Aequorea taiwanensis Zheng et al., 2009
|
publication ID |
https://doi.org/10.35929/RSZ.0049 |
|
DOI |
https://doi.org/10.5281/zenodo.5710633 |
|
persistent identifier |
https://treatment.plazi.org/id/D0118A7C-5B4F-0051-FC41-FDF1FA8D7919 |
|
treatment provided by |
Felipe (2021-11-02 22:36:03, last updated by Carolina 2025-01-03 17:31:27) |
|
scientific name |
Aequorea taiwanensis Zheng et al., 2009 |
| status |
|
Aequorea taiwanensis Zheng et al., 2009 View in CoL View at ENA
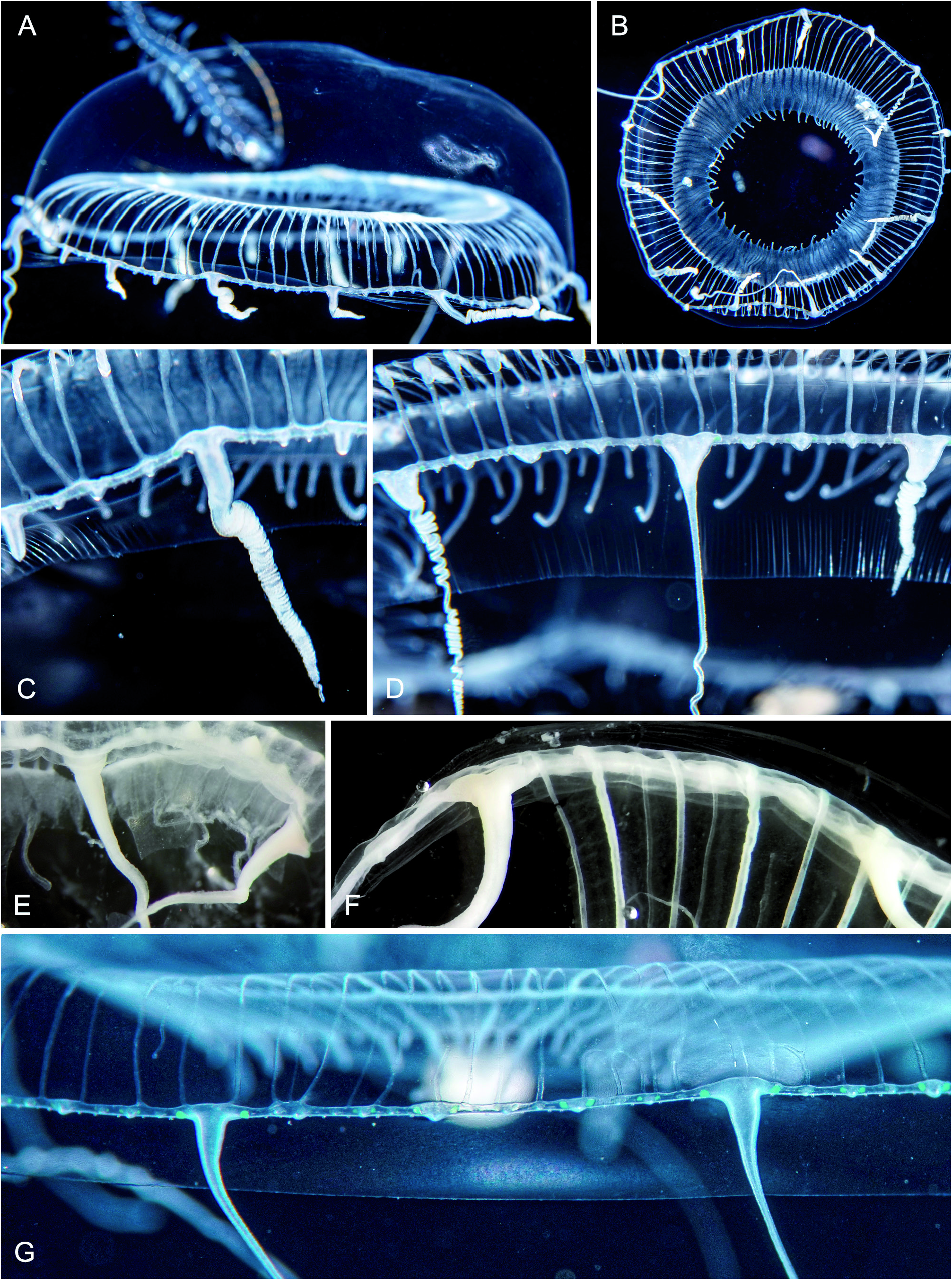
Fig. 41 View Fig A-G
Aequorea taiwanensis Zheng et al., 2009: 110 View in CoL , fig. 1. – Zheng et al., 2014: 63, 16S ML tree.
