Brycon orbignyanus ( Valenciennes, 1850 )
|
publication ID |
https://doi.org/ 10.5281/zenodo.257769 |
|
publication LSID |
lsid:zoobank.org:pub:F0EC0A87-B1EE-4B5C-8F53-77A7EEA75F3A |
|
DOI |
https://doi.org/10.5281/zenodo.6025341 |
|
persistent identifier |
https://treatment.plazi.org/id/C033D710-4F2E-FF99-4EA4-FEA8FE87FD17 |
|
treatment provided by |
Plazi |
|
scientific name |
Brycon orbignyanus ( Valenciennes, 1850 ) |
| status |
|
Brycon orbignyanus ( Valenciennes, 1850) View in CoL
( Figs. 53–55 View FIGURE 53 View FIGURE 54 View FIGURE 55 )
Chalceus orbignyanus Valenciennes, in Cuvier & Valenciennes, 1850: 249 (Type locality: “Buénos Ayres... la Plata”). [not Kner, 1860: 11 –12].
Chalceus rodopterus Valenciennes, in Cuvier & Valenciennes, 1850: 249 –250 (Type locality: “Buénos Ayres”); Lahille, 1895: 271 (Argentina, La Plata, Puerto Viejo).
Chalceus carpophagus (not Valenciennes): Kner, 1859: 12 (short description; “Irisanga” [upper rio Paraná, São Paulo]).
S. (almo) matrincham Kner (ex Natterer), 1860: 12 (name not available, published as a synonym of Chalceus carpophagus Valenciennes ).
Brycon lineatus Steindachner, 1866a: 19 View in CoL (Type locality: “La Plata-Strom”); Steindachner, 1866b: 211 –212, pl. 2 (idem); Howes, 1982: Géry & Mahnert, 1992: 807 –808 (synonymization with B. orbignyanus ).
Brycon orthotaenia View in CoL (not Günther): Günther, 1880: 13 (Rio de la Plata).
Brycon orbignyanus: Berg, 1895: 123 View in CoL –125 (Buenos Aires, Rio Paraná, Rio Uruguay); Devincenzi, 1924: 174 –175 (Uruguay, rio Uruguay); Bertoni, 1939: 55 (Paraguay); Ringuelet, 1940: 105 (Argentina: Rio Paraná, Rosario); Devincenzi & Teague, 1942: 73 –74 (Río Uruguay, Uruguay; common name, description, migrations, diet, fisheries, size); Thormählen de Gil, 1949: 351 – 440, 5 pls. (common name, description, osteology; Argentina: Río de la Plata, Rosario, Palo Branco, San Pedro, Posadas); Amaral Campos, 1950: 139 (Rio Aguapeí; rio Piracicaba; rio Mogi Guaçú, Pirassununga); Ringuelet et al., 1967: 135 –137 (common names, growth, size, migrations, reproduction, fisheries; Argentina: Rio de la Plata, San Pedro, Brazo Largo, Delta Bonaerense, Puerto Iguazú, Misiones; Isla Apipé Grande, depto. Ituzaingó, Corrientes; Laguna Ituzaingó, Corrientes); Britski, 1972: 89 (Rio Paraná basin, São Paulo state); Howes, 1982: 40 –41 (comparison with B. orthotaenia View in CoL ); Oldano & Tablado, 1985: 53 (Argentina, Laguna “La Cuarentena” Isla Carabajal, médio rio Paraná, 31°42’S, 60°37’W); Miquelarena, 1986: 5 (Argentina, rio Uruguay “frente a la desembocadura del Gualeguaychú”); Godoy, 1986: 75 –76, fig. (rio Paraná, Ilha Grande, Paraná); Quirós & Cuch, 1989: 432 –433, 435 (fisheries, La Plata basin); Quirós, 1990: 445 (Rio Paraná basin, Argentina; fisheries, decline); Géry & Mahnert, 1992: 806 –811, figs.8–13 (description, literature compilation, sinonymy, lectotype designation; Paraguay: “Canendiyu, lac Itaipu à la hauteur du Salto Guiara”; “Canendiyu, lac Itaipu à la hauteur de l’arroyo Pyra-Pita”; “en face de Puerto Iguazu”); Vazzoler & Menezes, 1992: 629, 632–633 (reproduction); Agostinho et al., 1994: 178, 183 (Itaipu reservoir); Agostinho et al., 1997: 183, 203–204 (Rio Paraná, Paraná and Mato Grosso do Sul; rio Ivinheima; rio Piquiri; rio Iguatemi); Hahn et al., 1997: 212, 223 (rio Paraná; diet, activity period); Agostinho et al., 1997: 235 (rio Paraná; diet); Benedito-Cecílio et al., 1997: 58 –59 (Itaipu dam, rio Paraná; length-weigth relationships); Pavanelli & Caramaschi, 1997: 26, 28–29 (Paraná, Porto Rico, occurrence in small tributaries of rio Paraná); Sverlij et al., 1998: 16, fig. (Lower Río Uruguay, Salto Grande dam; common name, fisheries, biology); Agostinho & Júlio Jr., 1999: 379 (Rio Paraná, Paraná and Mato Grosso do Sul); Seixas-Filho et al., 2000: 313– 324 (morphology of the digestive tract); Ganeco et al., 2001: 131 –138 (oocitary development in captivity); Nakatani et al., 2001: 120 –124 (eggs, larvae; development; published picture actually portrays Salminus hilarii View in CoL ); Ganeco & Nakaghi, 2003: 227 –231 (morphology of oocyte); Agostinho et al., 2003: 31, 33, 40, 42, 45–46, 65, 67, 73, 75 (Upper rio Paraná; biology, fisheries, conservation); Zaniboni Filho & Schulz, 2003: 172 –174, 182 (Upper rio Uruguai: migrations, conservation); Reis et al., 2003: 123 –124 (Rio Uruguai, Rio Grande do Sul; conservation status); Pavanelli & Caramaschi, 2003: 276, 279 (Paraná, Porto Rico, occurrence in small tributaries of rio Paraná); Zaniboni Filho et al., 2004: 101 (Upper rio Uruguai, Brazil; biology; photograph); Abilhoa & Duboc, 2004: 608 –609 (Rio Paraná basin, Paraná state: conservation status); Menni, 2004: 48, 74 (figure; Rio Paraná basin, Argentina); Casciotta et al., 2005: 125 –126, 222, fig. 39 (Río Corriente, Esteros de Iberá, Corrientes, Argentina; abundance, common names, biology); Liotta, 2005: 114 –115 (list of known localities in Argentina; map of distribution in Argentina); Graça & Pavanelli, 2007: 78 (upper rio Paraná, Paraná; short description, picture); Antonio et al., 2007: 178 –180 (Rio Paraná, Porto Primavera dam, São Paulo; tagging); Makrakis et al., 2007: 191 (Canal da Piracema, Itaipu dam rio Paraná); Lopera et al., 2008: 1110 –1119 (genetic variation, rio Paraná, São Paulo); Baumgartner et al., 2008: 554 (Rio Paraná basin, Paraná; larvae); Agostinho et al., 2008: 54–56 (biology, conservation); Almirón et al., 2008: 86 (Parque Nacional Pre-Delta, Entre Ríos, Argentina; biology, picture in life); Ricken & Malabarba, 2009: 470, 473, fig. 15 (Rio Uruguai, Machadinho reservoir, zooarcheology); Santos et al., 2009: 491 –499 (experiments with native and non-native fish preys); López et al., 2009a: 4 –30 (compilation of pictures and drawings); Oyakawa et al., 2009: 362 (Rio Paraná basin, São Paulo; conservation status); Mirande, 2010: 483 (relationships within Characidae View in CoL ; list of purported autapomorphies); Mello et al., 2011: 44 (distribution and conservation status, Uruguay). [not Ramlow, 1989: 10].
