Bythotrephes arcticus Lilljeborg, 1901
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4138.2.2 |
|
publication LSID |
lsid:zoobank.org:pub:724ABC9B-9548-4E3A-A667-274F10A19C8E |
|
DOI |
https://doi.org/10.5281/zenodo.6085645 |
|
persistent identifier |
https://treatment.plazi.org/id/D5745718-9B4C-FFED-FF03-FC4EFAC0FC23 |
|
treatment provided by |
Plazi |
|
scientific name |
Bythotrephes arcticus Lilljeborg, 1901 |
| status |
|
Bythotrephes arcticus Lilljeborg, 1901 View in CoL
( Figs. 1–6 View FIGURE 1 View FIGURE 2 View FIGURE 3 View FIGURE 4 View FIGURE 5 View FIGURE 6 )
Bythotrephes longimanus Leydig, 1860 View in CoL : Stenroos 1897: 67 –68 (partim); Ekman 1904: 28 –29.
Bythotrephes longimanus var. arcticus Lilljeborg, 1901 View in CoL : Lilljeborg 1901: 612 –613, Tab. 81, Figs. 9 View FIGURE 9 –12; Mordukhai-Boltovskoi & Rivier 1987: 152, Fig. 44A-B; Rivier 1998: 173, Fig. 215a, b.
Bythotrephes longimanus var. arctica Lilljeborg: Levander 1901 : 24.
Bythotrephes arctica Lilljeborg : Sars 1903: 186, Pl. 8, fig. 6.
Bythotrephes longimanus arcticus Lilljeborg View in CoL : Ischreyt 1930: 321, Fig. 13d; Manuilova 1964: 292–293, Fig. 160–6; Vekhov 1981: 74, Fig. 2 View FIGURE 2 ; 1987: 28, Fig. 1 View FIGURE 1 .
Bythotrephes crassicauda Lilljeborg : Ischreyt 1934: 277, Abb. 16d; 1937: 56; 1939: 127.
Bythotrephes longimanus crassicaudus Lilljeborg : Litvinchuk 2001: 129.
Bythotrephes crassicaudus Lilljeborg, 1890 : Litvinchuk 2002: 84–86, 118–119, 127–128, Figs. 8 View FIGURE 8 , 10, 35, 36, 41B, 42B; 2007: 191–192, Figs. 3 View FIGURE 3 B, 4B; Litvinchuk & Litvinchuk 2016: 67–73, Fig. 9 View FIGURE 9 .
Type material. Syntypes. All specimens certainly used by Lilljeborg (1901) for investigation and description of B. arcticus and labeled “ B. crassicauda ” or “ B. longimanus arcticus ” can be considered as belonging to the type series of the taxon ( ICZN, 72.4.1., 72.5.) and named syntypes ( ICZN, 73.2.).
Lectotype. A parthenogenetic female with body length 4.34 mm (Karesuando, Norrbotten, Sweden, 30.7.1875, collected by Lilljeborg, sample N 2669) deposited in a tube with alcohol in the Museum of Evolution of Uppsala University (№ UPSZTY 163492).
Paralectotypes are deposited in the same Museum: slides № UPSZTY 2730, 2826–2830; samples № UPSZTY 163493– 163505.
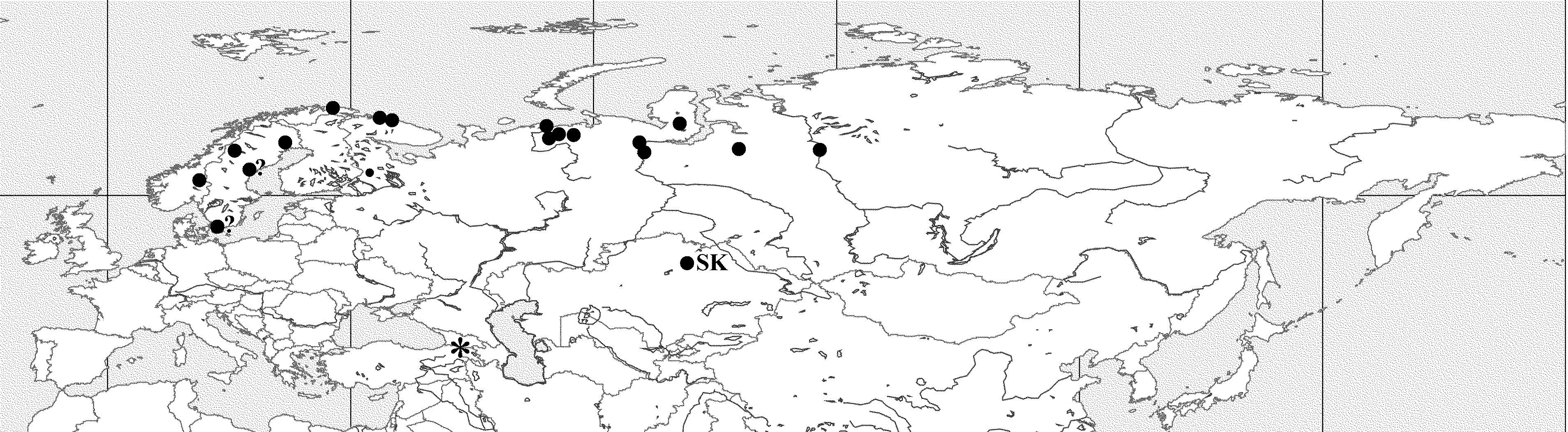
Material examined. Russia: Kola Peninsula (Murmansk region): 1) A slide ( MEUU, N 633c with specimens from sample N 2318) labeled “ Kola Penins., Karabella , 31/7 77, Sandeb. exp., Bythotrephes crassicauda n. sp. glacialis ” (the last name has probably been crossed out by Lilljeborg) (in a Museum catalog this material is designated as “ B. longimanus arcticus , syntypes ”), 2 ad, dried (paralectotypes); 2) a slide ( MEUU, N 633d with a specimen from sample N 2319) labeled “ Bythotrephes crassicauda n. sp., Kola Penins. Karabella , 5/9 77, Sandeb. exp.”, 1 ad dried and deformed (paralectotype); 3) sample ( MEUU, N 2672) designated in a Museum catalog “ B. l. arcticus , USSR, Kola peninsula, Karabella, 31.7.1877, Sandeberg”, numerous specimens (ad, gam, males) (paralectotypes,); 4) sample ( ZIN, without number) labeled “ Bythotrephes, Alexandrovsk (Murman), 1899 , A. Linko”. 1 ad of first generation; 5) sample ( ZIN, N 6852) labeled “ Bythotrephes longimanus Leydig , Verhne- Kildinskoe lake, coll. Knipovitch, 1898, det. Verestchagin”, 2 ad, 2 males; 6) a slide ( ZIN, without number) labeled “ Bythotrephes, Kildin, 1898 , N. Knipovitch”, 1 ad, dried; 7) a slide ( ZIN, N 73–15) labeled “ Bythotrephes longim v., swamps of Kildin Island, det. Verestchagin”, 1 ad, deformed; 8) a slide ( ZIN, without number) labeled “ Bythotrephes longim, Lake Kildinskoe, Knipovitch 1894 , det. Verestchagin”, 1 gam.