Material examined: BFLA4105 ; 1 specimen; 27-MAY- 2019; size 27 mm, beginning gonad development; part preserved in formalin and deposited as UF-013793, part preserved in alcohol for DNA extraction; 16S sequence MW528674 View Materials . – BFLA4173 ; 1 specimen; 09-AUG-2019; size 30 mm; part preserved in formalin and deposited as UF-013820 , part preserved in alcohol for DNA extraction; 16S sequence MW528685 View Materials . – BFLA4308 ; 1 specimen; 16-JAN-2020; size 31 mm, gonads visible; part preserved in formalin and deposited as UF-013846 , part preserved in alcohol for DNA extraction; 16S sequence MW528705 View Materials . – BFLA4332 ; 1 specimen; 31-JAN-2020; size 40 mm, gonads visible; part preserved in formalin and deposited as UF-013890 , part preserved in alcohol for DNA extraction; 16S sequence identical MW528705 View Materials . – BFLA4425 ; 1 specimen; 28-MAY-2020; size 50 mm, gonads visible; part preserved in formalin and deposited as UF-014051 , part preserved in alcohol for DNA extraction; 16S sequence identical MW528705 View Materials . – 1 specimen photographed 07-FEB-2020, not collected; size 30 mm.
The formalin samples are mostly strongly fragmented and damaged.
Observations: Subadult Aequorea medusae with bell diameters 30 to 50 mm, gonad development starts at about 30 mm size. Bell relatively high, apical jelly about 1/2 of bell height. Stomach wide, 7/10 of bell diameter. Mouth rim with numerous, long, thin fimbriae ( Fig. 41B View Fig ). Radial canals thin, 100 to 240, often also with 15 to 100 developing centrifugal canals. 14 to 25 fully developed tentacles, between pairs of tentacles 3-5 small bulbs without tentacles. Ratio of radial canals to tentacles 7.5 to 10, thus always many more radial canals than tentacles. Tentacles in life either with a distinct conical basal bulb with a slight depression of upper side ( Fig. 41D View Fig ) or widened laterally to give a T-shape ( Fig. 41C, G View Fig ). Abaxial spurs absent. Small, abaxial excretory papillae can be present, only seen in preserved material. Tentacle bases in preserved material usually also with lateral expansions ( Fig. 41F View Fig ), but some simply conical ( Fig. 41E View Fig ). Statocysts about as numerous as radial canals but not in phase with them, two statoliths per statocyst.
16S data: The three haplotypes had a range of base pair divergences of 0.2 to 0.5 % ( Table 1 View Table 1. 16 ). The maximum likelihood tree of the partial 16S sequences ( Fig. 37 View Fig ) identified them as very closely related to A. taiwanensis (p-distances 0.2-0.64%).
Distribution: Taiwan strait, Florida (this study). Type locality: Taiwan Strait.
Remarks: This material resembles very much Aequorea pensilis ( Haeckel, 1879) , only the lateral expansions of the bulbs are shorter and sometimes absent in preserved material, and small excretory papillae can be present. Our identification of the present material as A. taiwanensis was based on the strong similarity of the 16S sequences ( Table 1 View Table 1. 16 ). The haplotype divergences to the published sequences from Taiwan Strait were only 0.2 to 0.64%, while within the Florida population we found a maximal value of 0.5%, thus intra- and interpopulation divergences are in the same range. In the ML tree ( Fig. 37 View Fig ) the distance of the two population appears higher than the values obtained by pairwise comparisons. This is due to the fact that another substitution model was used for the distance calculation and more importantly, the sequences from the Taiwan Strait specimens were 112 bases shorter at the 3’ end, leading to a bias in the ML analysis. While the similarity of our sequences with A. taiwanensis almost certainly implies that our material is conspecific, it must be noted that so far no Aequorea pensilis 16S sequences are available. It might turn out that A. taiwanensis is in fact a synonym of Aequorea pensilis . Aequorea taiwanensis , according to the description in Zehng et al. (2009), resembles A. pensilis but lacks the diagnostic long lateral expansions of the tentacle base and it has excretory papillae (comp. Browne, 1905; Maas, 1905; Kramp, 1968: Fig. 268). In our material, the lateral expansions were present, but also not as wide as usually shown for A. pensilis . Our material is thus intermediate between A. pensilis and A. taiwanensis . The specimens of Zheng et al. (2009) measured only 25 mm, were thus likely younger than ours, which reached up to 50 mm diameter. Pacific Aequorea pensilis reach 100 mm in diameter ( Kramp, 1968).