Brycon lundii View in CoL (not Lütken): Magalhães, 1931: 160 –162, fig. 86 (rio Mogi Guaçú, rio Piracicaba, common name, diet, fisheries, reproduction); Schubart, 1943: 111 (fisheries, migrations, rio Mogi Guaçú).
Brycon travassosi Amaral Campos, 1950: 141 View in CoL , fig. 1 (Type locality: “Rio Bodoquena, Est. Mato Grosso”); Britski, 1969: 202 (holotype); Howes, 1982: 45 (as a possible synonym of B. orbignyanus View in CoL ); Géry & Mahnert, 1992: 811, figs. 14–15 (holotype data); Lima, 2003: 177 (primary type material; as a synonym of Brycon orbignyanus View in CoL ).
Triurobrycon lundii (not Lütken): Schubart, 1962: 27 (Rio Mogi Guaçu); Godoy, 1975: 288–307, figs. 45–50 (Rio Mogi- Guaçu, São Paulo; common names, description; size; migrations, diet, fisheries, decline).
Additional literature for the species in Argentina and Uruguay is listed in Géry & Mahnert (1992) and López et al. (2009b).
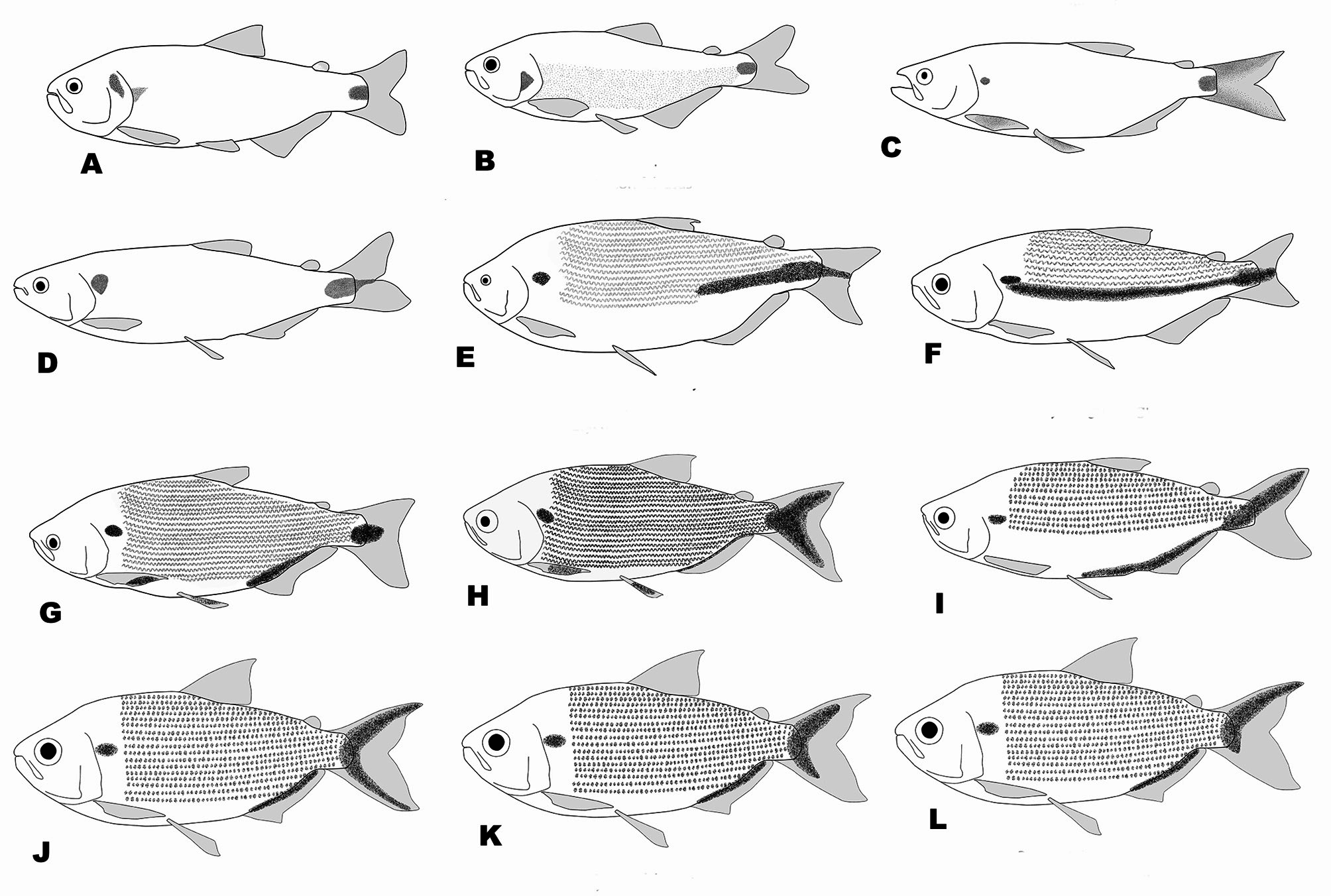
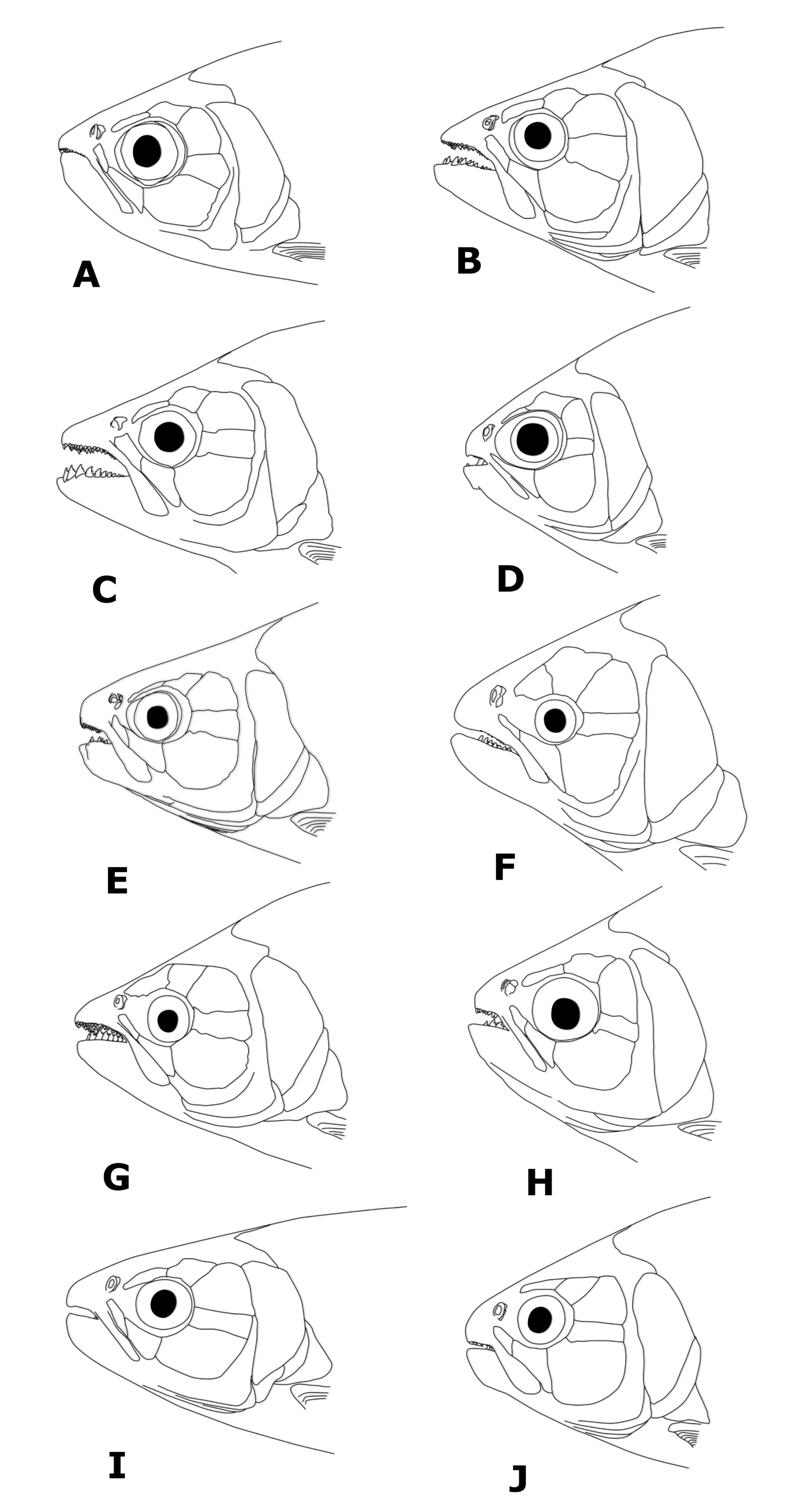
Diagnosis. Brycon orbignyanus can be distinguished from all remaining cis-andean Brycon species, except for B. orthotaenia , B. hilarii , B. whitei , and B. polylepis , by possessing a caudal peduncle blotch extending as a stripe into the distal portion of caudal-fin rays (caudal peduncle blotch, when present, limited to the caudal peducle or extending only into centralmost caudal-fin rays; Fig. 5 View FIGURE 5 ). It can be distinguished from these species by possessing dentary teeth decreasing gradualy in size (vs. four anteriormost teeth considerably larger than remaining teeth). It can be further distinguished from B. orthotaenia , B. hilarii , and B. whitei by possessing a relatively narrow and elongated dentigerous premaxilary surface (vs. a broad, relatively short dentigerous surface of the premaxillary). It can additionaly be distinguished from Brycon hilarii and B. whitei primarily by possessing lower scale counts (52– 63, modally 56 lateral