Arkhangel’skaya region, Bol’shezemelskaya tundra (north-east of European Russia): 9) sample ( NMK, N 2143), Nenetsky autonomous area, Srednayay Haryala, Lake Lolyalaty (67°4’ N; 56°3’ E), 11.7.2000, coll. A.V. Rzhavsky, numerous ad, 1 male; 10) sample ( NMK, N 2144), the same area, spring near Lake Lolyalaty, coll. A.V. Rzhavsky, 2 ad; 11) sample ( ZIN, N 6853) labeled “ Bythotrephes longimanus Leydig, Pechersky area, Pustozersk VII, coll. Zhuravsky, det. G. Verstchagin”, 2 males; 12) two slides ( ZIN, N 45-06) labeled “ Bythotrephes longimanus , Arkhangel’skaya guberniya, Pustozersk, Zhuravsky”, 2 ad; 13) basin of the River Tchernaya, lake 1 (68°22’18.5’’ N; 56°49’19.5’’ E) and lake 2 (68°29’59.4’’ N; 56°40’35.4’’ E), 9.7.2013, leg. E.B. Fefilova, 8 ad, numerous juv; 14) the same region, Lake Dubovskoi (67°36’032’’ N; 62°54’12.5’’ E), 20.7.2012, 1 gam, 2 males, leg. E.B. Fefilova; 15) two slides ( ZIN, without numbers) labeled “ Bythotrephes , tundra near mouth of the River Pechera, Peltsam”, 2 ad.
Western Siberia: 16) sample (ZIN, N 250-1936), Ob River, sor Vusrenohnor, Yurty Karvoki, 7.3.1931, 12 ad; 17) sample (ZIN, N 6849) labeled “v -? Bythotrephes longimanus, River Sos’va near Berezov town, 16.7.1896, coll. Varpahovsky, det. Verestchagin”, 1 ad; 18) a slide (ZIN, without number) labeled “lake near village Puiko, Yamal, det. G. Verestchagin”, 1 ad; 19) Lake Yan in the basin of the River Pur near town Tarko-Sale, 3 ad, 1 male, 5 juv.
Eastern Siberia: 20) sample (ZIN, N 6851) labeled “ B. longimanus v -?, Turuhansk, coll. Ulrih, det. Verestchagin”, 6 ad, 14 males, some juv; 21) a slide (ZIN, without number) labeled “ Bythotrephes longim . v?, Turuhansk, Ulrih”, 1 ad; 22) a slide (ZIN, without number), the same label, 2 gam; 23) a slide (ZIN, without number), the same label, 1 ad.
Kazakhstan: 1) sample (ZIN, N 6848) labeled “ Byth. arcticus Lilljeb, Akmolinskaya region, Lake Sabanty Kul, 6.6.1899, coll. Ignatov, det. G.O. Sars”, 6 ad, deformed; 2) a slide (ZIN, without number) labeled “ Bythotrephes arcticus, Akmolinskaya region (st. 15), P. Ignatov”, 3 ad, deformed.
Sweden: 1) a slide (MEUU, N 633a with specimens from sample N 2669) labeled “ B. longimanus arcticus, Sw, Lpl, Karesuando , 30/7 1875, Lillj.”, 2 ad (paralectotypes); 2) a slide (MEUU N 633b with specimens from sample N 2673) labeled “ B. l. arcticus, Sw, Lpl, Karesuando , 30.7.1875, Lillj.”, 4 ad (paralectotypes); 3) a slide (MEUU, N 633e with specimens from sample N 2320) labeled “ Bythotrephes crassicauda, Sw, Lpl, Karesuando , 30/7 75, Lillj.” with 2 dissected males (paralectotypes); 4) a slide (MEUU, N 633f with specimens from sample N 2321) labeled “ Bythotrephes crassicauda, Sw, Lpl, Karesuando , 30/7 75, Lillj.”, 2 ad, dissected (paralectotypes); 5) sample (MEUU, N 2648) labeled “ B. l. arcticus (junior), Sw, Sk, Vombsjön, 8.7.1880, Lillj.”, 1 juv (paralectotype); 6) sample (MEUU, N 2650) labeled “ B. l. arcticus, Sw, Hls, Råbosjön , 28.8.1895, E. Lönnberg”, numerous ad, gam, males (paralectotypes); 7) sample (MEUU, N 2651) labeled “ B. l. arcticus, Sw, Jmt, Undersüker Ottsjön , 21.8.1889, Schött H.”, 1 ad, 1 gam, 1 male, 3 juv (paralectotypes); 8) sample (MEUU, N 2658) labeled “ B. longimanus, Sw, Jmt., Östersund brook, 9.08.1889, coll. Lillj.”, 1 ad (paralectotype); 9) sample (MEUU, N 2669) designated in a Museum catalog “ B. l. arcticus, Sw, Lpl, Karesuando , 30.7.1875, Lillj.”, numerous ad, gam, males (paralectotypes, a lectotype was selected from this sample—see above); 10) sample (MEUU, N 2655) labeled “ B. l. arcticus, Sw, Sk, Vombsjön, Cederström G.C. ”, 1 ad (paralectotype); 11) sample (MEUU, N 2674) labeled “ B. l. arcticus, Sw, Lpl, Karesuando , 31.7.1875, Lillj.”, numerous ad, gam, males (paralectotypes).
