Parasabella aberrans, (AUGENER, 1926)
|
publication ID |
https://doi.org/ 10.1111/zoj.12308 |
|
DOI |
https://doi.org/10.5281/zenodo.10543464 |
|
persistent identifier |
https://treatment.plazi.org/id/E41687B4-7E49-FFBD-9E27-F967FAE9FA08 |
|
treatment provided by |
Felipe |
|
scientific name |
Parasabella aberrans |
| status |
|
PARASABELLA ABERRANS ( AUGENER, 1926) View in CoL SPP. COMPLEX ( FIGS 2C View Figure 2 , 4A View Figure 4 , 5B, J–L, 6 View Figure 6 , 7 View Figure 7 )
Sabella aberrans Augener, 1926: 245–253 View in CoL , fig. 18.
Sabella porifera View in CoL – Augener, 1914: 106–109 (in part).
Demonax aberrans – Knight-Jones & Perkins, 1998: 404.
Parasabella aberrans View in CoL – Tovar-Hernández & Harris,
2010: 14.
Holotype: ZMUC –POL–2115, Little Barrier Island , New Zealand, 55 m depth, 29.xii.1914, Dr Th. Mortensen Pacific Expedition.
Additional material examined (see Appendix for details): Western Australia: Bunbury (one); Ningaloo reef (three) ; Northern Territory: Darwin Harbour (one); Queensland: Lizard Island (three) ; New South Wales: Coffs Harbour (one), Port Stephens (eight), Newcastle (four), Malabar (one), Botany Bay (12), Port Kembla (one), Bass Point (one), Jervis Bay (two), Ulladulla (two), Tathra (three), Batemans Bay (one), Point Upright (one), Eden (eight), Twofold Bay (eight) ; South Australia: Kangaroo Island (nine), Port Hughes (two) .
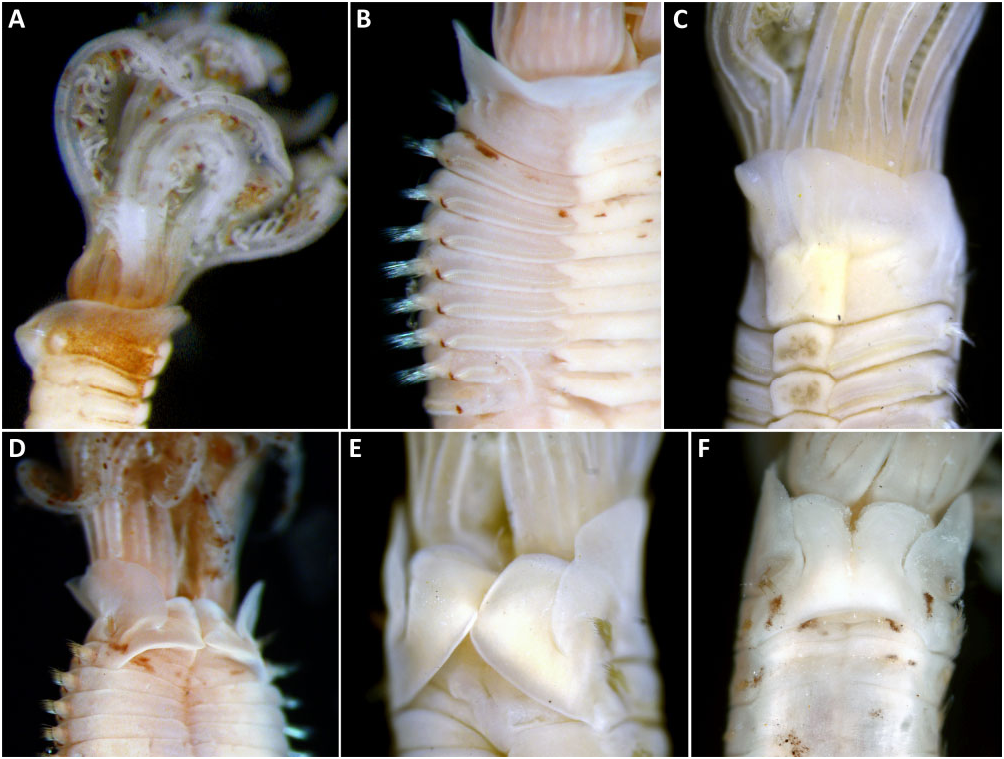
Diagnosis: Stiff, fleshy swelling occupying dorsum of first two chaetigers, with transverse ridge and forming posterior-facing pocket(s), either continuous across dorsum or separated by faecal groove (synapomorphy for this species complex). Radiolar eyes absent. Radioles supported basally by 14–20 rows of vacuolated cells in cross-section. Thoracic ventral shields in contact with neuropodial tori. Inferior thoracic notochaetae broadly hooded, of type B; hoods 1.5 times the width of shaft, and as long as four to five times maximum width. Thoracic uncini with medium-length handles.