line scales, vs. 67–82, modally 74`in B. hilarii , and 66–76, modally 70, in B. whitei ; 10–13, modally 12 scales between lateral line and dorsal-fin basis, vs. 12–17, modally 15, in B. hilarii , and 12–13, modally 13, in B. whitei ; 19–23, modally 20 circumpeduncular scales, vs. 20–28, modally 26, in B. hilarii , and 19– 24, modally 21, in B. whitei , respectively). Brycon orbignyanus can be further distinguished from B. whitei by lacking a midlateral dark stripe (vs. mdilateral dark stripe present). Brycon orbignyanus can be further distinguished from B. orthotaenia by possessing a pointed head profile (versus aproximately rounded to obtuse in the latter species; compare Figs. 6 View FIGURE 6 F and H), by possessing a higher number of teeth in the second, inner premaxilary row (not counting the teeth of the second row situated between the first and third rows) (5–9, modally 6, versus 3–5, modally 5, in B. orthotaenia ), and by possessing the dentary teeth decreasing gradualy in size (vs. anteriormost four dentary teeth considerably larger than the remaining teeth in B. orthotaenia ).
Description. Morphometric data are presented in Table 15 View TABLE 15 . Large-sized species, largest examined specimen 516.0 mm SL. Body moderately slender. Largest body height slightly ahead of dorsal-fin origin. Dorsal body profile slightly convex from upper lip margin to vertical through anterior naris, slightly concave to straight from latter point to basis of supraoccipital process, moderately convex from latter point to dorsal-fin origin, straight along dorsal-fin basis, and straight to slightly convex from dorsal-fin basis to adipose-fin origin. Dorsal profile of caudal peduncle slightly concave. Ventral profile slightly convex from lower lip to pelvic-fin insertion, straight to slightly convex from this point to anal-fin origin and approximately straight along anal-fin base. Ventral profile of caudal peduncle slightly concave.
