Paracanthopoma parva Giltay, 1935
|
publication ID |
https://doi.org/10.11606/1807-0205/2022.62.072 |
|
publication LSID |
lsid:zoobank.org:pub:A32FD3AF-C87F-4C75-9100-D695C3578283 |
|
DOI |
https://doi.org/10.5281/zenodo.10845458 |
|
persistent identifier |
https://treatment.plazi.org/id/A81A87C0-FFFA-FC60-FEED-17E92128A8D4 |
|
treatment provided by |
Felipe |
|
scientific name |
Paracanthopoma parva Giltay, 1935 |
| status |
|
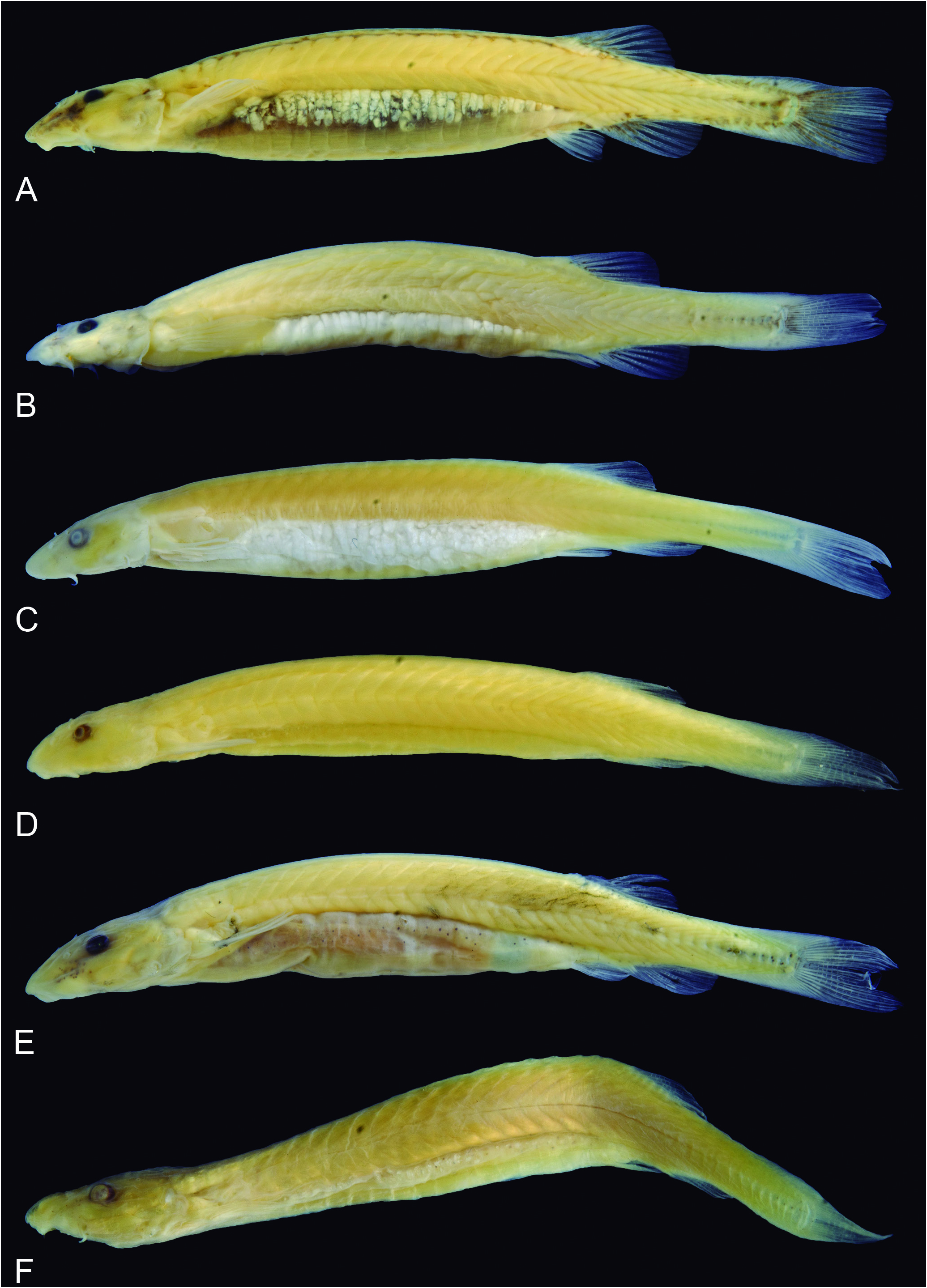
Paracanthopoma parva Giltay, 1935 View in CoL View at ENA ( Fig. 28 View Figure 28 )
Paracanthopoma parva Giltay, 1935: 1 View in CoL , figs. 1-3 [type locality: Brésil, Rio Catrymany supérieur, type: IRSNB 43, cotype: IRSNB 602 (1)] – Gosline, 1945: 66 [catalogue] – Walschaerts, 1987: 16-17 [type catalog] – Burgess, 1989: 324 [checklist] – Schmidt,1993 [in part, only c&s specimen AMNH 72898SW, later designated as paratype of Paravandellia alleynei Henschel et al., 2021b and recatalogued as AMNH 72899SW; occurrence in Essequibo drainage, Guyana; figs. 1, 3 (cephalic latero-sensory canals; jaws; dentition)]. – Eschmeyer et al., 1998: 1292 [catalog] – Spotte, 2002: 97 [historical account; summary of previously published information] – de Pinna & Wosiacki, 2003: 276 [checklist; geographical distribution] – Zuanon & Sazima, 2005 [feeding behavior; phoresis on body of host] – Ferreira et al., 2007: 190 [list, occurrence in rio Branco] – Ferraris, 2007: 410 [checklist] – Wosiacki & de Pinna, 2007: 73 [catalog]; Henschel et al., 2021b [redescription and data on type specimens; anatomy; comparisons;; CT-scan images of head skeleton; invalid lectotype designation].
Material examined
Type material: IRSNB 43 View Materials , 1 View Materials ex, holotype of Paracanthopoma parva Giltay, 1935 , 24.3 mm SL, Brazil, Roraima, upper rio Catrimani (trib. to rio Branco ), col., C. Lako and G. Salathé ; IRSNB 602 View Materials , 1 View Materials ex, paratype of Paracanthopoma parva Giltay, 1935 , 25.2 mm SL, collected with holotype ; AMNH 72899 About AMNH SW (originally AMNH 72899 About AMNH SW), 1 ex (c&s), 22.0 mm SL, paratype of Paravandellia alleynei .
Non-type material: BRAZIL: rio Araguaia: MZUSP 53594, 1 ex, 20.7 mm SL, Mato Grosso, rio Araguaia, near Araguaiana, col., unknown, Jul 1997; MZUSP 53824, 19 ex (4 c&s), 20.4-29.7 mm SL, Mato Grosso,rio Araguaia near Araguaiana (from the branchial chamber of Rhaphiodon sp. ), col., unknown, Jul 1997; MZUSP 89181, 5 ex 14.0- 17.5 mm SL, Mato Grosso, Cocalinho, rio Araguaia, col., unknown, 25 Jul 2005; MZUSP 104095, 5 ex, 24.5-26.4 mm SL, Tocantins, Ananás, rio Araguaia, near border of São Geraldo do Araguaia ( 06°08′24″S, 