Arbocuspis multicornis ( Hincks, 1881 ), 2016
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4282.1.5 |
|
publication LSID |
lsid:zoobank.org:pub:049026D4-2D2E-4443-98ED-FD7B4320A976 |
|
DOI |
https://doi.org/10.5281/zenodo.6001251 |
|
persistent identifier |
https://treatment.plazi.org/id/C11C8789-FFA7-FF99-FF6A-2001FB88FF39 |
|
treatment provided by |
Plazi |
|
scientific name |
Arbocuspis multicornis ( Hincks, 1881 ) |
| status |
|
Arbocuspis multicornis ( Hincks, 1881)
( Figs 26–38 View FIGURES 26 – 35 View FIGURES 36 – 38 )
Membranipora bellula var. multicornis Hincks, 1881: 149 , fig. 4b Electra bellula (Hincks) View in CoL : Hayward & Ryland: 1995, 537, fig. 4A.
Material examined. Holotype: NHMUK 1899.5 About NHMUK .1.690, lobes of a partly preserved colony on an algal thallus from unknown Australian locality, Recent. PMC. Additional specimens: Rosso Australia Collection, H.B.73 a: three colonies encrusting a soft algal lamina washed on the beach at Coral Bay , Western Australia, 23°142’ S, 113° 769’ E, Recent.
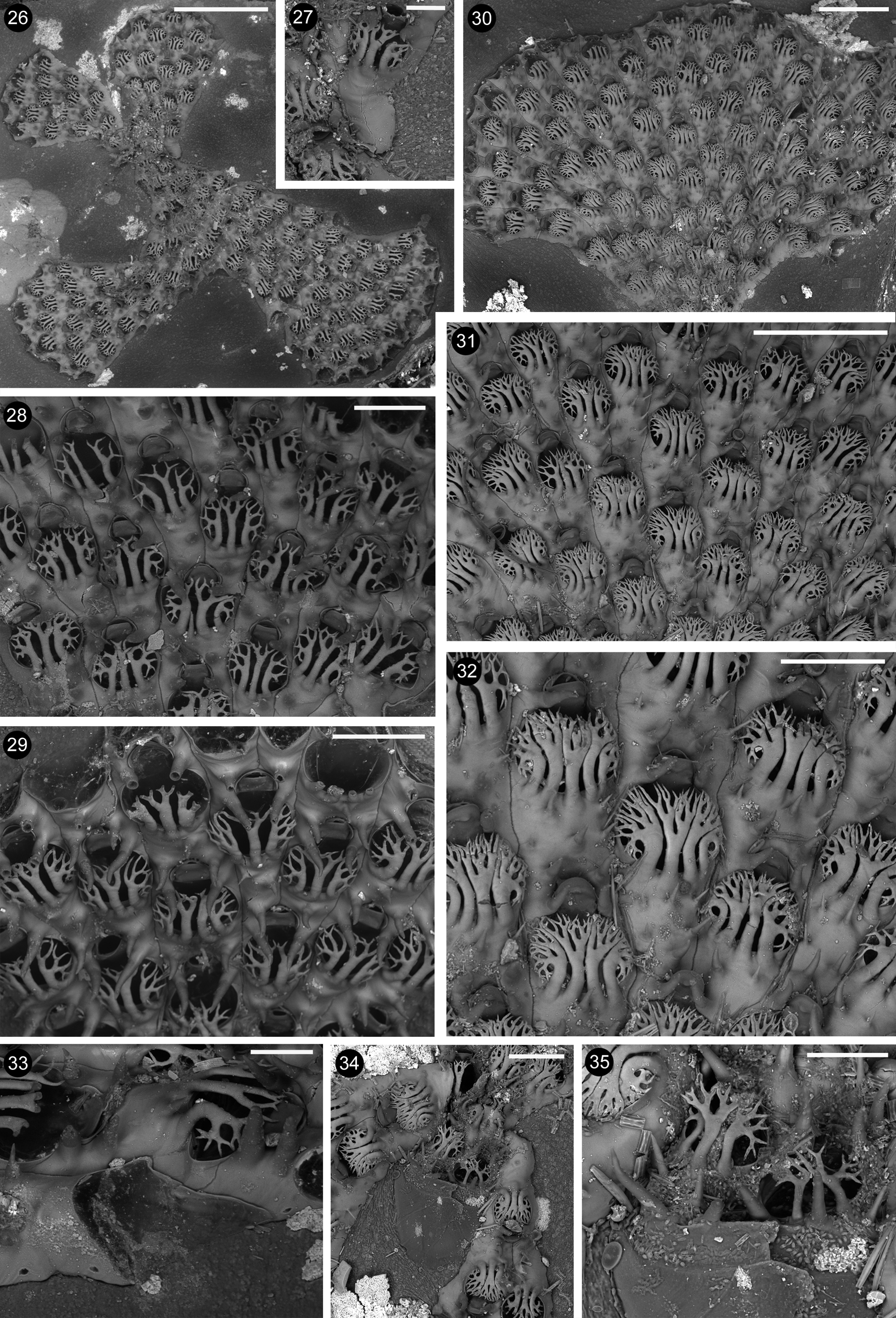
Description. Colony encrusting, unilaminar, pauci– to multiserial, fan–shaped to stellate ( Fig. 26 View FIGURES 26 – 35 ); lobes may rapidly increase in width, to include more than 20 transversal zooidal rows ( Fig. 30 View FIGURES 26 – 35 ); zooids usually alternating in contiguous rows ( Figs 28–32 View FIGURES 26 – 35 ). The first lobe is produced distally to the ancestrula and a second one grows in the opposite direction, budded from a lateral zooid from the first lobe ( Fig. 27 View FIGURES 26 – 35 ). Additional lobes (up to four in the present material) may start from near the ancestrula, produced from zooids situated along the edges of previous lobes.
Autozooids lightly calcified, whitish and vitreous, with lateral boundaries marked by thin furrows and transversal boundaries coincident with the distal rim of the opesia and opercula, but not visible along the edges of the lobes. Autozooids elongate, 0.331–0.537; 0.451± 0.048 mm long, and 0.171–0.228; 0.203± 0.013 mm wide, at their mid high. Opesium distal, pyriform, narrower distally than proximally, and rounded to elongated 0.154– 0.261; 0.222± 0.026 mm long and nearly as wide as zooids. Opesia of zooids located along the lobe edges, varying in a narrower