Norway: 1) sample (MEUU, N 2657) labeled “ B. l. arcticus, Norw, Tönset, Tönsettjarn , 28.7.1881, Esmark B.”, about 20 ad, gam, males (paralectotypes); 2) sample (MEUU, N 2659) labeled “ B. l. arcticus, Norw, Tönset Lonsö , 28.7.1881, B. Esmark”, 1 ad, 1 gam, 1 male (paralectotypes); 3) sample (MEUU, N 2668) labeled “ B. l. arcticus, Norw, Porsanger , 21.7.1887, G. Kolthoff”, numerous ad and males (paralectotypes); 4) sample (MEUU, N 2670) labeled “ B. l. arcticus, Norw, Porsanger Stora, Tumsö , 1.8.1887, G. Kolthoff”, numerous ad, gam, males (paralectotypes); 5) sample (MEUU, N 2675) labeled “ B. l. arcticus, Norw, Porsanger lake, 4.8.1887, G. Kolthoff”, numerous ad, gam, males (paralectotypes); 6) sample (MNB, N 18908 View Materials ) labeled “ Bythotrephes longimanus Leydig, 1860 , Norwegen, aus Forellenmägen, leg. Huitfeldt-Kaas, ded. Weltner, Oct. 1900 ”, numerous ad, males.
Data on body and body parts measurements of specimens of some populations are presented in Table 1. Data from other populations have been taken into consideration for comparison.
BL, mm HL:BL TlL:BL CPL:BL PCL:BL CPCL:BL% ICD: BL ICTh:BL
% % % % % %
1. Lake Lolyalaty (Arkhangel’skaya region, Bol’shezemelskaya tundra, NE European Russia), females, n = 15
3.5–4.0 35.7–44.4 86.7–110.3 155.8–216.1 13.0–17.6 9.9–14.7 11.3–17.0 8.5–14.8
3.7 41.3 95.9 188.5 15.5 11.9 13.9 10.5
2.6 6.9 3.6 1.3 1.4 1.4 1.7 6.3 7.2 1.9 8.3 11.7 10.2 16.5
2. Sor Vusrenohnor, Yurty Karvoki, Ob River (Western Siberia), females, n = 13
3.5–4.8 37.9–45.7 70.2–85.8 143.5-201.8 9.2–18.7 6.6–13.9 8.7–16.7 5.8–8.6
4.1 42.0 79.0 168.5 12.8 9.8 12.2 7.1 2.4 4.9 15.6 2.6 2.1 2.6 0.9 5.6 6.3 9.3 20.4 21.2 21.4 12.6
3. Karesuando (Norrbotten, Sweden), females, n = 15
3.6–5.4 33.8–43.6 65.1–82.5 114.7-183.4 8.6–19.3 4.5–12.2 8.0–15.6 5.6–8.0
4.9 38.0 71.3 131.5 11.8 8.7 12.1 6.8 2.8 4.6 17.7 2.8 2.0 2.2 0.8 7.4 6.4 13.4 23.4 23.3 18.1 12.4
4. Combined data on six populations, females, n = 25
2.8–6.1 31.0–51.5 63.1–92.5 80.7–190.0 7.2–18.6 5.2–16.0 9.9–15.9 5.5–9.9
4.6 39.9 78.8 132.6 12.7 9.2 12.1 7.7 3.5 2.6 34.5 2.8 2.5 1.6 1.2 8.8 3.3 26.0 22.3 27.5 13.0 15.6
5. Combined data for males, n = 16
2.5–4.4 35.6–46.5 51.1–80.3 141.8–214.0 10.8–21.6 8.5–18.4 8.2–13.5 6.1–10.6
3.6 39.8 65.2 172.2 16.2 14.1 10.6 8.8 3.3 7.8 24.6 2.9 2.8 1.9 1.3 8.2 11.9 14.3 17.7 19.7 18.0 14.8
Bythotrephes transcaucasicus, Lake Chaldyr ( Turkey) , n = 16
2.3–4.2 34.0–38.5 68.0–77.9 127.1 -157.9 6.0–11.1 5.0–9.6 7.6–9.9 4.4–7.2
3.8 36.8 73.5 145.5 8.5 7.4 8.8 5.8 1.4 2.6 8.6 1.5 1.3 0.7 0.8 3.8 3.5 5.9 17.8 17.1 7.4 12.9 Diagnosis. Body elongated and divided into four parts: head, thorax, abdomen and postabdomen with long caudal process ( Fig. 1 View FIGURE 1 A). Its longitudinal axis is conspicuously incurved when anterior part of head is located at almost right angle to the thorax. Also, highly movable abdomen can be either in a straight line with the thorax or at an angle to it. Head large with rounded anterior part filled by the enormously developed compound eye and bearing small antennules ventrally. Posterior part of head bears long swimming antennae and mouth parts consisting of mandibles, maxillules (mx I), and upper lip (labrum). Thorax with strongly developed muscular ventral side bearing four pairs of thoracic limbs of different size directed antero-ventrally or ventrally. Dorsally, thorax bears sack-like carapace transformed into brood pouch sometimes reaching large size. Abdomen (metasome) is elongated, cylindrical, inconspicuously three-segmented and flexible, connected with small postabdomen, bearing ventrally a pair of straight claws and posteriorly a very long straight caudal process with a pair of similar claws proximally. Generally body length of females (without caudal process) can reach 6.0 mm or slightly more (in the examined specimens it ranges from 2.3 to 6.1 mm), while the length of caudal process may be shorter or surpasses the body length considerably.
Description. Parthenogenetic female. Head. Comparatively very large (31.0–51.5 % of body length) and subdivided into two parts: rounded anterior part mostly filled by large compound eye and posterior part bearing dorsally a large saddle-shaped neck organ, swimming antennae and mouth parts. Large pigment spot occupies about one-third or at most a half of the eye’s volume (see also Ekman (1904)). Ocellus (naupliar eye) is absent ( Elofsson 1966).