48°19′47″W), col., G. Baumgartner et al., May 2009; rio Branco: CAS 118205, 1 ex, 24.1 mm SL, Amazonas, upper rio Catrimani, col., S. Lako; INPA 8158, 2 ex, 14.9-15.7 mm SL, Roraima, rio Tacutu (= Itacutu), next to the market, col., J.A.A. Gomes and J. Zuanon, 26 Mar 1992; INPA 16555 (mixed with 6 ex of Pc.alleynei ), 2 ex (1 c&s), 21.2-22.4 mm SL, Roraima, Boa Vista, Maracá, rio Branco,col.,O.Bitar, May1988; rio Jari: MZUSP 100234, 1 ex, 19.1 mm SL, Amapá / Pará, rio Jari, above Cachoeira de Santo Antônio, between Porto Sabão and 5 km above mouth of rio Uiratapuru ( 00°37′02″S, 52°31′35″W), col., C. R. Moreira & F.A. Bockmann, 20-24 Feb 2008; MZUSP 126876, 2 ex, 17.4-17.7 mm SL, Amapá, Laranjal do Jari, Igapó on left margin of rio Jari, upstream from mouth of rio Iratapuru, upstream from Cachoeira de Santo Antônio ( 00°35′05″S, 52°36′59″W), col., J. Birindelli et al., 22 Feb 2009 (collected with Pc. irritans, MZUSP 103511); MZUSP 104874, 2 ex, 21.3-21.6 mm SL, Pará, Monte Dourado, rio Jari, right margin, in front of mouth of Igarapé Carrapatinho,upstream from Cachoeira de Santo Antônio ( 00°35′39″S, 52°38′36″W), col., M.C. Soares & M. R. Carvalho, 02 May 2009; rio Madeira: MNRJ 15422, 11 ex, 20.7-25.0 mm SL, Rondônia, Nova União, rio Urupá (trib. to rio Machado), col., unknown, 13 Jul 1986; MZUSP 13994, 5 ex, 26.2-29.0 mm SL, Rondônia, Paraíso, channel of rio Machado (from body of Brachyplatystoma filamentosum ), col., M. Goulding, 06 May 1978; MZUSP 30397, 1 ex, 26.8 mm SL, Rondônia, Paraíso,rio Machado (from dorsal fin of Brachyplatystoma filamentosum ), col., M. Goulding, 20 May 1978; MZUSP 30400, 10 ex (3 c&s, 1 head removed for SEM), 26.6-30.4 mm SL, Rondônia, Independência,rio Machado (main channel) (from body of Brachyplatystoma filamentosum , 39 kg), col., M. Goulding, 06 May 1978; MZUSP 30407, 1 ex, approx. 9 mm SL (specimen damaged), Mato Grosso, Aripuanã, rio Madeira (probably rio Aripuanã) (from Brachyplatystoma filamentosum ), col., M. Goulding, 31 Dec 1979; rio Negro: MPEG 3329, 1 ex, 31.7 mm SL, Amazonas, Santa Isabel do rio Negro, rio Negro at Ilha de Tamaquaré, col., M. Goulding, 10 Oct 1979; MZUSP 29793, 2 ex, 20.3-25.2 mm SL, Amazonas, rio Negro at Cachoeira de São Gabriel (from body of"surubim″, 40 cm), col., M. Goulding, 18 May 1979, MZUSP 100230, 2 ex, 19.2-25.0 mm SL, rio Negro at Cachoeira de São Gabriel, col., M. Goulding, 18 May 1979; rio Tapajós: LIRP 7668, 10 ex, 13.7-22.1 mm SL, Mato Grosso, Sapezal, rio Juruena ( 12°54′22.00″S, 58°55′01.00″W), col., R, Ilário, 21 Jan 2009; LIRP 12696, 3 ex 19.0- 24.8 mm SL, Pará, Jacareacanga, rio Teles Pires ( 08°51′28″S, 57°25′10″W), col., M. Carvalho & A. Datovo, 04 Dec 2005; MZUSP 24277, 1 ex 12.6 mm SL, Pará, São Luis, rio Tapajós, col., EPA, 05 Nov 1970; MZUSP 63076, 25 ex (4 c&s), 15.5-32.6 mm SL, Mato Grosso, Nova Mutum, rio Arinos, col., H.F. Mendes, 16 Jan 1998; MZUSP 64923, 1 ex, 12.3 mm SL, Pará, Monte Cristo, rio Tapajós, lake on island in front of Monte Cristo, col., EPA, 08 Dec 1970; MZUSP 95662, 6 ex, 13.4-27.4 mm SL, Mato Grosso, Paranaíta, rio Teles Pires near ferryboat dock at road MT-416 ( 09°27′07″S, 56°30′46″W), col., L.M. Sousa & A.L. Netto-Ferreira, 27 Sep 2007; MZUSP 95934, 1 ex, 27.8 mm SL, Mato Grosso, Itauba, rio Teles Pires ( 10°58′30″S, 55°44′03″W), col., J.L.O. Birindelli & P. Hollanda-Carvalho, 01 Oct 2007; MZUSP 96148, 1 ex, 15.5 mm SL, Mato Grosso, Paranaíta, submerged rocks at middle of rio Teles Pires ( 09°26′58″S, 56°29′19″W), col., L.M. Sousa & A.L. Netto-Ferreira, 28 Sep 2007; MZUSP 99703, 17 ex (2 mol), 21.3-27.4 mm SL, Mato Grosso, Itauba, rio Teles Pires, near Pousada Ana Lima (a local lodge) ( 11°00′40″S, 55°23′47″W),col., R.A.G.Fuentes, 23 Mar 2008; MZUSP 101366, 8 ex (1 SEM), 23.7-32.0 mm SL, Mato Grosso,rio Arinos (from Brycon sp. and Pseudoplatystoma sp. ),col., H.F. Mendes, 14 Jan 2000; MZUSP 109848, 2 ex, 23.5-25.5 mm SL, Mato Grosso, Sapezal, rio Juruena at PCH (pequena central hidrelétrica) telegráfica ( 12°51′01″S, 58°55′38″W), col., R. Ilário, 31 Jan 2009; MZUSP 116411, 1 ex, 35.2 mm SL, Mato Grosso, Paranaíta, rio Apiacás (trib. to rio Teles Pires) ( 09°11′42.39″S, 57°05′05.31″W), col., W. Ohara, 19 Nov 2014; MZUSP 119276, 2 ex, 26.9-29.8 mm SL, Pará, Novo Progresso, rio Jamanxizinho (trib. to rio Jamanxin) ( 07°02′28.50″S, 55°41′20.72″W), col., O. Oyakawa et al., 10 Aug 2015; MZUSP 126271, 8 ex, 22.1-26.3 mm SL, Mato Grosso, Novo Mundo, rio Rochedo (trib. to rio Teles Pires), under bridge on dirt road from Vila Cinco Mil and Vila do Rochedo ( 09°44′02.31″S, 55°42′02.70″W), col., O. Oyakawa et al., 18 Oct 2017. rioTocantins: MZUSP 40585, 29 ex (5 c&s), 18.2-27.2 mm SL, Goiás, Monte Alegre de Goiás, rio Paranã above the mouth of rio Bezerra, col., J.C. de Oliveira & W.J.E.M. da Costa, 10 Jan 1989; MZUSP 114443, 1 ex, 16.1 mm SL, Tocantins, Aurora do Tocantins, rio Palmas, at encounter with rio Sombra, at Balneário Douradas ( 12°48′11.3″S, 46°22′08.4″W), col., Oyakawa et al., 01 Dec 2012; rio Trombetas: INPA 12420, 1 ex, 23.5 mm SL, Igarapé Caxipacoré, col., E. Ferreira, M. Jegú, 20 Apr 1985; MZUSP 15715-23, 6 ex (2 c&s), 19.6-27.2 mm SL, Pará, Trapiche da Sede da Reserva Biológica de Trombetas (from body of Brachyplatystoma filamentosum , 130 cm SL), col., R.M.C. Castro, 11 Jul 1979; rio Xingu: MZUSP 74624, 13 ex (1 head