The Atlantic occurrence of a rare Aequorea medusa of the Western Pacific Ocean is surprising, but not unparalleled. Pruski & Miglietta (2019) recently found Aequorea australis in the Gulf of Mexico, a species formerly only known from the Indo-Pacific Ocean.
The presence of A. taiwanensis in the Atlantic Ocean must not be interpreted as a possible recent introduction. While we found three different haplotypes, the four samples from Taiwan Strait ( Zheng et al., 2014) represented only a single one. The higher haplotype diversity in the Altantic argues against a recent introduction from the Pacific. The species has likely a wide distribution.
Browne E. T. 1905. Hydromedusae with a revision of the Williadae and Petasidae. Fauna and geography Maldives and Laccadives Archipelagoes 2 (3): 722 - 749, pls 54 - 57.
Haeckel E. 1879. Das System der Medusen. Erster Teil einer Monographie der Medusen. Denkschriften der Medicinisch- Naturwissenschaftlichen Gesellschaft zu Jena 1: I-XX, 1 - 360, 20 pls.
Kramp P. L. 1968. The hydromedusae of the Pacific and Indian oceans. Sections II and III. Dana Report 72: 1 - 200.
Maas O. 1905. Die Craspedoten Medusen der Siboga-Expeditie. Siboga Expeditie 10: 1 - 84, pls 1 - 14. DOI: 10.5962 / bhl. title. 11301
Pruski S., Miglietta M. P. 2019. Fluctuation and diversity of Hydromedusae (Hydrozoa, Cnidaria) in a highly productive region of the Gulf of Mexico inferred from high frequency plankton sampling. PeerJ 7: e 7848. DOI: 10.7717 / peerj. 7848
Zheng L., Lin Y., Li S., Cao W., Xu Z., Huang J. 2009. Aequorea taiwanensis n. sp. (Hydrozoa, Leptomedusae) and mtCOI sequence analysis for the genus Aequorea. Acta Oceanologica Sinica 28: 109 - 115.
Zheng L., He J., Lin Y., Cao W., Zhang W. 2014. 16 S rRNA is a better choice than COI for DNA barcoding hydrozoans in the coastal waters of China. Acta Oceanologica Sinica 33: 55 - 76.
Fig. 41. Aequorea taiwanensis. (A-B) BFLA4308, size 31 mm. (C) BFLA4308, bell margin. (D) BFLA4332, bell margin. (E) BFLA4308, bell margin after preservation. (F) BFLA4332, bell margin after preservation. (G) Bell margin of animal photographed 07-FEB-2020, size 30 mm; the green dots flanking the bulbs are likely due to interference effects and not a pigment.
Fig. 37. 16S maximum likelihood phylogenetic tree of the genus Aequorea and related genera obtained with PhyML (GTR+G+I model) using about 600 bp positions of the mitochondrial 16S gene. Node-support values are bootstrap values of 100 pseudoreplicates (shown only if> 70%). Sequence labels start with the GenBank numbers (except for identical haplotypes) permitting the retrieval of more information. Red labels are new sequences from this study, for the taxa in bold either a voucher specimen or photos of it have been examined. Notes: *1) Unpublished, L. Leclère, pers. comm. *2) See Material & Methods. *3) Could be a misidentification. *4) Unpublished, A. Hosia & L. Martell, pers. comm., see Fig. 39A.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Aequorea taiwanensis Zheng et al., 2009
| Schuchert, Peter & Collins, Richard 2021 |
Aequorea taiwanensis
| Zheng L. & He J. & Lin Y. & Cao W. & Zhang W. 2014: 63 |
| Zheng L. & Lin Y. & Li S. & Cao W. & Xu Z. & Huang J. 2009: 110 |
1 (by felipe, 2021-11-02 22:36:03)
2 (by ExternalLinkService, 2021-11-02 22:47:33)
3 (by tatiana, 2021-11-04 20:38:56)
4 (by felipe, 2021-11-05 00:44:58)
5 (by tatiana, 2021-11-09 19:41:39)
6 (by tatiana, 2021-11-16 13:41:43)
7 (by tatiana, 2021-11-18 14:02:52)
8 (by ExternalLinkService, 2021-11-18 14:16:41)
9 (by ExternalLinkService, 2021-11-22 19:15:25)
10 (by ExternalLinkService, 2021-11-22 19:55:07)
11 (by ExternalLinkService, 2023-05-08 20:51:13)
12 (by plazi, 2023-11-08 05:13:57)
13 (by ExternalLinkService, 2023-11-08 18:18:25)
14 (by guilherme, 2024-10-09 15:17:54)