Celleporina joannae, Almeida, Ana C. S., Souza, Facelucia B. C., Menegola, Carla & Vieira, Leandro M., 2017
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4290.2.3 |
|
publication LSID |
lsid:zoobank.org:pub:0AE2706B-F77D-4903-B3A6-BB11891CD67B |
|
DOI |
https://doi.org/10.5281/zenodo.5701223 |
|
persistent identifier |
https://treatment.plazi.org/id/BF6087E4-8141-B518-999D-FC2F941A2F35 |
|
treatment provided by |
Plazi |
|
scientific name |
Celleporina joannae |
| status |
sp. nov. |
Celleporina joannae n. sp.
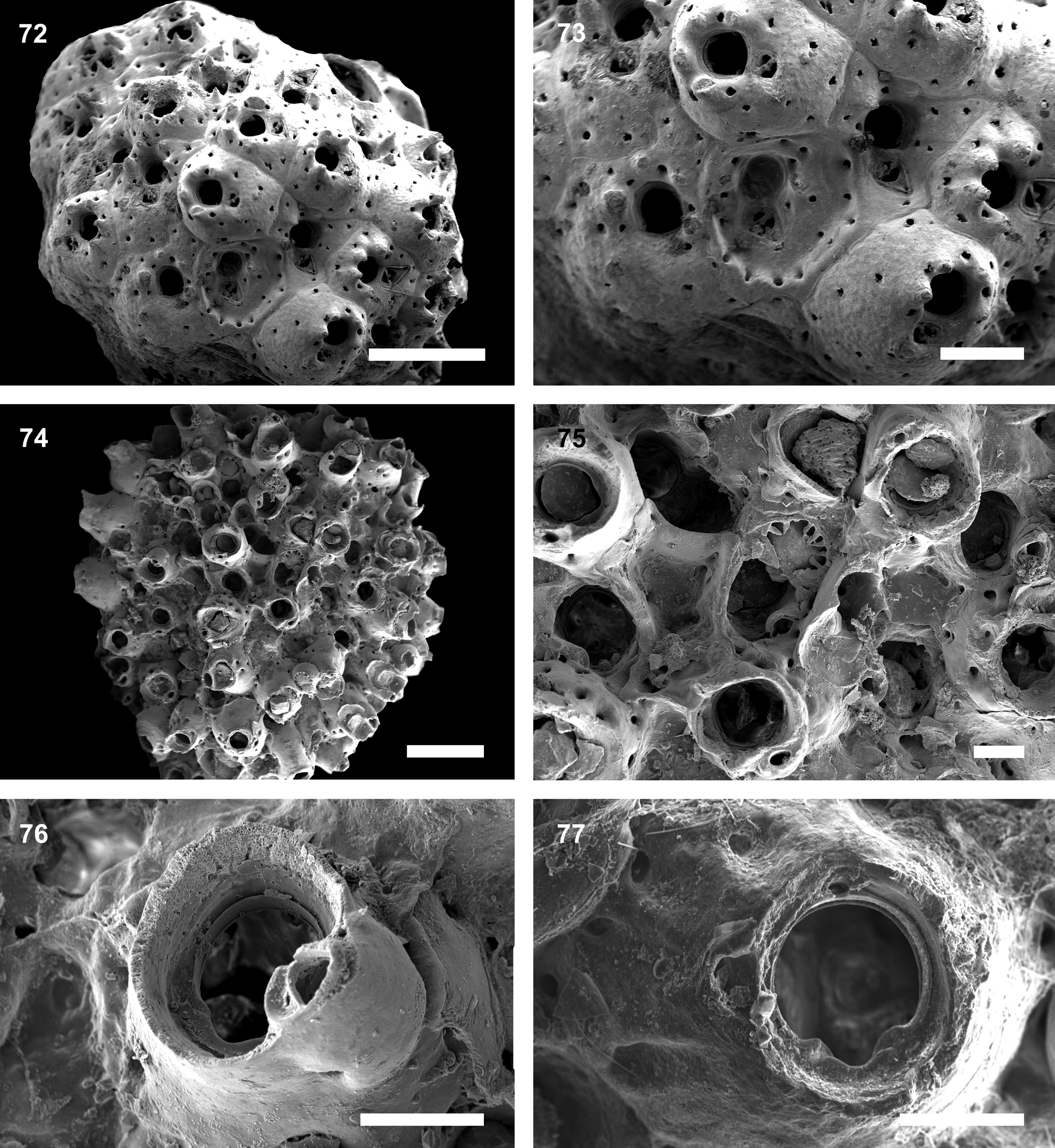
( Figs. 74–77 View FIGURES 72 – 77. 72 – 73 ; Table 8)
Celleporina costazii: Almeida et al. 2015b: 5 View in CoL .