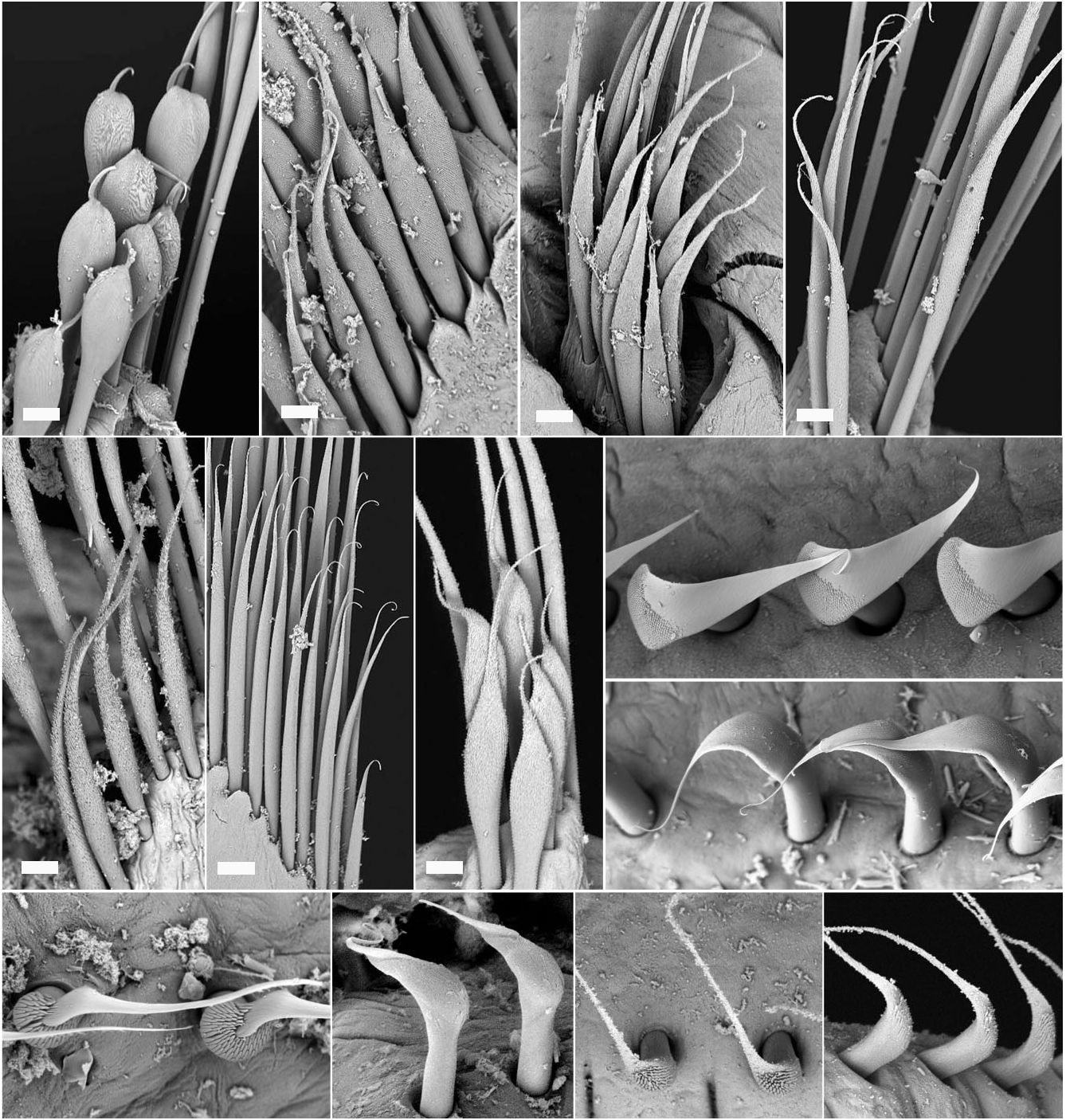
Description of Australian specimens: 2.3 to 35 mm long, 0.5 to 3 mm wide, 5–8 thoracic chaetigers, 20 to> 60 abdominal chaetigers. Radiolar crown with long basal lobes (∼ two thoracic segments), radioles arranged in two semicircles, curling inward in larger specimens. Six to 14 pairs of radioles, each supported basally by 14–20 rows of vacuolated cells in cross-section ( Fig. 4A View Figure 4 ). Radioles with wide, tapering tips, bare for the length of two thoracic segments. Radiolar flanges and eyes absent. Dorsal lips with radiolar appendages as long as 4–6 thoracic segments; 0–2 dorsal pinnular appendages. Posterior peristomial ring collar of even length all way round ( Figs 6B View Figure 6 , 7A, B View Figure 7 ) or oblique laterally ( Fig. 7C View Figure 7 ), reaching junction of crown and thorax ( Figs 6A View Figure 6 , 7C View Figure 7 ), with midventral incision and broad ventral lappets with rounded anterior margins ( Figs 6C View Figure 6 , 7A View Figure 7 ); dorsal margins subquadrate ( Figs 6D–F View Figure 6 , 7D, E View Figure 7 ). Peristomial eyes shallowly embedded under base of radiolar crown. Fleshy swelling with transverse ridge, occupying dorsum of first two chaetigers, forming either two posteriorfacing sinuses or pockets separated by faecal groove ( Figs 6D, E View Figure 6 , 7D View Figure 7 ), or continuous across faecal groove forming one large posterior-facing pocket ( Figs 6F View Figure 6 , 7E View Figure 7 ). Ventral glandular shields similar in width to each other, in contact with tori ( Figs 6B, C View