Mortogenesia mesopotamica
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3741.2.5 |
|
publication LSID |
lsid:zoobank.org:pub:EA93BFB6-CE2F-4FDF-862E-18E1935DD6B4 |
|
DOI |
https://doi.org/10.5281/zenodo.6147937 |
|
persistent identifier |
https://treatment.plazi.org/id/D0644B6B-EC3A-D66B-29DD-FF33FE069987 |
|
treatment provided by |
Plazi |
|
scientific name |
Mortogenesia mesopotamica |
| status |
|
Biology of Mortogenesia mesopotamica View in CoL
Habitat preferences. Like other genera of the family Palingeniidae (note that only Palingenia and perhaps Anagenesia Eaton, 1883 are known from the ecological/ethological point of view), larvae of M. mesopotamica occur solely in large permanent lowland rivers and adequate substrate. These rivers exhibit rather variable physical-chemical characteristics. The river depth ranges within at least 3–5 m, with maximum in April and May. Maximal depth can reach exceptionally more than 10 m (e.g. the Tigris River in spring 1987). Naturally, this fluctuation is accompanied by changes of current velocity and turbidity. Tubes of larvae do not occur at places with current velocity lower than about 10 cm.s -l or at places with intensive sedimentation of solid particles and organic debris. Water temperatures reach 22–23°C in summer and drop to 14°C in winter; water remains slightly alkaline or neutral all year round (Dr. Emad Almukhtar, personal communication).