range (0.162–0.211; 0.188±0.012). Proximal gymnocyst occupying one third to half of zooidal length, 0.176–0.285; 0.228±34 mm long, proximally ending as one or two deeply wedged portions lateral to the opesium of the preceding zooid. Gymnocyst narrow (0.010–0.020 mm) and deeply sloping laterally, usually absent distally in correspondence of the opesia. Gymnocyst more extensively developed along outer sides of zooids forming the lobes’ edges. Surface smooth, occasionally slightly undulated transversally. Cryptocyst absent. Opesium bordered by a thin raised gymnocystal rim.
In most zooids three large calcified branching spines are placed close to the proximal rim of the opesia ( Figs 28–29 View FIGURES 26 – 35 ), overarching the frontal membrane, and bending distally towards the base of the operculum, as a cage–like shield protecting the frontal membrane. Spines show robust and flat main branches, which are longitudinally parallel to each other or slightly curved laterally. Central spines branch after their mid–length, bifurcating two-tothree times, usually giving raise to eight pointed tips. Lateral spines branch at about the same level to the central ones, forming median larger branches bifurcating often nearly symmetrically, plus smaller lateral branches, which bifurcate less and more irregularly, with several tips bending laterally and even proximally. Four and even five spines have been observed on some zooids clustered in the older parts of some lobes ( Figs 31–32 View FIGURES 26 – 35 ). These spines are in close contact to each other to form a nearly continuous roof over the frontal membrane; each spine extensively branches, including a trifurcation pattern. Two spines were seen only on the first couple of periancestrular zooids.
In addition, robust elongate conical spines, up to 0.060–0.070 mm long, usually forming at a right angle to the frontal surface, are located proximally on the gymnocyst of each zooid (usually reducing from four to two on coupled zooids at the beginning of new rows), the most proximal one(s) placed lateral to the orifice of the preceding zooid, and slightly converging towards midline. These spines give the impression of being oral spines during initial observations. One to five additional, shorter and thinner spines may be present on the gymnocyst.