Head profile slightly acute anteriorly, mouth terminal. Jaws approximately isognathous to slightly anisognathous, outer row of premaxillary teeth partially exposed when mouth is closed. Maxillary moderately long, extending posteriorly to anterior third to middle of pupil. Adipose eyelid well developed. Premaxillary teeth in three rows; teeth of third row largest. Nine (3), 10 (8), 11 (15), 12 (18), or 13 (5) relatively small teeth in outer series. Teeth generally tricuspidate, some pentacuspidate in larger (> 470 mm SL) specimens. Five (8), 6 (24), 7 (17), 8 (2), or 9 (1) tri- to pentacuspidate teeth in second, inner premaxillary row, plus 2 (7), 3 (35), or 4 (10) tricuspidate teeth between the first and third rows. Two teeth in third premaxillary row, medial teeth largest, symphyseal teeth smaller, slightly tilted towards each other, penta- to hexacuspidate. Maxillary margins approximately parallel, straight in profile. Eleven to 20 maxillary teeth, slightly smaller than teeth of first premaxillary row, anterior teeth tri- to pentacuspidate, posterior teeth unicuspidate. Dentary with 8 (3), 9 (4), 10 (7), 11 (9), 12 (14), 13 (12), or 14 (5) teeth in main series. Teeth at dentary symphysis larger, pentacuspidate, remaining teeth decreasing gradually in size, larger teeth near dentary symphysis penta-, tetra-, tri- to unicuspidate. Inner (lingual) series consisting of a small, single unicuspid symphyseal tooth, situated immediately posterior to symphyseal dentary teeth of main series, plus row of 12–15 small, aciculated, unicuspidate teeth, originating on lingual crest of dentary replacement trench at the level of fifth to eighth main series dentary teeth.
Scales cycloid. Lateral line complete, from supracleithrum to caudal-fin base. Fifty-two (3), 53 (5), 54 (4), 55 (7), 56 (11), 57 (5), 58 (6), 59 (4), 60 (5), 61 (1), or 63 (1) scales in lateral line series. Laterosensory tube simple in specimens smaller than 100 mm SL, ramified in specimens larger than 100 mm SL. Tubules ramification increasing in complexity along ontogeny, specimens between 150–200 mm SL with tubules with two or three branches, four to seven branches in specimens between 220–270 mm SL, and with more than 10 branches and developing a dendritic pattern of ramification, with tubules overlapping each other in larger (> 300 mm SL) specimens. Horizontal scale rows between dorsal-fin origin and lateral line 10 (1), 11 (20), 12 (26), or 13 (4). Horizontal scale rows between lateral line and pelvic-fin 5 (4), 6 (18), 7 (24), 8 (4), or 9 (1). Circumpeduncular scales 19 (3), 20 (25), 21 (13), 22 (11), or 23 (1).
Dorsal-fin rays ii, 9. Dorsal fin origin slightly ahead middle of SL. First dorsal-fin pterygiophore inserting behind neural spine of 14th (1) vertebra. Anal-fin rays iii (not including first, small unbranched ray only visible in the cs specimen), 21 (4), 22 (6), 23 (6), 24 (15), 25 (15), 26 (3), or 27 (2). First anal-fin pterygiophore inserting behind haemal spine of 28th (1) vertebra. Anal-fin rays decreasing only slightly in size towards anal-fin end. Sheath of scales covering basis of anal-fin rays composed of four scale rows, lower scale row formed by 24–25 rectangular scales. Pectoral-fin rays i, 12 (4), 13 (40), 14 (8), or 15 (2). Pelvic-fin rays typically i, 7, a single specimen i, 6. Main caudal-fin rays 10/9. Caudal fin slightly forked, distal margin slightly concave. Central caudalfin rays with a small, pointed middle projection extending beyond primary margin of fin. Laterosensory tube extending over interradial membrane between upper and lower caudal-fin lobes to the distal portion of fin at the middle caudal-fin projection. Laterosensory tube on caudal fin with dorsally and ventrally oriented side branches across its length.
Four branchiostegal rays, three on anterior ceratohyal and one on posterior ceratohyal. First branchial arch with 14 (3), 16 (1), 17 (15), 18 (12), 19 (6), or 20 (2) lower, 1 at angle, and 14 (1), 15 (4), 16 (4), 17 (16), 18 (10), or 19 (2) upper gill rakers. Vertebrae 47 (1), 49 (1). Supraneurals 11 (1).
Coloration in alcohol. Top of head, snout, supraorbital, and sixth infraorbital light- to dark-brown. Dorsal portion of body light-brown to dark-brown. Second, third, fourth, and fifth infraorbitals, and opercle silvery.
Dentary, maxillary, gular area and lower portion of body light brown. Lateral portion of body light brown, with a silvery hue. Humeral blotch present, conspicuous, approximately rounded in shape, situated immediately above lateral line, its anterior margin at level of second, extending longitudinally to posterior margin of fifth lateral line scales, and vertically one and half scales high. Dark, wavy longitudinal stripes formed by dark pigment concentrated on upper and lower scale margins extending along trunk. Stripes more discernible dorsally. Caudal peduncle with broad median stripe, originating 4–6 scales from hypural joint and continuing posteriorly over 4 central principal caudal-fin rays to caudal-fin distal margin. Remaining caudal-fin rays, and remaining fins, clear.
Coloration in life. Description based on photographs of freshly caught or living specimens from middle and upper rio Paraná in Brazil and Argentina (published pictures in Godoy, 1986: 76; Reis Filho, 1998: 54; Zaniboni Filho et al., 2004: 101). Overall coloration silvery, dorsum dark-grey, with a silvery hue. Top of head dark grey. Midlateral dark stripe extending across caudal peduncle and middle caudal-fin rays very conspicuous. Caudal-fin rays dorsal and ventral to middle dark stripe reddish to intense red. Anal, dorsal, and pelvic fins reddish. Pectoral fins orangish or dark.
Sexual dimorphism. None of the examined specimens possess anal-fin hooks, but its presence was reported as a dimorphic feature of mature males in the species ( Zaniboni Filho et al., 2004: 101).
Common names. Brazil: “piracanjuba”, “piracanjuva” ( Magalhães, 1931: 160; Godoy, 1975: 289); Argentina: “pirapitá”, “salmón”, “salmón criollo”, “salmón del Paraná ”, “salmonete”, “pracanjuva”, “pirapitanga” ( Ringuelet et al., 1967: 136), “pirapitá blanco” (F. Baena, pers. comm.); Uruguay: “pirapitá”, “salmón”, “salmón criollo”, “pirapitanga” ( Sverlij et al., 1998: 16). Juvenile specimens were formerly called “piracanjuvira”, and adult, breeding specimens, “piracanjuba arripiada”, at the rio Mogi-Guaçú ( Magalhães, 1931: 161; Godoy, 1975: 293).
Distribution. Originally widespread throughout the rio Paraná (both above and below the Sete Quedas waterfalls, the former barrier between the upper and lower sections of the rio Paraná) and rio Uruguai basins, in Brazil, Argentina, Paraguay, and Uruguay ( Fig. 56 View FIGURE 56 ; see also Liotta, 2005: 100, for a map of known localities in Argentina). It has now disappeared from most of its former range (see item “Conservation”, below). Brycon orbignyanus does not occur in the rio Paraguai basin, where it is replaced by the congener B. hilarii (see under item “Distribution” of this species), though both species occur syntopically in the middle rio Paraná in Brazil and Argentina.