Figure 6 , 7A View Figure 7 ); first ventral shield one to two times length of following, with anterior margin m-shaped ( Fig. 6C View Figure 6 ). Collar chaetae elongate, narrowly hooded ( Fig. 7F View Figure 7 ). Superior thoracic notochaetae elongate, narrowly hooded, inferior group with two rows of shorter broadly hooded chaetae of type B ( Figs 2C View Figure 2 , 5B, 6G View Figure 6 ), with hoods as long as 4–5 times maximum width and maximum width 1.5 times width of shaft. Thoracic neuropodial tori slightly diminishing in width posteriorly ( Figs 6B View Figure 6 , 7A, B View Figure 7 ). Uncini with about 10 rows of similar-sized teeth above main fang, covering more than half length of main fang ( Fig. 7I, J View Figure 7 ), neck as long as breast, well-developed breast and medium-length handles (∼1.5 times distance from main fang to breast) ( Fig. 5J, K). Companion chaetae with enlarged subdistal end, conspicuous microtubercles forming hood, resulting in dentate appearance, with thin distal mucro compressed laterally ( Fig. 7K, L View Figure 7 ). Abdominal neuropodial chaetae narrowly hooded in both anterior and posteri- or rows ( Fig. 7M View Figure 7 ). Abdominal uncini with about 10 rows of similar-sized teeth above main fang covering more than half length of main fang ( Fig. 7N View Figure 7 ), neck shorter than breast, well-developed breast and short handles (0.5 times distance between breast and main fang, Fig. 5L). Pygidium as rounded rim around ventral anus ( Fig. 7O View Figure 7 ), with scattered eyespots present on both sides, only visible in some specimens.
Colour pattern: Brown pigmentation on bases of radioles and on groups of pigmented pinnules ( Fig. 6A, D View Figure 6 ) in some specimens. Scattered pigment spots present along longitudinal axis of radioles ( Fig. 6A, D View Figure 6 ). Some specimens with brown pigment on thorax, absent from ventral shields and tori ( Fig. 6A–C View Figure 6 ). Spots present between neuro- and notopodial rami, superficially resembling interramal eyespots ( Fig. 6B View Figure 6 ), faded in some specimens.
Reproductive features: Gravid specimens with body lengths of 10–35 mm were found, with eggs in last thoracic and in abdominal segments.
Genetic data: Sequences from two specimens from Western Australia and four from New South Wales show wide genetic variation, congruent with their geographical distribution, with the physically most distant specimens showing the largest genetic divergence (of up to 23.6% in cox1 and 10.2% in ITS sequences). Genetic distance in cox1 sequences to the other Parasabella species is 29.6–31.5% ( Table 3) and 20.9–28.4% in ITS ( Table 4). All members of this clade exclusively show the nucleotide sequence TGGA in positions 173–176 of the ITS alignment, amongst several scattered onenucleotide synapomorphies along the nuclear and mitochondrial fragments .
Remarks: This species is easily distinguished from other congeners by the fleshy swellings on the dorsum of the first two chaetigers. Augener (1926: 247, fig. 18A, B) illustrated this very distinctive feature in his original description. However, variations have been found amongst Australian specimens, as some show the swelling as a continuous transverse ridge with a single large posterior-facing sinus and without the faecal groove and cilia running longitudinally across this structure. The two morphological conditions (continuous, or dual dorsal swelling across anterior thoracic segments) are not congruent with the two subclades that show maximum genetic divergence. PS26 (from New South Wales) is the only specimen included in the genetic analyses with a continuous transverse dorsal swelling whereas the rest of specimens (New South Wales and Western Australia) present the dual split structure across the dorsum.
Parasabella aberrans View in CoL was recorded in Australia, albeit as part of the material of Sabella porifera Grube, 1878 View in CoL , by Augener (1914) from Shark Bay in Western Australia, before he subsequently described it from Little Barrier Island in New Zealand (see Augener, 1926). Further collecting within the distribution range will determine if this species is broadly distributed but with great genetic population structure, or whether there has been a lack of gene flow between two populations from distant localities that can be interpreted as an indication of separate, closely related species that show no morphological variation (cryptic species).
Type locality: Little Barrier Island , New Zealand, 55 m depth .
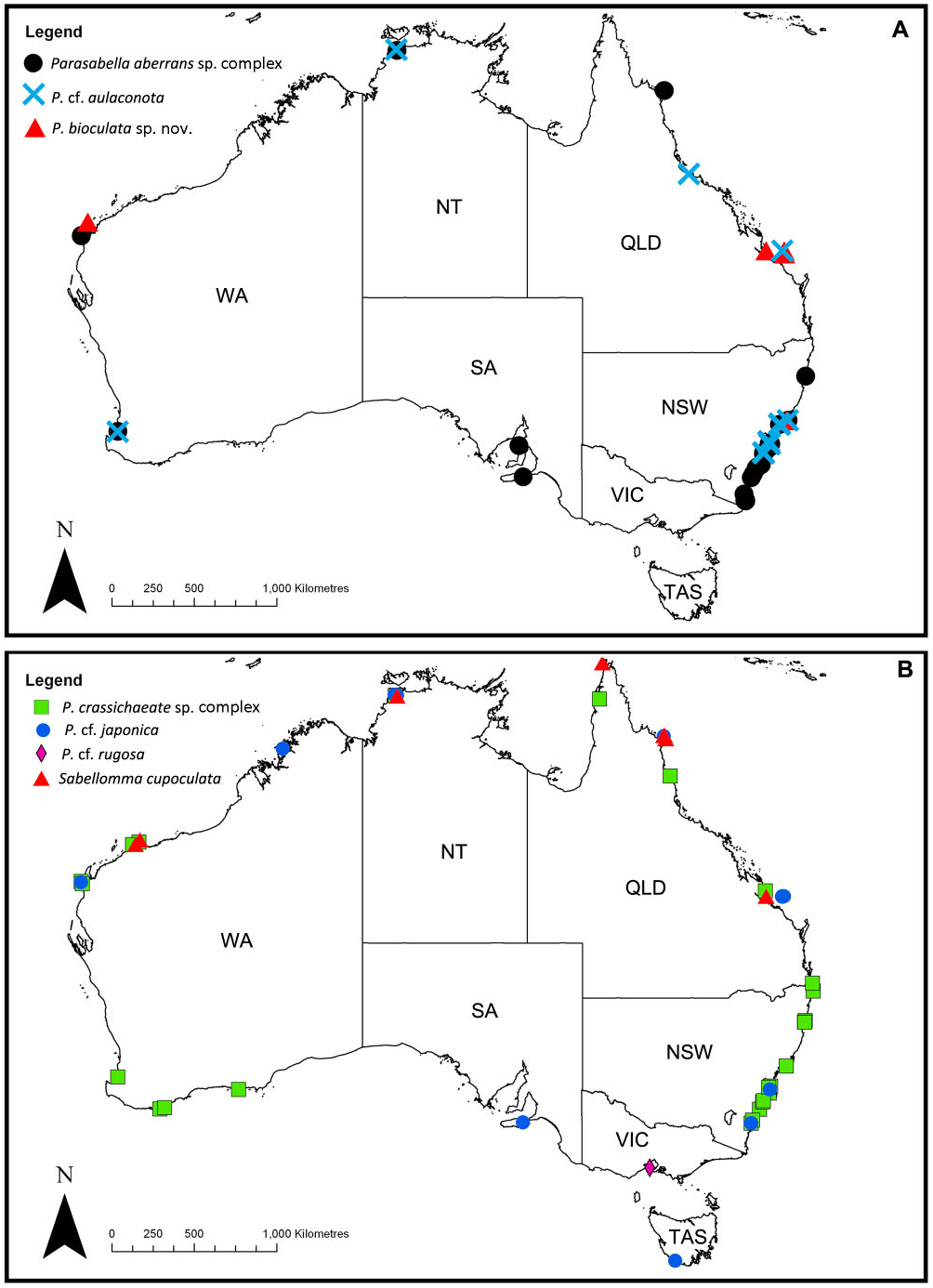
Distribution: New Zealand, Australia (Queensland, New South Wales, South Australia, Western Australia; Fig. 1A View Figure 1 ).
Ecological notes: The species is widespread in New Zealand but usually occurs as single individuals on wharf piles (G. Read, pers. comm.). It was reported in New Zealand Port surveys from 2005–2008 ( Inglis et al., 2005, 2006, 2008). In Australia, it has been recorded not only in the fouling communities from wharf piles in port areas (Port Kembla, Eden, Botany Bay and Bunbury Harbour, see Appendix), but has also been collected from more pristine coastal and offshore environments such as Lizard Island in Queensland, Kangaroo Island in South Australia, and south of Jervis Bay, New South Wales, from the intertidal to 80 m depth. In future, genetic studies of New Zealand populations should also be included to evaluate the broad distribution range reported for the species to determine if this can be explained through natural means or if unintentional translocations could be responsible for such a wide distribution (not detected in this study).
PARASABELLA SP. CF. P. AULACONOTA ( VON
| ZMUC |
Zoological Museum, University of Copenhagen |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Parasabella aberrans
| Capa, María & Murray, Anna 2015 |
Demonax aberrans
| Knight-Jones P & Perkins TH 1998: 404 |
Sabella aberrans
| Augener H 1926: 253 |
Sabella porifera
| Augener H 1914: 106 |