Kenozooids not observed, but incomplete abutting zooids ( Fig. 33 View FIGURES 26 – 35 ) may be present along the lobes’ edges, generated distolaterrally to a zooid and coupled with a zooid. They have an extensive gymnocyst usually with a single proximal spine and are closed by a distal, askew placed, membrane.
Communication pores were not observed. Budding loci scattered along the walls of marginal zooids ( Fig. 33 View FIGURES 26 – 35 ). New branches seem to arise from them, starting with a single zooid growing opposite to the growing direction of the previous lobe.
Ancestrulae were damaged and only visible in one colony ( Figs 34–35 View FIGURES 26 – 35 ), consisting of a large rounded (0.345 mm long and 0.348 mm wide) flat lightly calcified bulge ending distally in a fissure-like opening ornamented with spines. Possible vertical walls seem to be present internally, only visible through the light microscope. From the ancestrula two zooids are distally budded, which are decidedly smaller than the subsequent ones.
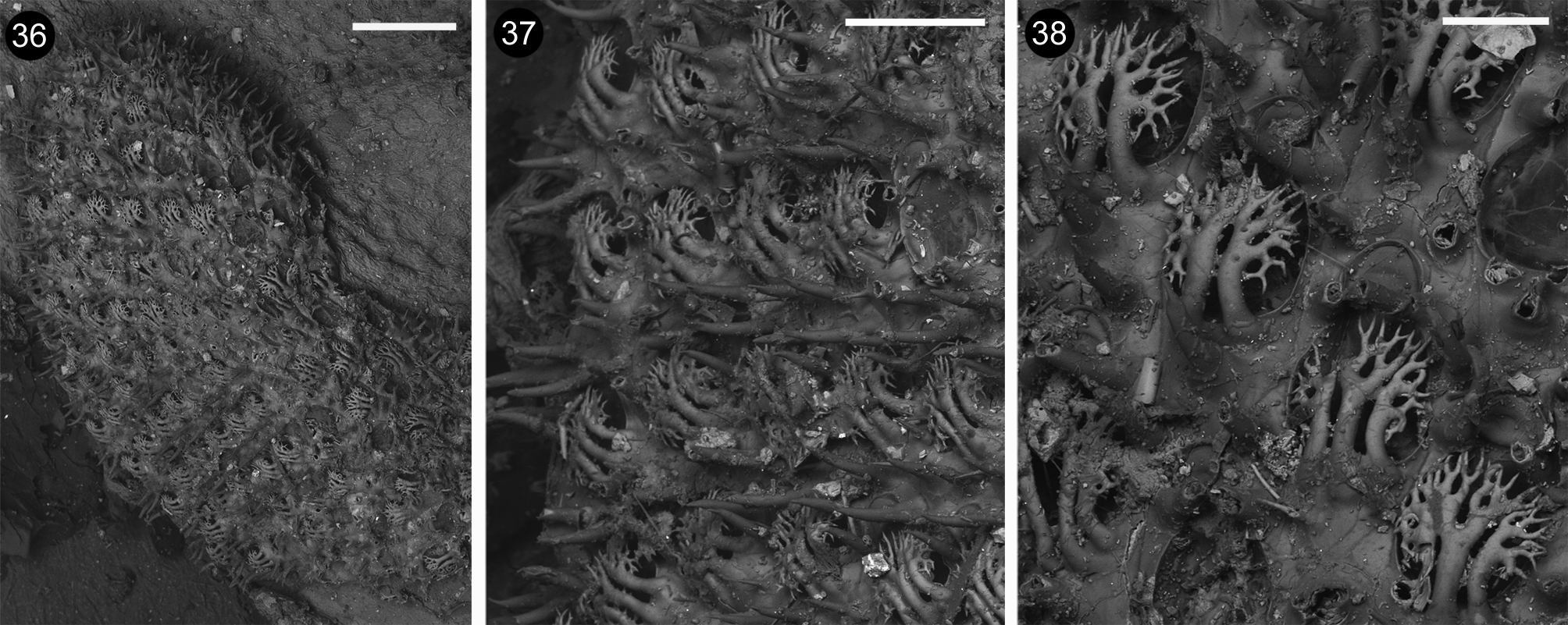
Remarks. The recently collected specimens from Coral Bay , Western Australia, fit well to the characters observable on the type material ( Figs 36–38 View FIGURES 36 – 38 ), and to the original description of Membranipora bellula var. multicornis Hincks, 1881 for the ‘ Opercular spines 3–5, placed closely together, their numerous dichotomous branchlets combining to form a beautiful protective shield, which extends to the base of the oral valve’ ( Hincks , 1881). Indeed , three-to-five spines have been observed on zooids from Coral Bay . Nevertheless, some zooids placed near to the growing edge, in the holotype, show only two branched spines, which still exhibit strong and flattened longitudinally elongated main branches, and the branching pattern characteristic of A. multicornis . A certain difference relates to the length of the gymnocystal spines encircling the orifices, which are somewhat smaller than those visible on the holotype ( Fig. 37 View FIGURES 36 – 38 ) and mostly than those figured by Hincks (1881: fig. 4b). Hincks (1881) also illustrated a sporadic very long gymnocystal spine, which has been never observed in the examined material from Coral Bay, and is actually lacking on the holotype of A. multicornis . In contrast, the morphology of the branched spines forming a heavy cage–like roof above the opesia is remarkably distinctive for A. multicornis and allows to easily distinguish the species from all other congeners. The opesium is more rounded in the material from Coral Bay, and more elongated in the holotype ( Figs 37-38 View FIGURES 36 – 38 ), although not so markedly as suggested by Hincks’ (1881) drawings. However, the examination of further material would be useful for evaluating the possible erection of a separate species.