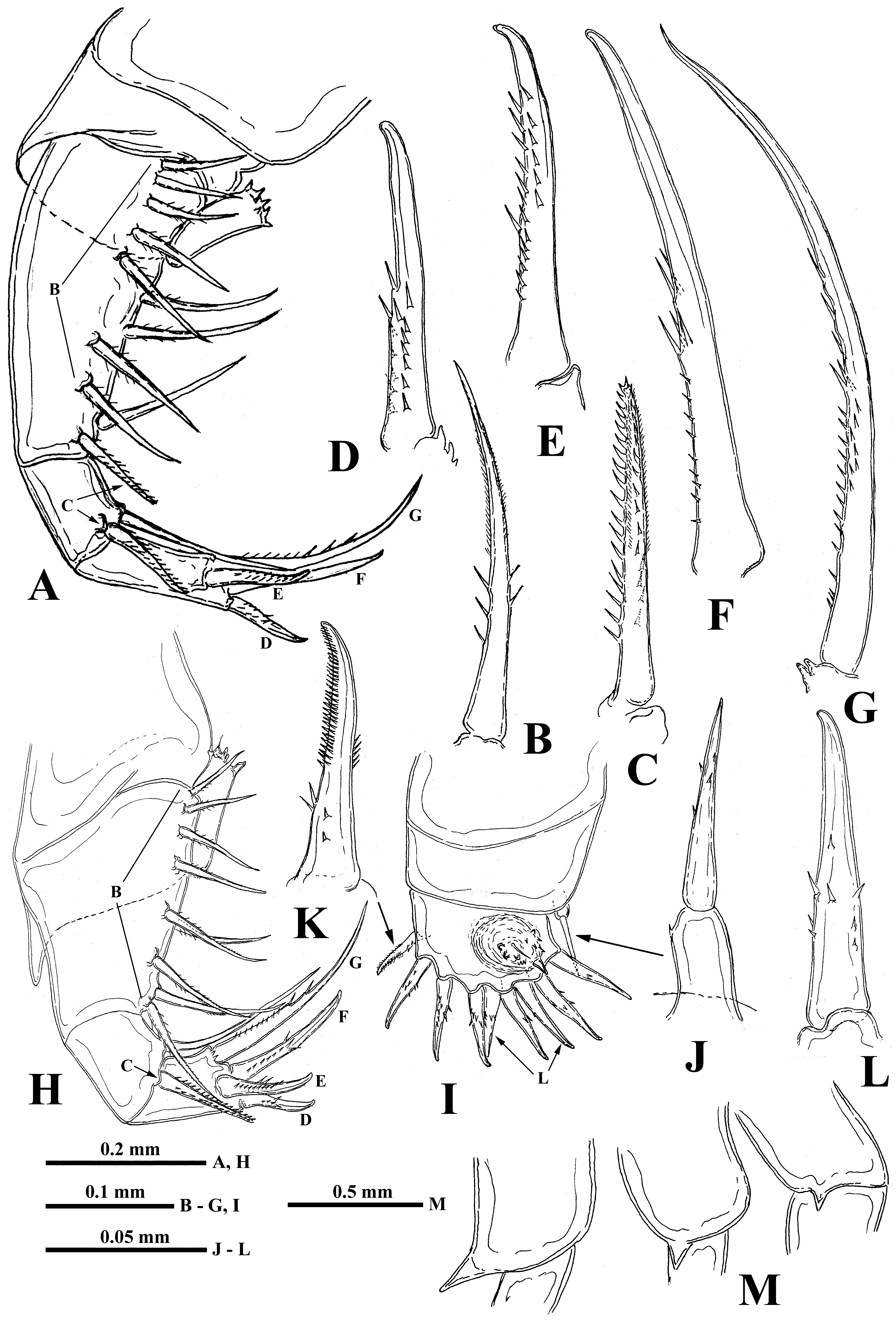
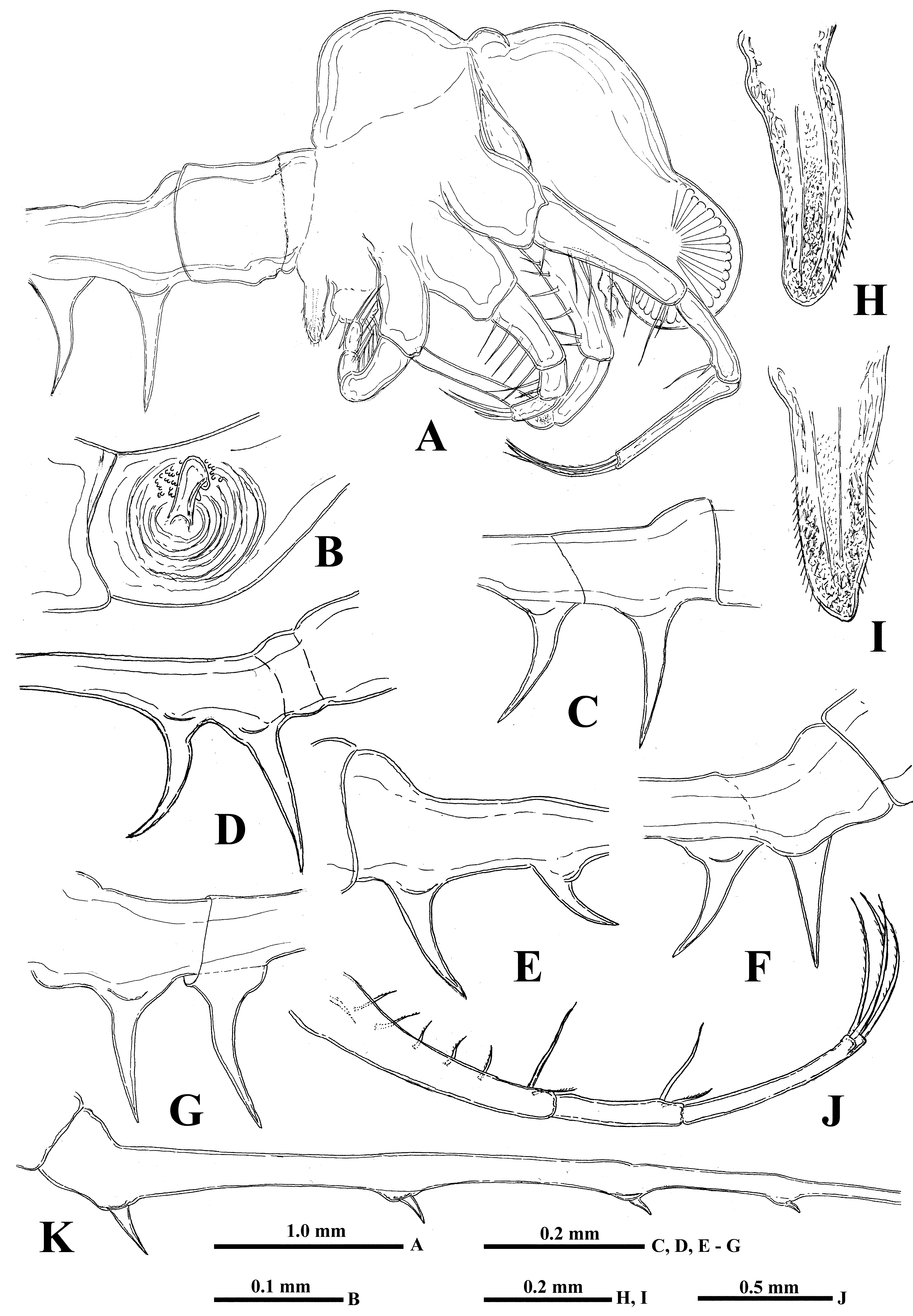
Antennules. Small and situated on the ventral side of the anterior head part beneath the eye. They are bulbous ( Fig. 1 View FIGURE 1 B) and sit on the joined basis slightly split anteriorly. Terminally they bear five regular aesthetascs in two groups, with three and two in each one, one shorter and thinner aesthetasc-like structure situated in a group with two regular aesthetascs, and having slightly widened apical end with dark granular structure inside (“accessory simple seta” according to Scourfield (1896)).