Ecological notes. Brycon orbignyanus originally occurred in middle to large sized rivers, and at floodplain lakes and channels associated with large rivers ( Agostinho et al., 1997: 183). The species prefered forested rivers and was considered to be mainly frugivore ( Magalhães, 1931; Ringuelet et al., 1967). Godoy (1975: 304–305) examined stomach contents of nine specimens collected at the rio Mogi-Guaçú and found vegetal matter (mostly leaves), insects, and small fishes. Hahn et al. (1997: 212) examined stomach contents of 46 specimens collected in the floodplains of the rio Paraná at Paraná state and, similarly to Godoy (1975), found insects and other invertebrates, vegetal matter, and fishes. Reis et al. (2003: 123) reported seeds but predominantly aquatic plants as the diet of six specimens collected in the upper rio Uruguai. Based on the findings by Hahn et al. (1997), Agostinho et al. (1997: 235) considered the species as being mainly insectivore, though that conclusion is probably mainly a result of the relative small size of the specimens examined by Hahn et al. (1997). Interestingly, the unique dentition of Brycon orbignyanus among congeners, with graduated-sized and relatively small and compressed anterior teeth on the dentary suggests a folivore diet for the species (pers. obs.). Size at first maturation is reported to be 30 cm TL, being reached at 2–3 years of age ( Agostinho et al., 2003). Brycon orbignyanus is well-known as a migratory species. At the lower rio Paraná and rio Uruguai at Argentina and Uruguay, Brycon orbignyanus migrated downstream during October, moving upstream during March. Breeding in the southern part of the range of the species took place between December and January ( Devincenzi & Teague, 1942: 73; Ringuelet et al., 1967: 137). On other hand, at the upper rio Paraná basin the reproductive migration is undertaken upstream ( Ihering, 1929; Magalhães, 1931; Godoy, 1975). The upstream migration in rio Mogi-Guaçú started by the end of Setember, and spawning took place between November and January ( Godoy, 1975: 296, 299). At the rio Piracicaba, Magalhães (1931: 162) reported reproductive schools moving upstream the river between December and January, and, on one occasion, hundreds of specimens were observed entering a flooded area, the males then apparently chasing the females. Ihering (1929: 81) estimated that females ranging between 570 and 690 mm in total length possessed a total fecundity of 500,000 to 1,000,000 oocytes. After spawning, Brycon orbignyanus move downstream, sometimes considerably far from the spawning site. Godoy (1975: 299–300) reported that two specimens captured and tagged at Cachoeira de Emas (rio Mogi Guaçú) were recaptured two to four months later 601–637 km downstream, into the rio Grande. Thormählen de Gil (1949: 414–437) studied annuli rings in scales and calculated that specimens between 90–116 mm SL are less than two years old, specimens between 123–215 mm SL are 2–3 years old and specimens between 229–245 mm SL are 4–5 years old. Thormählen de Gil (1949: 414–437) estimated that specimens with 264, 374, 530 and 604 mm SL were respectively about 6, 11, 13, and 16 years old. Though age estimation through annuli rings can be misleading as an age determinator ( Casselman, 1990), so are other biological markers ( Campana, 2001), and Thormählen de Gil’s (1949) data representa at the very least the only attempt to infer growth and longevity in wild populations of this now threatened fish. Godoy (1975: 294) reported that the largest female specimen recorded in the rio Mogi-Guaçú reached 79.5 cm TL and weighted 8.2 kg, while the largest male measured 68.0 cm TL and weighted 3.62 kg.
Conservation. Brycon orbignyanus was in the past one of the most valued and important fishes in commercial and sport fisheries throughout its range (e.g., Ringuelet et al., 1967; Schubart, 1943, 1949; Monteiro, 1953; Machado et al., 1968; Godoy, 1975). Godoy (1975) documented the gradual decline of the species in the rio Mogi- Guaçu in the upper rio Paraná basin, where the species was once one of most important target of fisheries ( Schubart, 1943, 1949). The species is now extirpated from the rio Mogi-Guaçu, and, in fact, from almost the entire upper rio Paraná basin, with the exception of the last natural stretch of the rio Paraná in Brazil, the floodplains situated between the Porto Primavera and Itaipu dams (Agostinho et al., 2008). Populations of Brycon orbignyanus are in fact steeply declining throughout its entire range ( Quirós, 1990; Agostinho et al., 2008; Mello et al., 2011). Though pollution and removal of the riparian forest were certainly important factors that contributed to the decline of the species, the disappearance of this previous widespread species throughout most of its range should be primarily imputed to the drastic alterations in flow regime caused by damming. The species clearly is unable to survive in reservoirs or river stretches with flow regimes regulated by hydroelectric dams. There is almost no freeflowing river stretches in the upper rio Paraná basin ( Agostinho et al., 2003), and a similar fate is under way for the upper rio Uruguai basin. Brycon orbignyanus is officially considered a threatened species in both Brazil (Agostinho et al., 2008) and Argentina, and should in fact be considered as globally threatened. There is a relatively intense stocking program for the species in Brazil (e.g., Dumont-Neto et al., 1997; Senhorini et al., 2002; Lopera et al. . 2008).
Remarks. Chalceus orbignyanus was described by Valenciennes (in Cuvier & Valenciennes, 1850: 249), based on two specimens (MNHN 9835) collected at “Buénos-Ayres”, collected by Alcides d’Orbigny. Géry & Mahnert (1992: 811) designated the larger syntype of the lot MNHN 9835 (161 mm SL) as the lectotype. As defined in the present study, Brycon orbignyanus possess three junior synonyms. Chalceus rodopterus Valenciennes (in Cuvier & Valenciennes, 1850: 249–250), described subsequently to Chalceus orbignyanus based on two juvenile specimens also collected at “Buénos-Ayres”, is, as already remarked by Géry & Mahnert (1992: 807–808), a synonym of B. orbignyanus . Brycon lineatus , described by Steindachner (1866a: 19) and redescribed and illustrated by the same author ( Steindachner, 1866b: 211–212, pl. 2), was also considered by Géry & Mahnert (1992: 807) as a possible synonym of B. orbignyanus , differing, however, by possessing a lower number of precaudal vertebrae (21) and supraneurals (8). Those counts are indeed lower than the usual counts for the species (28 to 29 pre-caudal vertebrae, and 11–12 supraneurals). Géry & Mahnert (1992), which have only examined the radiograph of the holotype of Brycon lineatus , supposed that it could be a teratological specimen. The examination of the purported holotype of Brycon lineatus (NMW 62943) revealed it to be rather a specimen of Brycon falcatus , a species actually quite distinct from B. orbignyanus , which explains the discrepancy in pre-caudal vertebrae and supraneurals noticed by Géry & Mahnert (1992). Since the description and illustration by Steindachner (1866b) clearly depicts a specimen of Brycon orbignyanus , which is additionaly the sole species of Brycon occurring in the southern portion of the La Plata basin, it seems that at some point the holotype of Brycon lineatus was inadvertently exchanged with this Brycon falcatus specimen. Although the “true” holotype of Brycon lineatus is apparently lost, we concur with Géry & Mahnert (1992) in concluding that this nominal species clearly represents a synonym of B. orbignyanus .