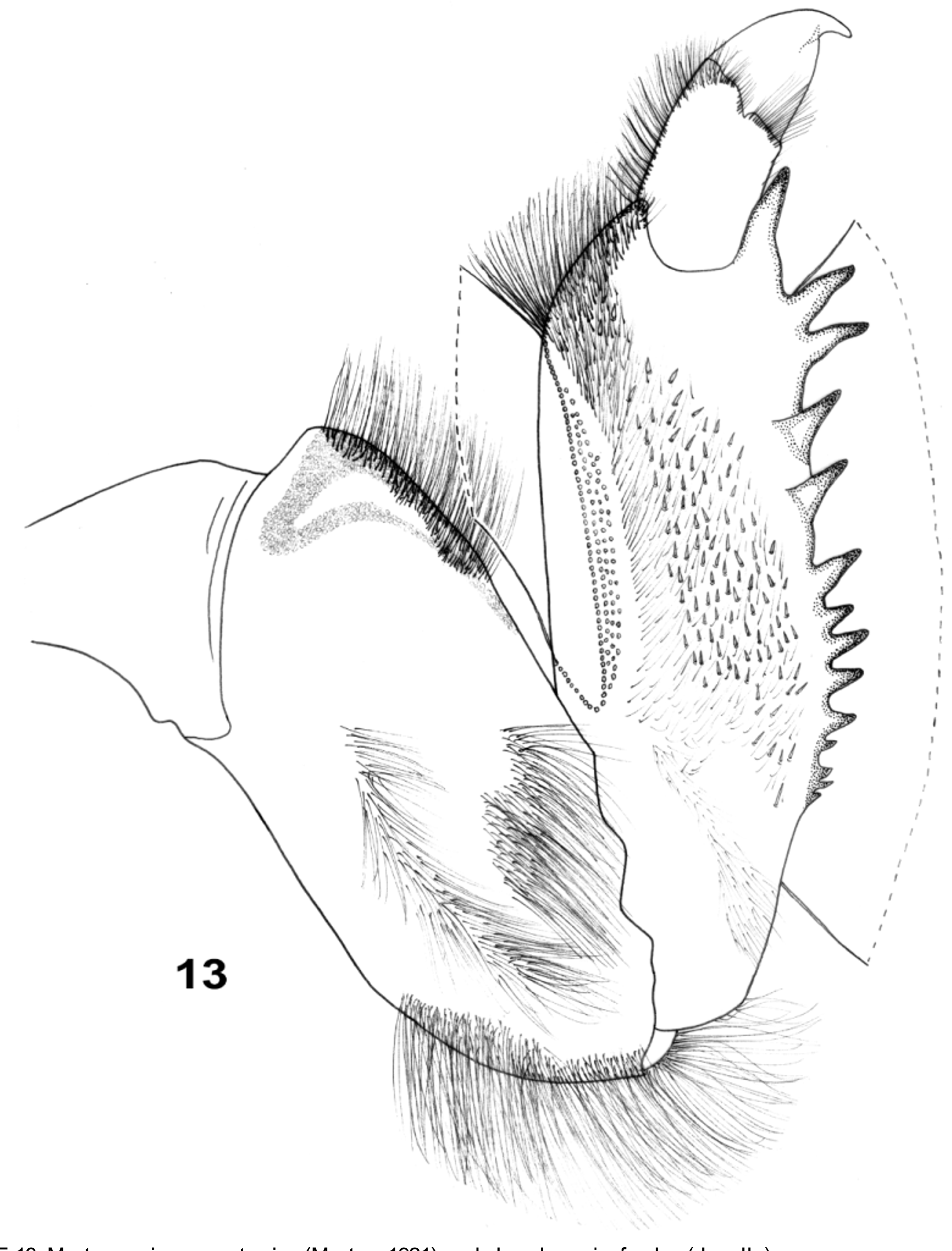
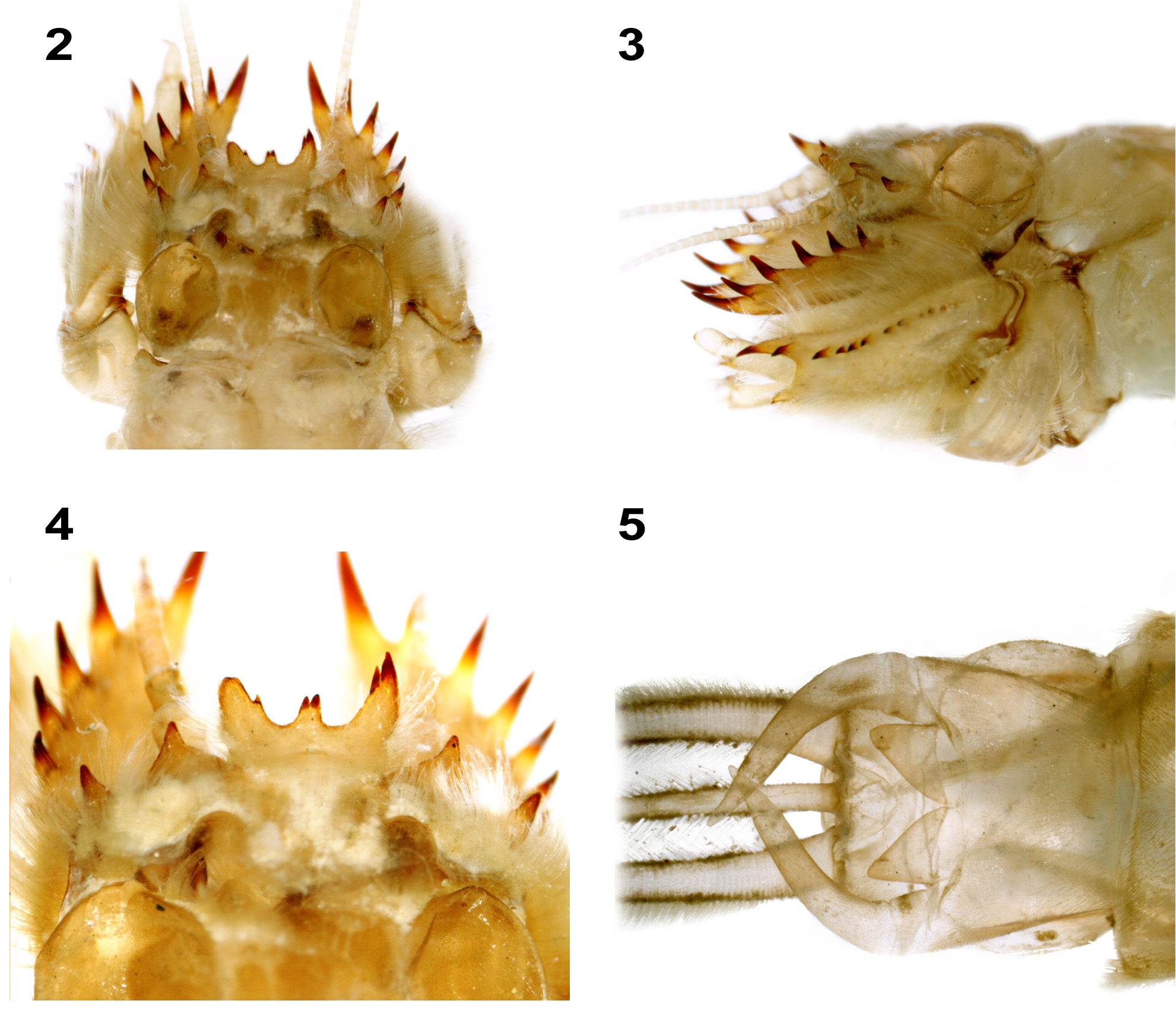
Judging from morphological characters, the larvae of Mortogenesia belongs, analogously to other genera of the family (and superfamily), to the “burrowing” larval type (see mandibular tusk and heavily sclerotized spines of foretibiae in Figs 2–4 View FIGURES 2 – 5 , 7, 8 View FIGURES 6 – 8 , 13 View FIGURE 13 ). They make U-shaped tubes in clayey or muddy sediments. At some places the whole bottom surface of the Tigris River is covered with pairs of tube openings. However, larvae do not inhabit sediments with particles exceeding 0.025–0.075 mm, simply because they are not able to construct their tubes.
Nevertheless, the question of the density of larvae in respective habitats is rather enigmatic. Despite mass occurrence of adults and locally numerous tubes, which are quite analogous to those made by Palingenia larvae, nobody has managed to sample fully grown larvae. The characters described above are based on exuviae collected by chance at Huwaish locality, because despite a considerable collecting effort and time spent there, we failed to collect living larvae. Moreover, several tens of dredge (Ruttger) samples regularly taken from the Tigris River bottom have not included any larvae of M. mesopotamica (Dr. Emad Almukhtar, personal communication). Nevertheless, larval density can be roughly estimated at least one or two hundreds per square meter in places with dense occurrence of tubes.
Life history. Our knowledge on life history of M. mesopotamica is very fragmentary. Judging from approximately the same size of the eggs and females body in Palingenia and Mortogenesia , the fecundity of females is most probably equal (about 6000–8000 eggs per female; Soldán & Landa 1986), probably being smaller in populations from Iran and the Euphrates River. Morphological characters of egg surface (presence of an adhesive membrane) indicate that the mechanism of fixing the eggs to substrate is the same as in Palingenia (cf. Soldán & Landa 1986; Russev 1987; Gaino & Bongiovanni 1993; Landolt et al. 1995).
Nothing is known about the embryonic and larval development. Within the Palingeniidae , there are 3-years cycles in Palingenia , much shorter, probably one-year cycle at least in some species of Anagenesia and tropic genera Cheirogenesia and Plethogenesia (Clifford 1982; Soldán & Landa 1986; Russev 1987; Sartori & Elouard 1999; Soldán et al. 2009). It is also possible that some aspects of Mortogenesia biology, e.g. feeding and swarming behavior, characteristics of growth and spatial distribution, habitat preference of larvae, as also as the data on adults biology, are closely related to those of the North American palingeniid’ genus Pentagenia (Keltner 1983; Keltner & McCafferty 1986; Braaten & Guy 1997).