Similarities exist mostly between A. emanuelae n. sp. and A. multicornis that have a high number of spines starting proximally to the opesium. Nevertheless, in A. emanuelae n. sp. only the two lateral–distal spines branch whereas the other ones end in a single point. Furthermore, the opesial rim is thin and elevated but not crenulated.
Originally described in 1881 from an unknown Australian locality ( Hincks, 1881), A. multicornis has been virtually never recorded since then. The material described and figured by Hayward & Ryland (1995) as Electra bellula (Hincks) (their Fig. 4 View FIGURES 2 – 4 A), however, clearly belongs to this species. Its origin from Heron Island, off East Australia in the Pacific Ocean, suggests that Hincks’ material (from an unknown Australian locality) was collected near that area. Hayward & Ryland (1995) also accepted the suggestion of Livingstone (1927) that Membranipora cervicornis Haswell, 1881 , reported again from Queensland, is conspecific with M. bellula Hincks. Indeed, Haswell (1881) described M. cervicornis as having ‘the aperture protected by three to five closely approximated branching, antler–like process (sic), which arise from one side of the cell and almost entirely hide the mouth’. Consequently, it is possible that those colonies were conspecific with A. multicornis . The identity of populations encrusting the thallus of Amphibolis sp. and Dictyopteris sp. algae from Perth, analysed for clearance capacity by Lisbjerg & Peterson (2000), and indicated as the variety multicornis of E. bellula , remains to be ascertained. The specimen from Saint Francis Island, South Australia figured by Bock (2016) has been considered similar to A. multicornis . Nevertheless, only two, particularly slender and relatively poorly branched spines, which only partly cover the markedly elongated opesia, are present on these zooids. Owing to these differences, this specimen could belong to a different species whose identity needs to be ascertained after examination of further material.
The ancestrula is figured for the first time not only for the species, but also for the genus. It differs greatly from already known ancestrulae in cheilostome bryozoans and from that reported by Vieira et al. (2016) for A. bellula , which is described as having a gymnocyst smaller than autozooids and possessing eight spines. Single ancestrulae have been also reported ( Vieira et al., 2016) although not figured for A. bicornis and A. ramosa . Somewhat similar ancestrulae were recognized and described by Cook (1985) from not clearly identifiable colonies belonging to ‘ E. bellula’ from the ‘British Museum Collection’. Nevertheless, the swollen bulging ancestrula from Coral Bay is larger than those described by Cook (1985) as 0.200-0.300 mm long.