Brycon travassosi Amaral Campos (1950) View in CoL was described based on a single specimen, said to have been collected at the “rio Bodoquena, Est. Mato Grosso” in Brazil. Amaral Campos (1950) only compared Brycon travassosi View in CoL with B. lineatus View in CoL , from which it was said to be distinct based on the possession of tricuspidated dentary teeth and by the size of the scales. The examination of the holotype of Brycon travassosi View in CoL (MZUSP 3811) showed that it rather possess pentacuspidated dentary teeth, which is in fact the usual condition for this character in the genus Brycon View in CoL . As for the size of the scales, the holotype of Brycon travassosi View in CoL possess 55 lateral-line scales, a count within the range of variation of B. orbignyanus View in CoL . In fact, Howes (1982: 45) already considered B. travassosi View in CoL as a possible synonym of B. orbignyanus View in CoL . However, Géry & Mahnert (1992: 811) suggested that the holotype of Brycon travassosi View in CoL as possessing two differences when compared to specimens of B. orbignyanus View in CoL , which were a lower body depth and and a lower anal-fin ray count (iii, 21). However, the examination of a large sample of Brycon orbignyanus View in CoL in the present study demonstrated that both the body depth and the anal-fin count of the holotype of Brycon travassosi View in CoL are within the range of variation of B. orbignyanus View in CoL . We consequently consider Brycon travassosi View in CoL as a synonym of B. orbignyanus View in CoL . The exact provenance of the holotype of Brycon travassosi View in CoL is uncertain. The Serra da Bodoquena (Bodoquena ridge) is a small carbonate plateau lying at the southeastern border of the Pantanal wetland (see Ribeiro et al., 2007). There is apparently no “rio Bodoquena” in Brazil, and in fact the original label of the holotype of Brycon travassosi View in CoL records only “Bodoquena”, the word “rio” being apparently an addition by A. Amaral Campos. The town of Bodoquena (20°32’47’’S, 56°40’33’’W) lies at the northern portion of the Bodoquena ridge and it is close to the village of Salobra (20°12’S, 56°30’W), a locality visited by the collector of the holotype of Brycon travassosi View in CoL , the helmintologist Lauro Travassos, between 1939–1941 ( Travassos & Freitas, 1940), which could be consequently identified as the presumable type-locality of Brycon travassosi View in CoL . This area is drained by upper tributaries of the rio Miranda, itself a major tributary of the rio Paraguai. There are, however, no other records of Brycon orbignyanus View in CoL for the rio Paraguai basin, the sole Brycon View in CoL species occurring in this area (including the rio Miranda basin) being B. hilarii View in CoL (see under this species). There are in fact some Brycon hilarii View in CoL specimens collected by Lauro Travassos at Salobra during 1940–1941 (MZUSP 2986, MZUSP 3073, MNRJ 2878). During 1941, the entomologist Lauro Travassos Filho, Lauro Travassos´son, made a field expedition in the rio Paraná at Porto Cabral ( Travassos Filho, 1945), where he collected some Brycon orbignyanus View in CoL specimens (MNRJ 4767, MNRJ 4775, MNRJ 4776, MNRJ 4779). We suggest that the holotype of Brycon travassosi View in CoL might have been rather collected at this latter locality, being subsequently mislabeled. We consequently consider the locality “Bodoquena” for the holotype of Brycon travassosi View in CoL as very probably incorrect.
Kner (1860: 4) mentions Chalceus carpophagus as occurring at “Irisanga” (presently Estiva Gerbi, rio Orissanga, a tributary of the rio Mogi-Guaçú, São Paulo). Howes (1982: 17) noticed that the meristic data provided by Kner (1860) “closely matches that of B. orbignyanus View in CoL ”. We have not re-examined the specimen studied by Kner (1860), but we concur with Howes (1982) that this record refers with all likelihood to B. orbignyanus View in CoL .
Some authors ( Magalhães, 1931; Schubart, 1943, 1962; Godoy, 1975) erroneously identified B. orbignyanus from the upper rio Paraná basin in São Paulo (and, particularly, from the rio Mogi Guaçú basin) as Triurobrycon lundii (= Brycon orthotaenia ). All specimens examined in the present study from the rio Mogi-Guaçu basin (MZUSP 2070, MZUSP 3830, BMNH 1946.12.23.136-137) are undoubtedly Brycon orbignyanus , B. orthotaenia being restricted to the rio São Francisco basin (see under this species).
Material examined. Type material: MNHN 9835 (2, 142.7– 160.9 mm SL): “Buenos-Ayres”; A. d’Orbigny. Lectotype (larger specimen) and paralectotype (smaller specimen) of Chalceus orbignyanus Valenciennes, 1850 (see Géry & Mahnert, 807, 811). MNHN A.9834 (2, 78.8–79.5 mm SL): “Buenos-Ayres”; A. d’Orbigny. Syntypes of Chalceus rodopterus Valenciennes, 1850 . MZUSP 3811 (1, 241.0 mm SL): Mato Grosso [=Mato Grosso do Sul?], rio Bodoquena [=? Bodoquena; see “Remarks”, above]; L. Travassos-Filho, 1941. Holotype of Brycon travassosi Amaral Campos, 1950 .