Swimming antennae. Comparatively long, with elongated cylindrical basipodite ( Fig. 1 View FIGURE 1 A) which has a dorsal thin naked seta on its folded proximal part. Of two antennal branches, the lower three-segmented one (endopodite), sitting on the apical basipodital prominence, is slightly longer than upper branch. The upper branch is foursegmented and lower branch is three-segmented. Proximalmost segment of the upper branch is rudimentary and clearly visible only externally, all other segments of both branches are much more developed. Small spine with a row of neighboring minute prominences on the end of second segment of upper antennal branch ( Fig. 1 View FIGURE 1 F), similar apical prominences and spines on the distal segments of both branches ( Figs. 1 View FIGURE 1 D, 1E). Small proximalmost segment of upper branch lacks setae, while other segments possess two-segmented swimming setae of more or less similar size except distalmost of them which are shorter. All setae bilaterally armed with rows of uniform thin setules. General formula of antennal setae: 0–1–2–5 / 1–1–5.
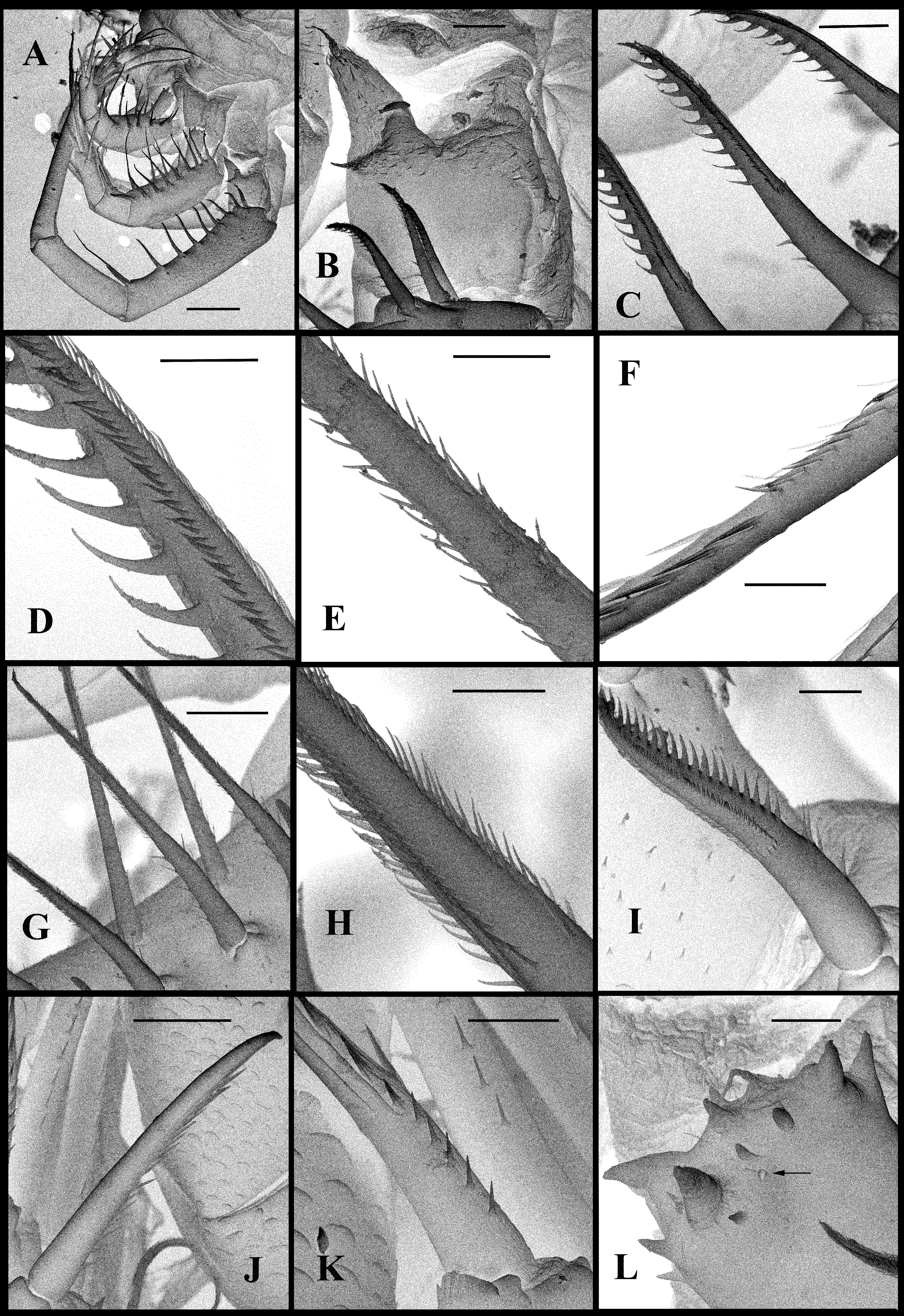
Mouth parts. They are represented by upper lip (labrum), mandibles, and maxillules (maxilla I). The upper lip ( Fig. 1 View FIGURE 1 C) is composed of two parts: a posterior thick and slightly flattened triangular lobe and anterior large proboscis-like outgrowth. The latter is separated from the former one by a deep indention of the cuticle. The triangular lobe bears numerous papillae along its posterior-interior (oral) margin while the outgrowth is armed with tiny spinules. Mandibles are bilobed and adapted for biting ( Fig. 4 View FIGURE 4 H), with a toothed, blade-like posterior lobe and small anterior lobe (mandibular process) armored with a cluster of about 30 long outgrowths, differing in size and bearing some prominences distally ( Figs. 4 View FIGURE 4 J, 4K). Posterior lobe is strongly sclerotized and divided into two toothshaped parts, the larger (posterior) of which has a small additional tooth about midway along its border ( Fig. 4 View FIGURE 4 H). Maxillules (mx I) look like two cylindrical structures situated posterior to mandibles. Distally, they bear a short central seta and some papillae near it. Maxillae (mx II) are absent; the openings of maxillar glands are situated near the bases of tl I laterally (see Olesen et al., 2003).