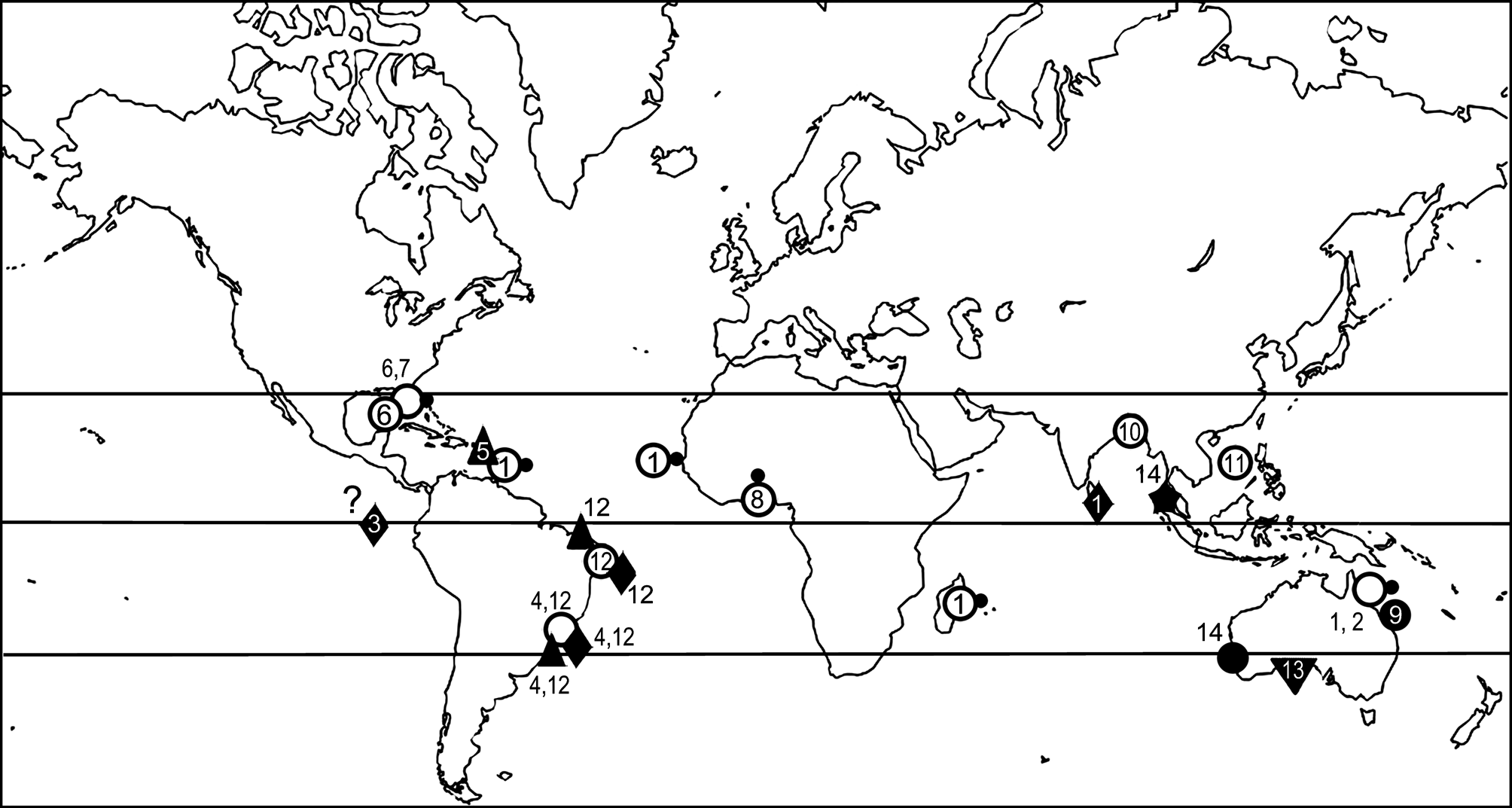
Distribution. Based on previous records A. multicornis appeared restricted to the eastern Australian coasts in the Pacific Ocean. The present finding extends its distribution to the western coasts of Australia, in the Indian Ocean ( Fig. 1 View FIGURE 1 ). The species seems associated to macroalgae in shallow waters.
| NHMUK |
Natural History Museum, London |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Arbocuspis multicornis ( Hincks, 1881 )
| Rosso, Antonietta, Sciuto, Francesco, Sanfilippo, Rossana & Jones, Mary Spencer 2017 |
Membranipora bellula var. multicornis
| Hayward 1995: 1995 |
| Hincks 1881: 149 |