Non types. Brazil, Goiás: NUP 1125 (2, 223.9– 240.9 mm SL) : Caldas Novas, rio Corumbá (trib. rio Paranaíba ) at Corumbá reservoir, 17°43’37’’S, 48°32’54’’W; Nupélia team, 9 Apr 1999 GoogleMaps . São Paulo: MZUSP 16626 View Materials (1, 280.8 mm SL) : Rio Grande, Cachoeira do Onça (now under Ilha Solteira dam), 19°59’S, 50°53’W; P.E. Vanzolini et al., 6–7 Jan 1962 GoogleMaps . MZUSP 17169 View Materials (7, 200.8– 473.8 mm SL): Ilha Solteira, rio Paraná, 20°23’S, 51°21’W GoogleMaps ; Exc. Departamento de Zoologia, Sept 1965. MZUSP 67070 View Materials (1, 156.5 mm SL): rio Aguapeí (trib. rio Paraná), c. 21°03’S, 51°46’W GoogleMaps ; J. Canella, 27 Aug 1941. MZUSP 67069 View Materials (1, 167.1 mm SL): rio Paraná, mouth of rio Aguapeí , 21°03’S, 51°46’W GoogleMaps ; J. Canella, 7 Aug 1941. MNRJ 4776 (1, 209.0 mm SL); MNRJ 4767 (1, 187.8 mm SL); MNRJ 4775 (1, 198.2 mm SL); MNRJ 4779 View Materials (12, 99.6–289.0 mm SL): rio Paraná, Porto Cabral, 22°17’S, 52°38’W GoogleMaps ; L. Travassos Filho, March–April 1944. FMNH 119092 About FMNH (1, 200.0 mm SL): rio Tietê , Salto do Avanhandava, c. 21°13’S, 49°57’W GoogleMaps ; J.D. Haseman, 15 Sept 1908. FMNH 92082 About FMNH (1, 213.0 mm SL): Itapura, rio Tietê , 20°39’S, 51°30’W GoogleMaps ; J.D. Haseman, 27 Sept 1908. MZUSP 1474 View Materials (1, 147.9 mm SL): Itapura, rio Tietê , 20°39’S, 51°30’W GoogleMaps ; E. Garbe, 1904. MZUSP 79319 View Materials (1, 89.9 mm SL): Pereira Barreto, rio Tietê, Três Irmãos reservoir, 20°40’25’’S, 51°30’6’’W GoogleMaps ; W.S. Smith, Jan 2001. MZUSP 1523 View Materials (1, 171.9 mm SL): Piracicaba, rio Piracicaba , 22°43’S, 47°40’W GoogleMaps ; J. Lima, 1896. MZUSP 3900 View Materials (1 head and scapular girdle; 108.9 mm HL): Pirassununga, rio Mogi-Guaçú, Cachoeira de Emas , 21°55’S, 47°22’W GoogleMaps ; R. von Ihering, 1907. MZUSP 3830 View Materials (7, 115.3–195.0 mm SL): same locality; O. Schubart, 1945 GoogleMaps . BMNH 1946.12.23.136-137 (2, 120.4–234.0 mm SL): Rio Mogi-Guaçu (no precise locality but very likely Cachoeira de Emas) ; A.Amaral Campos, no date. MZUSP 3618 View Materials (1, 334.9 mm SL): same locality ; A. Amaral Campos, March 1943 . MZUSP 3378 View Materials (10, 2 cs, 109.9–129.4 mm SL): Teodoro Sampaio, rio Paranapanema at Porto Marcondes, 22°32’S, 52°2’W; E. Dente & D. Seraglia, 17–23 Nov 1946 GoogleMaps . Mato Grosso do Sul: MZUSP 18377 View Materials (1, 179.0 mm SL): Ilha Solteira, rio Paraná, opposite to Ilha Solteira (cofferdam), 20°23’S, 51°21’W GoogleMaps ; Exc. MZUSP, 25–28 May 1972. MZUSP 16714 View Materials (3, 211.3– 277.4 mm SL): Três Lagoas, rio Paraná, at Jupiá , 20°47’S, 51°38’W GoogleMaps ; Exc. Departamento de Zoologia, 15–23 Sept 1962. MZUSP 3039 View Materials (1, 213.8 mm SL): rio Verde (trib. rio Paraná), 21°11'S 51°53'W GoogleMaps ; J. Canella, 25 Aug 1941. Paraná: MZUEL 7440 (34, 107.1– 160.7 mm SL) ; ZUEC 7762 View Materials (4, 121.6– 146.9 mm SL): Icaraíma , rio Paraná, Ilha Maringá, c. 23° 21’S, 53° 47’W GoogleMaps ; O. A. Shibatta & M. Caetano Filho, 31 March–2 April 1995 . MZUEL 5387 (2, 188.0–240.0 mm SL): Altônia , rio Paraná, Porto Cerâmica, 23°51’40’’S, 50°0’48’’W GoogleMaps ; F. Yuldi, 25 March 2010. LBP 9210 (1, 190.0 mm SL): Altônia , rio Paraná, Porto Cerâmica, 23°51’40’’S, 54°0’48’’W GoogleMaps ; A.C.R. Casimiro & F. Yuldi, 25 Apr 2010 .
MZUSP 42353 View Materials (2, 179.0– 196.3 mm SL): Reservatório de Itaipu , c. 25°16’S, 54°28’W; no collector specified, Jan 1990 GoogleMaps . MZUSP 18840 View Materials (1, 237.7 mm SL): Guaíra, rio Paraná, 24°4’S, 54°15’W GoogleMaps ; CETESB, Jul–Aug 1977. MZUSP 21065 View Materials (2, 195.0– 204.2 mm SL): Guaíra, rio Paraná below Sete Quedas waterfalls, c. 24°7’S, 54°20’W GoogleMaps ; CETESB, 1977–1980. MZUSP 15364 View Materials (1, 443.6 mm SL): Porto Mendes, rio Paraná, 24°29’S, 54°19’W GoogleMaps ; CETESB, Nov–Dec 1977. Santa Catarina: MCP 21605 (1, 322.7 mm SL): Itapiranga, rio Uruguai, near pedra da Fortaleza, 27°11'42''S 53°38'34''W; L.F. Câmara et al., 5 Nov 1998 GoogleMaps . MCP 22458 (1, 373.5 mm SL); MCP 22457 (1, 326.1 mm SL): same locality; L. Hahn et al., 26 Feb 1999 GoogleMaps . Rio Grande do Sul: MCP 8746 View Materials (1, 516.0 mm SL) ; MCP 8747 View Materials (1, 506.5 mm SL); rio Uruguai (no specific locality); J. Bertoletti, June 1975 .
Paraguay: UMMZ 207060 View Materials (1, 515.0 mm SL): Pettirossi fish market (= Mercado Quatro) in Asuncion; J. Taylor et al., 14 Sept 1979.
Argentina: MZUSP 17115 View Materials (1, 111.1 mm SL): Rio de la Plata , Buenos Aires, c. 34°30’S, 58°26’W; H.P. Castello, 4 April 1965 GoogleMaps . BMNH 1927.2.9.7-9 (4, 188.9– 205.3 mm SL): Misiones, Puerto Iguazu, rio Paraná at mouth of rio Iguazu , 25°35’S, 54°35’W GoogleMaps ; G. Harrison, no date. BMNH 1872.6 .8.22 (1, 307.0 mm SL): “ Paraná ” (no precise locality); collector and date not specified.
Uruguay: MNRJ 11266 View Materials (1, 124.8 mm SL): Departamento Colonia, Arroyo Linetas , Estancia San Jorge, c. 34°10’S, 58°6’W GoogleMaps ; R. Pradori, 17 Apr 1965. MCZ 845 About MCZ (1, 309.6 mm SL): Rio Uruguay (no precise locality); J. Wyman, no date.