Since Mortogenesia adults emerge regularly each year, 1–2 year but strictly seasonal and fixed cycle can be supposed (see life cycle classification in Clifford 1982).
Adults are known to occur only several days in March in the Tigris river-basin. All the material being at disposal was collected solely in March in Iraq. However, in Iran (Karkheh River) the swarming evidently occurred in February. Although the emergence of adults was not studied in detail, a typical behaviour very similar to those of the genus Palingenia (cf. Russev 1987; Landolt et al. 1997) was observed by Dr. M. S. Abdul Rassoul (pers. comm.). Adult emerged from water surface using larval exuviae as rafts. Males exhibited a typical “patrol” flight in a very short distance from the water surface. Some specimens were observed to flight in 1–2 m above the river (most probably compensatory upstream flight of females after copulation). It is unknown if males are sitting during subimaginal molting like males in Palingenia .
Emergence, mating flight and oviposition take place at daylight from about 10 a.m. in the morning to the noon or very early in the afternoon. This pattern is similar to some subtropic and tropic species (e.g., Palingenia orientalis, Sartori 1992 ) and different from temperate representatives of Palingenia ( P. longicauda , P. fuliginosa ) which emerge and swarm before sunset or shortly later (Soldán 1978; Russev 1987). Emergence is very early also from the seasonal point of view; it occurs practically by the end of winter when both water and air temperatures are relatively very low.
The unique phenomenon in Mortogenesia seems to be possible reduction of imaginal stage. The only (subimaginal) winged instar is known to occur in Palingenia (Edmunds & McCafferty 1988; Kluge 2004), but solely in females. Also Kluge (2004: 254) noted that “some specimens [of males of Anagenesia ] do not moult at all”; on the other hand for all species of Chankagenesia the presence of male imago is confirmed (Buldovsky 1935a: 834; 1935b: 160; 1935c: 124; Tshernova 1952: 243–246). Preliminary dissection of some male body parts (wings, legs, cerci and forceps) of Mortogenesia seems to indicate that teneral adult cuticle is missing. Neoteny of males is documented also by simultaneous occurrence of male “subimagoes” and females (with no or only several eggs in abdominal cavity) after oviposition, which never occurs in related genus Palingenia (Soldán & Landa 1986; Russev 1987; Bauerfeind & Soldán 2012). This hypothesis should be verified by electron micrographs of transversal sections through male subimaginal cuticle.
The early daily and seasonal emergence, and neoteny could be explained as an adaptation to extreme climatic conditions (arid or even desert abiotic factors) and/or protection from predators. While the aquatic environment is relatively constant and comparable to that of other Palingeniidae , “terrestrial” conditions, where mating and compensatory flight is realized, exert a very strong environmental pressure of extreme temperatures (even more than 45°C) and extremely low humidity which can be tolerated by adult mayflies only for several minutes. Moreover, most predators (including fish and birds) are active after the sunset. These would be the reasons for the general elimination of subimaginal instar and shift of emergence to the morning and to early spring months. Similar tendency to reduce a subimaginal stage was documented in Cloeon species in arid areas (Soldán 1987).
Acknowledgements
We are grateful to Drs Zohair H. Mohsen, Emad H. Almukhtar and Nazair A. Ouda of the Biological Research Centre, Baghdad, Iraq who provided us with material and valuable information on Mortogenesia biology. The material and biology data provided by Dr. M.S. Abdul-Rassoul of the Iraq Natural History Museum, Baghdad are much appreciated. We would like to thank Dr. Jindřiška Bojková of the Masaryk University, Brno for valuable comments on the manuscript.
This study was supported by the Grant Agency of the Czech Republic (Project No. 206/08/1389) and was conducted with institutional support RVO:60077344; it was possible thanks to the interacademic exchange between the Academy of Sciences of Czech Republic and the Russian Academy of Sciences (Southern Scientific Center, Rostov-na-Donu, RF).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |