Dicksonia antarctica ( Dicksoniaceae ). Ecotonal regrowth habitats occupied by the nominate subspecies swainsonui are often characterized by Gahnia trifida ( Cyperaceae ), Sprengelia incarnate, Epacris gunna, and Monotoca sp. (all Ericaceae ), Boronia rhomboidea ( Rutaceae ), Leptospermum sp. (Myrtaceae) , Gleichenia alpina ( Gleicheniaceae ), Casuarina distyla (Casuarinaceae) , Eucalyptus gunnu ( Myrtaceae ), Poa caespitosa ( Poaceae ), Calorophus lateriflorus and Restio australis (both Restionaceae ), and Lepidosperma filiforme ( Cyperaceae ). Temperatures in these areas may vary from as low as —12°C in subalpine habitat to 35°C on the coast. The nominate subspecies swainsonii is found consistently in areas of dense cover and thick litter and is not trapped in treeless expanses of button grass ( Mesomelaena sphaerocephala, Cyperaceae ), where it is often replaced by the Swamp Antechinus . In New South Wales, the subspecies mimetes is found on and east of the Great Dividing Range in wet sclerophyll and rainforest gullies, heathlands, coastal sand dunes, and swamps, as well as subalpine woodland with dense shrubby understory.
Food and Feeding. In one study, Dusky Antechinuses ate beetles, spiders, and ants. Other dietary prey included cockroaches, weevils, and Lepidoptera larvae. The Dusky Antechinus uses its long claws and powerful limbs to unearth a variety of soil invertebrates, occasionally supplementing them with fruits such as blackberries. The Dusky Antechinus locates prey principally by smell and,to lesser degrees, sight and hearing. Prey is held in forepaws and passed voraciously to the mouth; water, when needed,is lapped up by the short tongue.
Breeding. During winter, female Dusky Antechinuses excavate nests in creek banks, just below the soil surface, or below the snowpack at higher elevations, often under cover of decaying logs or grass. Nest chamber is roughly spherical and c.10 cm in diameter; it is loosely lined with dried grass and leaves and has a single opening, which is less than 1 m long and leads to the outside. Tunnels are sometimes constructed along the topsoil-vegetation interface, but more commonly, Dusky Antechinuses use larger tunnels of Australian Bush Rats (Rattusfuscipes). Pathways of Common Wombats (Vombatus ursinus) may also be used as trails. Seasonal fluctuations in population size are due in part to the highly synchronized life history of the Dusky Antechinus , with mating restricted to a short period in winter. Copulation is brutal and not preceded by evident courtship. The (often much) larger male attempts to subdue the female immediately by biting fur on her neck. He tightly clasps her chest with his forelimbs before achieving intromission, which lasts for up to an astonishing 9-5 hours. As is the case for all congeners, male Dusky Antechinuses die within c.3 weeks of mating. Females give birth c.1 month after mating and carry their 6-10 young in an open pouch for up to eight weeks. Females then leave their young in nests until they can venture out on their own at ¢.3 months of age. Influx ofjuveniles into the population in spring coincides with a peak in availability of invertebrate food. Mortality is high at this time, likely due to predation on inquisitive and inexperienced young. In the subspecies swainsonii , females have 6-10 nipples in the pouch. Young have been recorded from early Octoberto the end of December, with lactation recorded until late February. In the subspecies mimetes, ovulation and mating occurs later at higher elevations; earliest mating has been recorded in May from the Central Coast district of New South Wales at an elevation of 380 m. In lowland Victoria, mating occurs in July-August, with a delay until September in subalpine habitats. Parturition occurs c.28 days after mating. Young remain in the pouch for up to 60 days, after which the mother leaves them in a nest until they are c.10 weeks old; young are fully independent at c.13 weeks of age. In the subspecies insulanus from the Grampians in Victoria, limited data suggest that mating occurs from late May to early June, with births in early July.