TABLE 15. Morphometric data of Brycon orbignyanus (A: lectotype and paralectotype of Chalceus orbignyanus, MNHN 9835; B: syntypes of Chalceus rodopterus, MNHN A. 9834; C: holotype of Brycon travassosi, MZUSP 3811). Mean and range does not includes syntypes of Chalceus rodopterus.
| A | B | C | n | Range | Mean | |
|---|---|---|---|---|---|---|
| Standard length (SL) | 142.7–160.9 | 78.8–79.5 | 241.0 | 52 | 109.9–473.8 | - |
| Percentages of standard length | ||||||
| Depth at dorsal-fin origin | 26.8–28.2 | - | - | 42 | 26.8–34.9 | 30.8 |
| Snout to dorsal-fin origin | 50.1–52.3 | 54.1–54.6 | - | 50 | 47.1–56.4 | 50.8 |
| Dorsal-fin base length | 10.6–12.2 | 11.9 | 10.7 | 52 | 10.3–13.6 | 11.7 |
| Posterior terminus of dorsal fin to adipose fin | 23.7–23.9 | 19.3–22.1 | 25.0 | 52 | 22.5–27.5 | 24.8 |
| Posterior terminus of dorsal fin to hypural joint | 39.0 | 34.4–36.5 | 35.8 | 52 | 34.6–40.6 | 37.9 |
| Snout to pelvic-fin insertion | 48.8–49.5 | - | - | 44 | 43.7–54.7 | 47.8 |
| Snout to anal-fin origin | 69.9–72.5 | 70.2–75.6 | - | 49 | 63.2–74.8 | 68.6 |
| Anal-fin base length | 22.6–23.1 | 21.8–22.1 | 22.1 | 51 | 21.3–25.2 | 22.9 |
| Caudal peduncle length | 11.4–12.9 | 12.3–12.8 | 13.2 | 52 | 11.4–18.8 | 15.3 |
| Dorsal-fin height | 21.1–22.3 | 22.3 | 21.1 | 43 | 18.0–23.0 | 20.4 |
| Pectoral-fin length | 18.4–19.4 | 18.5–20.8 | 18.3 | 45 | 14.3–20.1 | 16.8 |
| Pelvic–fin length | 16.0–16.6 | 17.1 | 16.6 | 48 | 13.0–19.3 | 16.4 |
| Caudal peduncle depth | 9.4–9.7 | 8.2–9.0 | 10.1 | 52 | 8.6–10.9 | 10.0 |
| Head length | 25.5–25.8 | 32.5–32.9 | 21.6 | 52 | 19.6–29.8 | 24.5 |
| Percentages of head length | ||||||
| Head height | 73.0–76.1 | 67.8–71.4 | 87.9 | 50 | 71.0–87.9 | 79.5 |
| Snout length | 29.9–30.8 | 30.1–30.2 | 33.1 | 52 | 27.6–36.5 | 31.7 |
| Upper jaw length | 46.0–47.5 | 46.9–49.0 | 45.8 | 52 | 44.4–52.8 | 46.8 |
| Horizontal eye diameter | 21.4–24.5 | 25.1–27.5 | 23.1 | 52 | 19.9–31.8 | 25.3 |
| Post-orbital length | 46.7–47.7 | 44.2–47.1 | 45.6 | 52 | 39.1–48.4 | 45.8 |
| Least interorbital width | 37.1–39.3 | 32.4–34.1 | 39.4 | 52 | 33.6–45.9 | 39.3 |
| MZUSP |
Museu de Zoologia da Universidade de Sao Paulo |
| MNRJ |
Museu Nacional/Universidade Federal de Rio de Janeiro |
| FMNH |
Field Museum of Natural History |
| ZUEC |
Museu de Zoologia da Universidade Estadual de Campinas |
| MCP |
Pontificia Universidade Catolica do Rio Grande do Sul |
| UMMZ |
University of Michigan, Museum of Zoology |
| MCZ |
Museum of Comparative Zoology |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Brycon orbignyanus ( Valenciennes, 1850 )
| Lima, Flávio C. T. 2017 |
Brycon travassosi
| Lima 2003: 177 |
| Gery 1992: 811 |
| Howes 1982: 45 |
| Amaral 1950: 141 |
Brycon lundii
| Schubart 1943: 111 |
| Magalhaes 1931: 160 |
Brycon orbignyanus:
| Mello 2011: 44 |
| Mirande 2010: 483 |
| Ricken 2009: 470 |
| Santos 2009: 491 |
| Lopez 2009: 4 |
| Oyakawa 2009: 362 |
| Lopera 2008: 1110 |
| Baumgartner 2008: 554 |
| Almiron 2008: 86 |
| Graca 2007: 78 |
| Antonio 2007: 178 |
| Makrakis 2007: 191 |
| Casciotta 2005: 125 |
| Liotta 2005: 114 |
| Zaniboni 2004: 101 |
| Abilhoa 2004: 608 |
| Menni 2004: 48 |
| Ganeco 2003: 227 |
| Agostinho 2003: 31 |
| Zaniboni 2003: 172 |
| Reis 2003: 123 |
| Pavanelli 2003: 276 |
| Ganeco 2001: 131 |
| Nakatani 2001: 120 |
| Agostinho 1999: 379 |
| Sverlij 1998: 16 |
| Agostinho 1997: 183 |
| Agostinho 1997: 235 |
| Benedito-Cecilio 1997: 58 |
| Pavanelli 1997: 26 |
| Agostinho 1994: 178 |
| Gery 1992: 806 |
| Vazzoler 1992: 629 |
| Quiros 1990: 445 |
| Quiros 1989: 432 |
| Ramlow 1989: 10 |
| Miquelarena 1986: 5 |
| Godoy 1986: 75 |
| Tablado 1985: 53 |
| Howes 1982: 40 |
| Britski 1972: 89 |
| Ringuelet 1967: 135 |
| Amaral 1950: 139 |
| Gil 1949: 351 |
| Devincenzi 1942: 73 |
| Ringuelet 1940: 105 |
| Bertoni 1939: 55 |
| Devincenzi 1924: 174 |
| Berg 1895: 123 |
Brycon orthotaenia
| Gunther 1880: 13 |
Brycon lineatus
| Gery 1992: 807 |
| Steindachner 1866: 19 |
| Steindachner 1866: 211 |
Chalceus orbignyanus Valenciennes, in Cuvier & Valenciennes, 1850 : 249
| Kner 1860: 11 |
| Cuvier 1850: 249 |
Chalceus rodopterus Valenciennes, in Cuvier & Valenciennes, 1850 : 249
| Lahille 1895: 271 |
| Cuvier 1850: 249 |