prepared for SEM), 17.2-25.4 mm SL, Mato Grosso, rio Xingu, Parque Indígena do Xingu, Posto Diauarum, col., G. R. Kloss, 08 Dec 1973; MZUSP 74650, 5 ex, 18.1-22.6 mm SL, same locality and collector as MZUSP 74624, 12 Dec 1973; MZUSP 87048, 1 ex, 27.4 mm SL, Mato Grosso, Gaúcha do Norte, rio Curisevo, Porto do Vitório, near Ribeirão Kevuaieli ( 13°02′05″S, 53°25′19″W), col., C. Moreira et al., 19 Oct 2004 (collected together with Pc. irritans MZUSP 87049); MZUSP 91959, 3 ex, 22.1-29.4 mm SL, Mato Grosso, Paranatinga, rio Culuene, at the planned site of upcoming hydroelectric dam Paranatinga II (approx. 13°49′S, 53°15′W),col.,J.Birindelli etal., 21 Aug2006; MZUSP 94143, 1 ex, 23.5 mm SL, Mato Grosso, Gaúcha do Norte, rio Culuene, Fazenda do Sr. Zezé ( ca. 2 km above bridge) ( 13°30′53″S, 53°05′40″W), col., F.C. T. Lima et al., 21-26 May 2007; MZUSP 97190, 2 ex, 26.8-28.5 mm SL, Pará, Altamira, rio Curuá (trib. to rio Iriri), at village of Castelo dos Sonhos ( 08°19′07″S, 55°05′23″W), col., J.L. Birindelli et al., 22 Oct 2007; MZUSP 111769, 1 ex, 14.9 mm SL, Pará,Altamira,rio Xingu,island beach immediately downstream from Vila de Belo Monte ( 03°05′52″S, 05°44′12″W), col., O. Oyakawa et al., 13 Nov 2011. COLOMBIA: FMNH 94767, 1 ex, 29.6 mm SL, Vichada, río Tomo near Puerto Borracho (río Orinoco drainage), col., W.W. Lamar, 17 Feb 1979. ECUADOR: FMNH 99519, 2 ex, 20.2-25.2 mm SL, río Aguarico, near Destacamiento Militar Cuyabeno and confluence of río Cuyabeno and río Aguarico (río Napo drainage), col., D. Stewart et al., 21 Oct 1983. GUYANA: ANSP 179207, 2 ex, 24.0- 29.7 mm SL, Rupununi (Region 9), Ireng River, 6.9 km WSW of village of Karasabai ( 04°01′10″N, 59°36′06″W), col., M.H. Sabaj et al., 01 Nov 2002; ANSP 180020, 3 ex, 10.5-13.3 mm SL, Rupununi (Region 9), Takutu River, 3.77 km SSW of Lethem ( 03°21′18″N, 59°49′51″W), col., M. Sabaj et al., 16 Nov 2003; MHNLS 21614, 1 ex, 14.3 mm SL, Essequibo River at Akuthopono Rocks ( 01°39′02,4″N, 58°37′40,5″), col., C.Lasso,J. Señaris,A. Eustace, 25-26 Oct 2006 (Mixed with 1 ex of Ochmacanthus cf. flabelliferus ). PERU: ANSP 180483, 1 ex, 22.8 mm SL, Madre de Dios, río Manuripe ( Orton-Madre de Dios drainage), road crossing at town of Mavila ( 11°55′44″S, 69°07′15″W), col., M. Sabaj et al., 31 Jul 2004; MUSM 4562, 3 ex 22.9-25.4 mm SL, Madre de Dios, Parque Nacional Manu, Manu, Pakitza, río Manu, col., H. Ortega, 22 Jun 1993 (collected together with Paravandellia sp. MUSM 20672); MUSM 20674, 1 ex 24.3 mm SL, Madre de Dios, Manu, Parque Nacional Manu, Panagua, rio Manu, col., W. Valle, 06 May 1991; INHS 42780, 1 ex 24.0 mm SL, Loreto, río Nanay, Sabolla Cocha, near Sabolla (río