Carapace. It looks like a comparatively low bag-like structure, strongly modified into a closed brood pouch ( Fig. 1 View FIGURE 1 A) widely connected at its base with the dorsal side of thorax.
Thoracic limbs. Four pairs of strongly chitinized, stenopodous limbs are densely situated along the muscular ventral side of thorax and directed antero-ventrally ( Figs. 1 View FIGURE 1 A, 3A). All of them have complex and variously setaceous armament along their inner side. Limbs of the three anterior pairs are five-segmented and those of the last fourth pair are three-segmented. Protopodites of all of them, covered by comparatively softer cuticle, are inconspicuously delimited into two parts (segments), coxa and basis, while the endopodites of limbs of the three anterior pairs are composed of three well developed segments and those ones of the fourth pair are unisegmented (Figs, 1I, 2A, 2H, 2I).
First pair of limbs (tl I) are especially long and strong; their length can surpass the body length but are usually shorter (63.1–110.3 % of body length) ( Figs. 1 View FIGURE 1 A, 1I, 3A). Terminally, the inner side of their protopodite bears a small triangular lobe, the pseudognathobasic process (see the explanation of the term in Korovchinsky (2015)), armed laterally and distally with two outgrowths with apical setae and numerous spinulae ( Fig. 3 View FIGURE 3 B). The external part of the protopodite is longer than the internal one and bears apically a small conical outgrowth ( Fig. 2 View FIGURE 2 M–right). The first segment of the endopodite is long (19.9–34.9 % of body length) and bears 6–10 (usually 7–8) anterior lateral setae (their number of limbs of one individual can vary) with a posterior row of incurved spines, and anterior and lateral rows of fine setulae ( Figs. 3 View FIGURE 3 C, 3D). Distally, this segment bears a shorter anterior seta of the same type and a long posterior finely setulated seta ( Figs. 1 View FIGURE 1 I, 1J, 1K, 3E). The second segment of the endopodite is conspicuously shorter (9.3–19.8 % of body length) and bears only two apical setae similar to those on the end of the previous segment, but shorter ( Figs. 1 View FIGURE 1 I, 1L). The terminal third segment of the endopodite is also long (17.0– 37.4 % of body length), almost equal or slightly surpassing the length of the first segment (1.09–1.13: 1.0), and always bears apically four long roughly spinulated setae, two of them terminally and two subterminally ( Fig. 1 View FIGURE 1 I). Basally, these setae are armed by a row of smaller spines, while distally, by larger lanceolate spines situated in two rows and directed terminally ( Fig. 3 View FIGURE 3 F).
Second pair of limbs (tl II) considerably shorter; their protopodite, again externally, is conspicuously longer and bears a conical outgrowth ( Figs. 2 View FIGURE 2 A, 2M-middle, 3A). The first, basal segment of their endopodite, bears a row of 7–10 (mostly 7–8) rather long anterior lateral setae of type “B” (their number also can vary in one individual) ( Figs. 2 View FIGURE 2 A, 2B, 3G, 3H). Also there are 2–3 posterior lateral setae of the same type on this segment. Terminal setae of the segment are different; the anterior one of type “C” is shorter and roughly armored ( Figs. 2 View FIGURE 2 A, 2C, 3I), while the posterior one is longer and finely setulated. Internally, this segment bears stout cylindrical pseudognathobasic process, possessing some prominences of different size, one small, thin seta, and a pore apically ( Figs. 2 View FIGURE 2 A, 3L). The second segment of the endopodite is short with only two setae, the anterior of which is of “C”- type while the posterior seta is longer and finely setulated. The distal, third segment of the endopodite of the limb bears four setae, two terminal and two subterminal ones ( Fig. 2 View FIGURE 2 A). Of the latter, the anterior seta is of type “E”; it is comparatively short, thick and armed with a number of thin lateral denticles, while its distal end is naked and slightly hooked apically ( Fig. 2 View FIGURE 2 E, 3J). Its neighboring posterior subterminal seta of type “G” is considerably longer and similar to the subterminal and terminal setae of tl I, having similar spine armament and sharp apex ( Figs. 2 View FIGURE 2 A, 2G). The anterior terminal seta of type “D” is thick and comparatively short with longitudinal ribs, few thin lateral denticles, and slightly hooked apical end ( Fig. 2 View FIGURE 2 A, 2D, 3K). The posterior terminal seta is similar to the neighboring anterior one but longer, has few lateral denticles and a slightly hooked apical end ( Figs. 2 View FIGURE 2 A, 2F).
Third pair of limbs (tl III) generally similar to those of the previous ones, differing in some details. The external outgrowth of their protopodite is conspicuously larger ( Fig. 2 View FIGURE 2 M– left) and lateral anterior and posterior setae of the first segment of the endopodite are fewer (5–7 and 1– 2, respectively) ( Figs. 2 View FIGURE 2 H, 3A). Distal setae of the segment are similar to other ones ( Fig. 2 View FIGURE 2 H). The pseudognathobasic process is also similar to that of tl II ( Figs. 2 View FIGURE 2 H, 4D). The anterior setae of the second segment is of “C”- type, being similar to the respective one of tl II ( Figs. 2 View FIGURE 2 H, 4A). Terminal and subterminal setae of third segment are similar to those of tl II but slightly shorter and bear fewer denticles ( Figs. 2 View FIGURE 2 H, 4B, 4C).
Fourth pair of limbs (tl IV) considerably reduced; their protopodite bears slightly spinulated seta sited on a short cylindrical base ( Figs. 2 View FIGURE 2 I, 2J, 3A). The unique segment of the endopodite has two rows of comparatively short spine-like setae. The external row (group) always consists of two setae, and the internal row has 7–9 setae, which differ in their appearance and armament ( Figs. 2 View FIGURE 2 K, 2L, 4E, 4F). Almost the whole internal part of the endopodital segment is occupied by the reduced pseudognathobasic process, also having a pore and armed by some denticles and thin seta ( Figs. 2 View FIGURE 2 I, 4G). The bulbous structure is situated just behind tl IV ( Fig. 1 View FIGURE 1 A).
Abdomen (metasome) ( Fig. 1 View FIGURE 1 A) is often deformed. It is inconspicuously delimited in two segments, a short proximal one and a long distal one with a prominent fold more or less in the middle of the dorsal side.