Activity patterns. Although young Dusky Antechinus up to c.6 months old sometimes climb in lower limbs oftrees, activity is largely restricted to the rich and friable topsoil typical of these habitats. It is here that they forage, perhaps as much by day as by night. Activity pattern of the Dusky Antechinus has been variously described as largely crepuscular or nocturnal, and diurnal, or occurring throughout the diel cycle. A recent study of the Dusky Antechinus in subalpine heathland at Perisher Creek, Kosciuszko National Park, southern New South Wales, found that individuals were active throughout the diel cycle; however, there was a shift in peak activity from around sunset in autumn to early morning in winter. As adults, Dusky Antechinuses are heavy-bodied, and captive individuals make little effort to match acrobatic feats of many congeners. When cage tops are lifted, rather than making standing leaps of 0-5 m or more attempting to escape, Dusky Antechinus either bury their heads in leaf litter or limit their activity to rearing onto their hindlegs and cautiously sniffing the air. They may use exercise wheels in captivity but will not run on the outer/underside of the wheel like its smaller congeners, preferring instead to indulge in shorter bouts of standard use.
Movements, Home range and Social organization. One study found that male and female Dusky Antechinuses used home ranges of up to 0-8 ha in autumn; home range size decreased in winter, with females using at a maximum of 0-2 ha and males using a maximum of 0-25 ha. The Dusky Antechinus may compensate for reduced home range size in winter by foraging more actively within available space. Food availability, however, may not be limiting; at another study site of similar elevation, numbers of invertebrates detected in pitfall traps peaked in September and included a substantial proportion of preferred dietary items ( Araneae , Coleoptera , and Hemiptera). The Dusky Antechinus has a wide variety of vocalizations, some of which are related to age or sex. When threatened, nestlings may utter a “siss” cry, which increases in frequency and intensity throughout nest life. Males and females utter “chits” (occasionally “whitter’) when they encounter an unfamiliar object. Fighting males utter shrills, openmouthed “siss” cries, wheezes, and cackle-laughs; adult males will also smear objects with secretions from a chest gland.
Status and Conservation. Classified as Least Concern on The IUCN Red List. In Victoria, the Dusky Antechinus is widespread throughout the Eastern Highlands, Snowfields, Gippsland Highlands, and East Gippsland, but more restricted in the Midlands to the Wombat State Forest and Mount Cole and in coastal heaths to the Wannon region, Otway Range, and Wilsons Promontory. Although the Dusky Antechinus is not threatened, local populations have been reduced by controlled burns and replacement of native forests by pine plantations that removes the complex understory and thus prey diversity and also tree hollow availability. Farming has a smaller influence, but domestic and feral cats and Red Fox (Vulpes vulpes) are very effective in reducing local numbers. One study examined implications of snow-based recreation (skiing and snowboarding) on populations of the Dusky Antechinus in Kosciuscko National Park. When snowpack was experimentally compressed at 22 sites, destroying the subnivean (under snow layer) space, detections of Dusky Antechinuses significantly declined by 75-80%. Dusky Antechinuses clearly remain active below snow throughout winter and depend on presence of adequate subnivean space. Furthermore, removal of vegetation by fire reduced size of subnivean space, regardless of habitat type. Vegetation clearing occurs in the process of ground preparation prior to establishing ski runs. Researchers predicted that “supergrooming,” in which surface soil is also disturbed, is likely to have similar if not worse effects. They concluded that nival areas used for snow-based recreation should be managed to minimize negative effects on the Dusky Antechinus , by maintaining natural features associated with subnivean space formation (dense shrubs, boulders, and microtopography) and confining human developments to areas where such features are not present. The Dusky Antechinus is notoriously difficult to catch in consistent numbers. At certain times and in some habitats,it is readily caught, but in other locations that appear to be suitable, it may not be captured in less than 300 trap nights, even during dispersal or pre-mating phases ofits life cycle. It is unknown if Dusky Antechinuses occur at lower densities in many places or if they are wary of Elliott traps; the former may be more likely given that they are largely ground dwelling and can be repeatedly caught in certain areas with high densities.
Bibliography. Baker et al. (2012), Davison (1986, 1991), Dickman (1986, 2008b), Green, K. (2001), Green, R.H. (1972), Iredale & Throughton (1934), Lunney et al. (2001), Menkhorst (1995a), Sanecki, Green, Wood & Lindenmayer (2006), Sanecki, Green, Wood, Lindenmayer & Sanecki (2006), Thomas (1924), Van Dyck (1997), Van Dyck & Ogilvie (1977), Wakefield & Warneke (1963), Waterhouse (1840), Williams & Williams (1982).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
SubClass |
Metatheria |
|
Order |
|
|
Family |
|
|
Genus |
Antechinus swainsonii
| Russell A. Mittermeier & Don E. Wilson 2015 |
Phascogale swainsonii
| Waterhouse 1840 |