Material examined. Holotype: UFBA 1664 , Camamu Bay, 13°53’S, 38°59’W, 18–20 m, coll GoogleMaps . October 2012 (on sponge Spongosorites sp.). Paratypes: UFBA 1665 , Camamu Bay , 13°53’S, 38°59’W, 18–20 m, coll GoogleMaps . October 2012 (on sponge Spongosorites sp.); UFBA 1671 , UFBA 2396–97 , Camamu Bay , 13°53’S, 38°59’W, 18–20 m, coll GoogleMaps . October 2012 (on sponge Spongosorites sp.).
Type locality. Camamu Bay , Bahia State, NE Brazil.
Etymology. Named after JoAnn Sanner (Smithsonian Institution, National Museum of Natural History), for her contribution to bryozoology.
Description. Colony encrusting, multilaminar, forming small spherical nodules. Autozooids mound-like, globular to sub-erect. Frontal shield heavily calcified, smooth, marginally punctured by 6–8 large widely-spaced pores. Sometimes additional frontal pseudopores are seen surrounding the peristome, often in young zooids with low peristomes that expose the primary orifice. Primary orifice terminal, circular, sunken, with a broadly U-shaped sinus; condyles rounded, thickened and conspicuous. Secondary orifice formed by a deep tubular peristome, margin usually smooth but sometimes somewhat crenulated. Single or paired peristomial avicularia, proximolateral to the orificial border, placed at peristomial lobes; rostrum triangular, with complete cross-bar, margins smooth, oriented upwardly and distolaterally. Additional frontal avicularia absent. Ooecia slightly wider than long, frontal surface in level with peristome; tabula occupying most of the frontal surface, bordered by a single row of 8– 12 distolateral pores; ooecial surface with distinct ribs in later astogeny. Ooecia open into distal part of peristome, just above the operculum; aperture quadrangular, edged by a broad band of smooth ectooecium.
Remarks. Celleporina joannae n. sp. can be distinguished from other species of Celleporina by the combination of a tubular peristome with 1–2 avicularia placed at lobes, a widely U-shaped sinus with strong rounded condyles, no frontal avicularia, and ooecia with a quadrangular aperture and a tabula bordered by a single row of 8–12 distolateral pores.
At least eight species of Celleporina are reported to the Western Atlantic ( Vieira et al. 2008; Bock 2016). Three of them are readily distinguished from C. joannae n. sp. in having ooecia with entirely porous tabula, viz. Celleporina diota ( Marcus, 1938) , Celleporina langei ( Marcus, 1939) and Celleporina lucida ( Hincks, 1880) . Two other species, Celleporina abtrusa Winston & Vieira, 2013 and Celleporina bicostata Hayward, 1980 , have smooth ooecial tabula. Celleporina joannae n. sp. most closely resembles Celleporina lacrimula Hayward, 1992 and Celleporinas surcularis ( Packard, 1863) in having ooecial pores commonly separated by distinct ribs, and a peristome with lobes. The species are distinct, however, in the primary orifice (drop-shaped in C. lacrimula and almost circular in C. surcularis ; with a broadly U-shaped sinus and strong condyles in C. joannae n. sp.) and in their vicarious avicularia (present in C. lacrimula C. lacrimula and C. surcularis ; absent in C. joannae n. sp.).