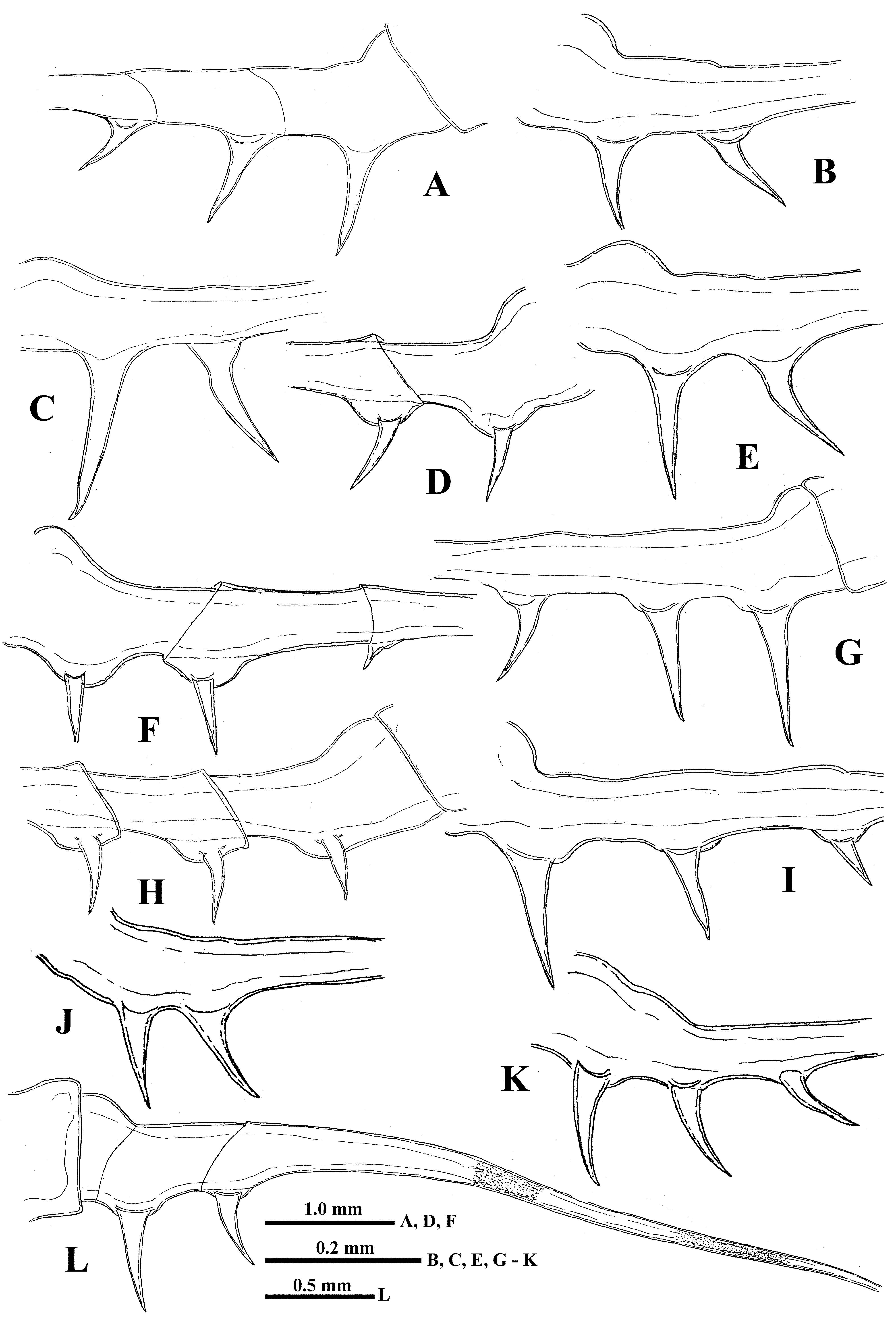
“ Postabdomen”, actually consisting of two parts: the last small abdominal segment and the postabdomen per se (see Korovchinsky (2015)). It is comparatively small; the anal opening is situated between the postabdominal claws ( Fig. 4 View FIGURE 4 I). Postabdominal claws are comparatively large (7.2–19.3 % of body length), slightly curved backwards or more or less straight, directed downwards, sometimes with apex curved slightly forward ( Figs. 5 View FIGURE 5 A- K).
Caudal process is directly connected with postabdomen; it is long, a rather thick and curved proximally, then straight and spine-like ( Fig. 1 View FIGURE 1 A, 1G, 5L), variable in its length (80.7–216.1 % of body length), but typically up to twice the body length. Generally, the caudal process is strongly chitinized and its surface is covered by numerous minute spinulae ( Fig. 4 View FIGURE 4 I), but sometimes it has one or two darker colored and probably more strongly sclerotized sections ( Fig. 5 View FIGURE 5 L). Basally, the caudal process bears one or two pairs of claws similar to those of the postabdomen but usually smaller (e.g., proximal claws reach 4.5–17.0 % of body length), and apically two minute setae arise from a common base ( Fig. 1 View FIGURE 1 H) (they were observed only in juvenile specimens). Pairs of claws are closely situated (e.g., distance between postabdominal claws and proximal claws of caudal process (interclaw distance) constitutes 8.0–17.0 % of body length). Between these pairs of claws, the thickness of the structure is considerable ( Fig. 5 View FIGURE 5 ), reaching 5.5–14.8 % of body length. Borders separating old molted integuments of caudal process with claws either are barely visible or inconspicuous.
Gamogenetic female differ from parthenogenetic one only in the presence of large yellow-brownish resting eggs (0.48–0.59 mm in diameter) in their brood pouches (up to 14 in one female).
Female of first generation hatched from resting egg. Only one such specimen was found in the old sample from Alexandrovsk (Murman) ( Kola Peninsula, northern European Russia) taken in 1899. It had body length 4.70 mm, head length 34.7 %, comparatively shorter tl I (63.7 %) and shorter caudal process (109.3 %) of body length. The distal segment of tl I is comparatively short (17.3 % of body length) ( Fig. 6 View FIGURE 6 J). There are four pairs of small claws (from proximal to distal: 6.8–1.7 % of body length), one on the postabdomen and three on the comparatively thinner caudal process, distance between which is considerably larger (28.1–14.2 % of body length) than in females described above (see Table 1); the posteriormost claws are located much closer to the end of the process ( Fig. 6 View FIGURE 6 K). The setae armament of tl I seems similar to that of females of later generations.
Juvenile females. The investigation of few juvenile females (with only a pair of postabdominal claws) has revealed that they differ from adults in the presence of a comparatively longer caudal process (163.2–298.0 % of body length) and longer postabdominal claws (14.2–19.0 % of body length). On the other hand, their setae on the end of the second endopodital segment of tl I are short ( Fig. 1 View FIGURE 1 M).
Males. Measurements of male specimens are shown in Table 1. Males ( Fig. 6 View FIGURE 6 A), on average, are smaller than females. Thoracic limbs of the first pair (tl I) are comparatively shorter (51.1–80.3 % of body length) as well as each segment of them, especially the distal one (12.2–21.4 % of body length), which is slightly swollen proximally and bears on its inner side a small strongly chitinized hook with two inner denticles, and a field of tiny prominences situated under it ( Fig. 6 View FIGURE 6 B). The copulatory appendages are small (8.0–11.5 % of body length) and armed with numerous minute spinules terminally ( Figs. 6 View FIGURE 6 H, I). Males always have only two pairs of claws which are generally larger and closer to each other than in females ( Table 1, Figs. 6 View FIGURE 6 A, 6C, 6D, 6F, 6G), though sometimes they can be more moved apart ( Fig. 6 View FIGURE 6 E).
Size. Body length of parthenogenetic females 2.8–6.1 mm. Body length of gamogenetic females 2.4–4.1 mm. Body length of juvenile females 2.2–3.8 mm. Body length of males 2.5–4.4 mm.
Differential diagnosis. In comparison with B. longimanus (see Korovchinsky (2015)), the species under consideration possesses much larger body size, shorter tl I (however, in some populations of B. arcticus , the length of the thoracic limbs is comparable with that of the former species) with large setae on the distal ends of their first and second segments. Further, in B. arcticus , the lateral anterior setae of the first segment of tl I–tl III and internal setae of tl IV are more numerous (6–10 vs. 4–7 in B. longimanus ) and the caudal process is conspicuously shorter (80.7– 216.1 vs. 186.0–301.0 in B. longimanus ). Also, the adult females of B. arcticus have more numerous claws (two–three pairs instead of only two pairs in B. longimanus ; females of the first generation of the former species have four pairs of claws instead of three pairs in the latter one) of larger size (mean 11.8–15.5 % and 8.7–11.9 % of body length in B. arcticus vs. 6.2–9.2 % and 7.8–9.9 % of body length in B. longimanus ), which are usually curved backwards, not straight and directed downwards as in B. longimanus .
Another recently redescribed species, B. cederströmii ( Korovchinsky 2015) possesses very prominent differences from B. arcticus , having very long caudal process with a denticulated curve. Its postabdominal and caudal claws are much larger, curved forward and sit distantly from each other. The representatives of the supposed hybrid form B. cederströmii x B. arcticus ( B. cederströmii x “ B. crassicaudus” in Korovchinsky (2015)) differ from B. arcticus in the presence of a similar long caudal process with large claws and denticulated curve, though the shape of the latter can vary considerably up to its disappearance and presence instead of only enlarged denticles.
Remarks. Lilljeborg (1901) correctly described the morphological peculiarities of B. arcticus (“ B. l. arcticus ”): large body size; presence of numerous setae on the first segment of endopodite of thoracic limbs; comparatively short and basally thick caudal process; comparatively low and basally wide brood pouch; and inconspicuous borders of old molted integuments of caudal process. Sars (1903) specified these features, emphasizing the specificity of the claw’s shape and occurrence of the species in small and shallow water bodies.
Ischreyt’s (1930, 1934) individuals of B. arcticus from Lappland (Northern Sweden) were of medium size (mean length = 2.93 mm in females and 2.91 mm in males) with characteristic not long tl I, especially in males, and number and structure of their setae. He mentioned long and strong claws of the caudal spine, the anterior of which are directed forward, and which sometimes was observed in B. arcticus during the present study (see Figs. 5 View FIGURE 5 C, 5G).
The following descriptions of B. arcticus (Manuilova 1964; Mordukhai-Boltovskoi & Rivier 1987; Rivier 1998) were sporadic, brief and partly incorrect. Only Vekhov (1981, 1987) presented new data on the species (“ B. l. arcticus ”) focusing on ecology, life cycle, growth, and reproduction, while his morphological analysis was limited. In particular, he described especially large females of the first generation with four pairs of claws and well developed brood pouch, and mentioned the presence of red-cherry blossom color of caudal spine with 3-4 darker colored stripes, which probably represent stronger sclerotized sections of the spine.
Litvinchuk (2002, 2007) erroneously included the hybrid forms (“ B. cederströmii x B. crassicaudus ”) in the synonyms of B. arcticus , thus confusing the understanding of the taxon. At the same time, her brief morphological diagnosis of the species was correct.
Intra- and interpopulation variability. The variability of claw size, interclaw distance and interclaw thickness is especially high ( Table 1). This is especially prominent in specimens from Sor Vusrenohnor (Western Siberia) and Karesuando (Northern Sweden). The length of the caudal spine is highly variable in Karesuando and in specimens from the six combined populations. The latter index stresses the significance of interpopulation variability of this parameter. The longest tl I, caudal spine, and claws are present in specimens from Lake Lolyalaty (north-east of European Russia). Unusual claws with slightly forward curved apical tips were noted in specimens from Pustozersk (north-east of European Russia) and Puiko (Jamal Peninsula, north of Western Siberia).
Specimens from some Scandinavian populations (Forelenmägen ( Norway), Råbosjön (Hälsingland, Sweden), Undersäker Ottsjön (Jämtland, Sweden), and Vombsjön (Skåne, Sweden)) differed from the others by comparatively small body size (2.6–3.0 mm) and small claws. One specimen from Undersäker Ottsjön had an unusually long caudal process (251.5 % of body length) and tl I (108.3 % of body length) with short setae on the end of the second segment of the endopodite. Because of these differences and occurrence in more southern latitudes, in central and southernmost part of Scandinavian Peninsula, the representatives of these and other populations of the regions should be investigated in more detail to check their conspecificity with B. arcticus .
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Bythotrephes arcticus Lilljeborg, 1901
| Korovchinsky, Nikolai M. 2016 |
Bythotrephes longimanus crassicaudus
| Litvinchuk 2001: 129 |
Bythotrephes crassicauda
| Ischreyt 1934: 277 |
Bythotrephes longimanus arcticus
| Vekhov 1981: 74 |
| Ischreyt 1930: 321 |
Bythotrephes arctica
| Sars 1903: 186 |
Bythotrephes longimanus var. arcticus
| Rivier 1998: 173 |
| Mordukhai-Boltovskoi 1987: 152 |
| Lilljeborg 1901: 612 |
Bythotrephes longimanus var. arctica
| Lilljeborg 1901: 24 |
Bythotrephes longimanus
| Ekman 1904: 28 |
| Stenroos 1897: 67 |