Specimens from the Brazilian coast described by Marcus (1937, 1939, 1949) under the name Celleporina costazii ( Audouin, 1826) differ from C. joannae n. sp. in having a median peristomial umbo and vicarious avicularia and ooecia becoming entirely porous.
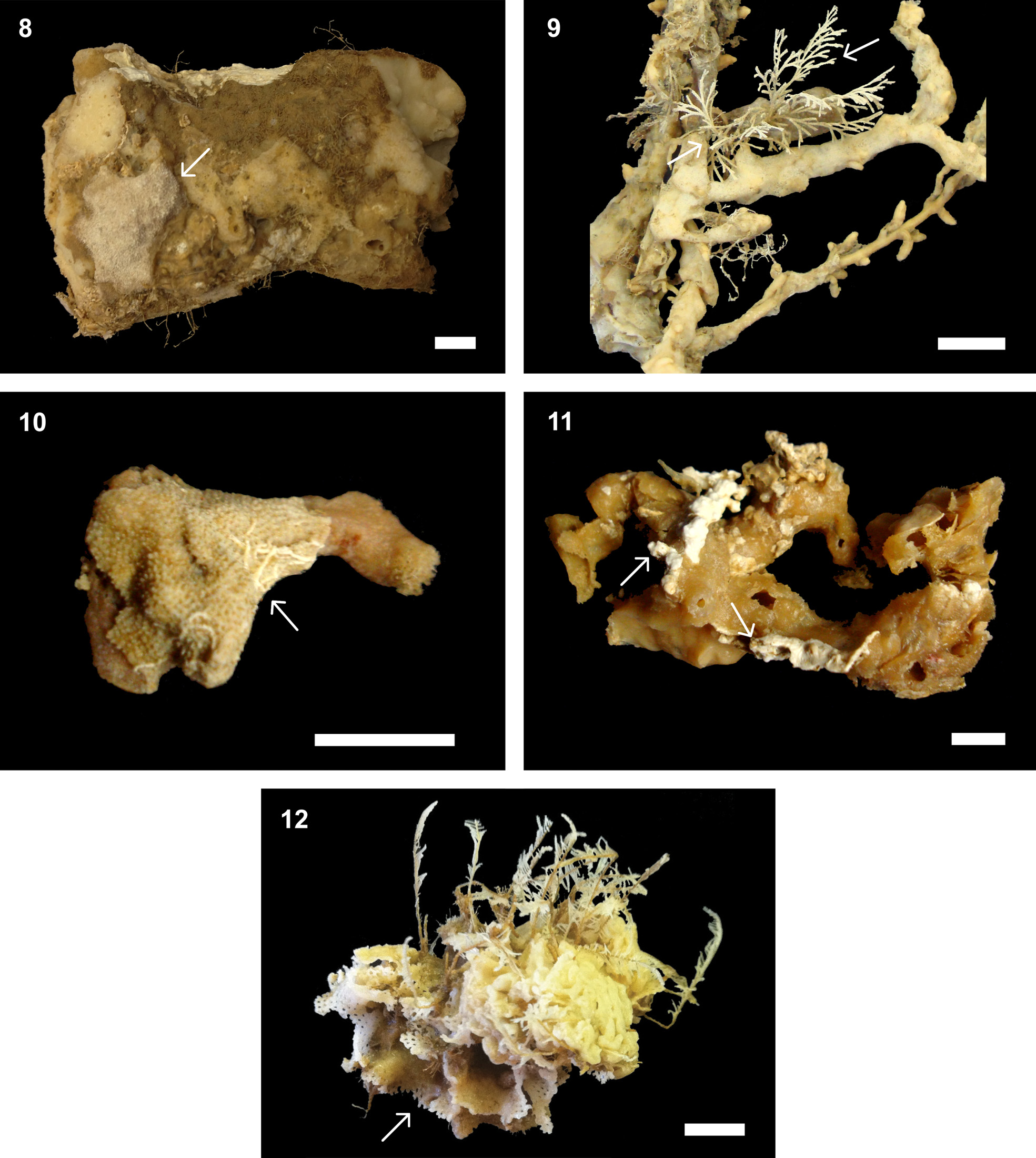
Species of Celleporina are commonly found encrusting shells, other bryozoans, corals and hydrozoans, worm tubes and algae (e.g., Cook 1985; Marcus 1938, 1939; Hayward & Ryland 1999; Ramalho et al. 2011; Winston & Vieira 2013). Here we found spot-like colonies with 2–3 mm in diameter encrusting the rugose-textured sponge Spongosorites sp. ( Fig. 11 View FIGURES 8 – 12 ).
Distribution. Atlantic: Brazil (Bahia).
Material examined. Holotype: UFBA 1185 , Camamu Bay, 13°53’S, 38°59’W, 18–20 m, coll GoogleMaps . October 2012 (on sponge Haliclona (Soestella) melana ). Paratypes: UFBA 1667 , Camamu Bay , 13°53’S, 38°59’W, 18–20 m, coll GoogleMaps . October 2012 (on sponge Haliclona (Soestella) melana ); UFBA 2398 , Camamu Bay , 13°53’S, 38°59’W, 18–20 m, coll GoogleMaps . October 2012 (on sponge Haliclona (Soestella) melana ).
Type locality. Camamu Bay , Bahia State, NE Brazil.
Etymology. Honorific for Iara Coelho Sousa, Ana C.S. Almeida’s mother.
Description. Colony encrusting, multilaminar, nodular. Autozooids semi-erect, hexagonal, limited by distinct grooves. Frontal shield heavily calcified, smooth to slightly rugose, convex, raised distally, imperforate centrally, marginally punctured by 5–20 large pseudopores. Primary orifice small relative to frontal shield, sunken, with arcuate distal edge and broad concave proximal sinus, with two triangular condyles placed at proximal third of the orifice. Secondary orifice oval to orbicular, often obscuring primary orifice and forming a smooth tubular peristome, particularly in ovicelled zooids. Avicularia absent. Ooecia globular, partially framed by the tubular peristome, punctured by 10–25 pseudopores, opening into distal part of peristome, just above the operculum.
Remarks. Turbicellepora iarae n. sp. is the only species of the genus that lacks any type of avicularium. However, its colony form, frontal calcification, primary and secondary orifices, and distinct porose ooecia fully agrees with the definition of the genus. Other species reported from Brazilian waters are Turbicellepora brasiliensis Winston, Vieira & Woollacott, 2014 , Turbicellepora pourtalesi Winston, 2005 and Turbicellepora winstonae Vieira, Gordon, Souza & Haddad, 2010 . Besides the absence of avicularia, T. iarae n. sp. can be also distinguished from T. brasiliensis by the peristome (tubular in T. iarae n. sp.; broad and flat-rimmed in T. brasiliensis ), and primary orifice (without sinus in T. iarae n. sp.; with shallower U-shaped sinus in T. brasiliensis ). Other differences between T. iarae n. sp. and T. pourtalesi include the bluntly pointed frontal mucro, primary orifice with a median U-shaped sinus, sinuate peristome and avicularia of T. pourtalesi , all absent in T. iarae n. sp. Turbicellepora winstonae is distinct from T. iarae n. sp. in having an orificial sinus, oral avicularia and a short peristome (the peristome of T. iarae n. sp. is well-developed and tubular).
Turbicellepora View in CoL species are frequently found on hard substrata such as calcareous nodules but also encrusting algae, worm tubes, bryozoans and hydrozoans (e.g., Hayward 1978; Winston 2005; Vieira et al. 2010). Colonies studied here were attached to the smooth-textured sponge Haliclona (Soestella) melana View in CoL ( Fig. 15 View FIGURES 13 – 17 ). Distribution. Atlantic: Brazil (Bahia).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Celleporina joannae
| Almeida, Ana C. S., Souza, Facelucia B. C., Menegola, Carla & Vieira, Leandro M. 2017 |
Celleporina costazii: Almeida et al. 2015b : 5
| Almeida 2015: 5 |