Amazonas drainage), col., C. Chuquipiondo Guardia, 28 Jul 1997; USNM 357993, 2 ex, 22.4-24.8 mm SL, Madre de Dios, Manu, Parque Nacional Manu, Pakitza, río Manu close to mouth of río Panahua, col., W. Valle, 06 May 1991 (collected together with Paravandellia sp. USNM 317775). VENEZUELA: MCNG 22882, 1 ex, 25.1 mm SL, Amazonas, Atabapo, Ventuari, río Paru, 10 km upstream from confluence with río Asisa ( 04°40.00′N, 65°58.00′W), col., L. Nico, E. Guayamore, 02 Oct 1989.
Diagnosis: Distinguished from all congeners except Pc. carrapata , Pc. daemon , and Pc. truculenta by the presence of nine ( Pc. daemon occasionally with 10) teeth on the median premaxilla (vs. 3 to 5 or 11 and more); by the presence of a single median s6 pore, visible on the middle of skull posterior to eyes (vs. paired s6 pores, distant from midline of skull), and by the supraoccipital anteriorly produced into large pointed spike (vs. either anteriorly concave or straight across skull roof). Distinguished from all congeners except Pc. carrapata and Pc. truculenta by the posterior margin of the anal fin well posterior to vertical through that of the dorsal fin (vs. margins of two fins approximately at same vertical or that of anal fin only slightly posterior to that of dorsal fin); and by the deeply emarginate, bilobed caudal fin (vs. truncate with round corners or only slightly concave). Distinguished from Pc.carrapata and Pc. truculenta by lacking an extensive invasion of the skull roof by head musculature, with widest exposed part of neurocranium wider than interorbital (vs. exposed part of neurocranium reduced, approximately equivalent to interorbital); and by the larger opercular and interopercular odontodophores, approximately as large as eye (vs. odontodophores smaller than eye). Distinguished from Pc. carrapata by having smaller median premaxillary teeth, with the distal (post-bend) portion as large as the basal portion (vs. distal portion larger than basal one); by the longer interopercular odontododes, where the largest odontode is longer than the long axis of the interopercle (vs. largest odontode smaller than the long axis of the bone); and by the interopercular odontodes inserted partly towards the ventral margin of the interopercle, with their insertions tilelike at that area (vs. odontodes clustered at the distal end of the bone, with their insertions at approximately the same plane). Distinguished from Pc. truculenta by having four or five opercular odontodes, clearly visible on surface of skin (vs. opercular odontodophore very reduced, bearing only one or two odontodes not protruding from surface of head, sunk in small slit of integument).
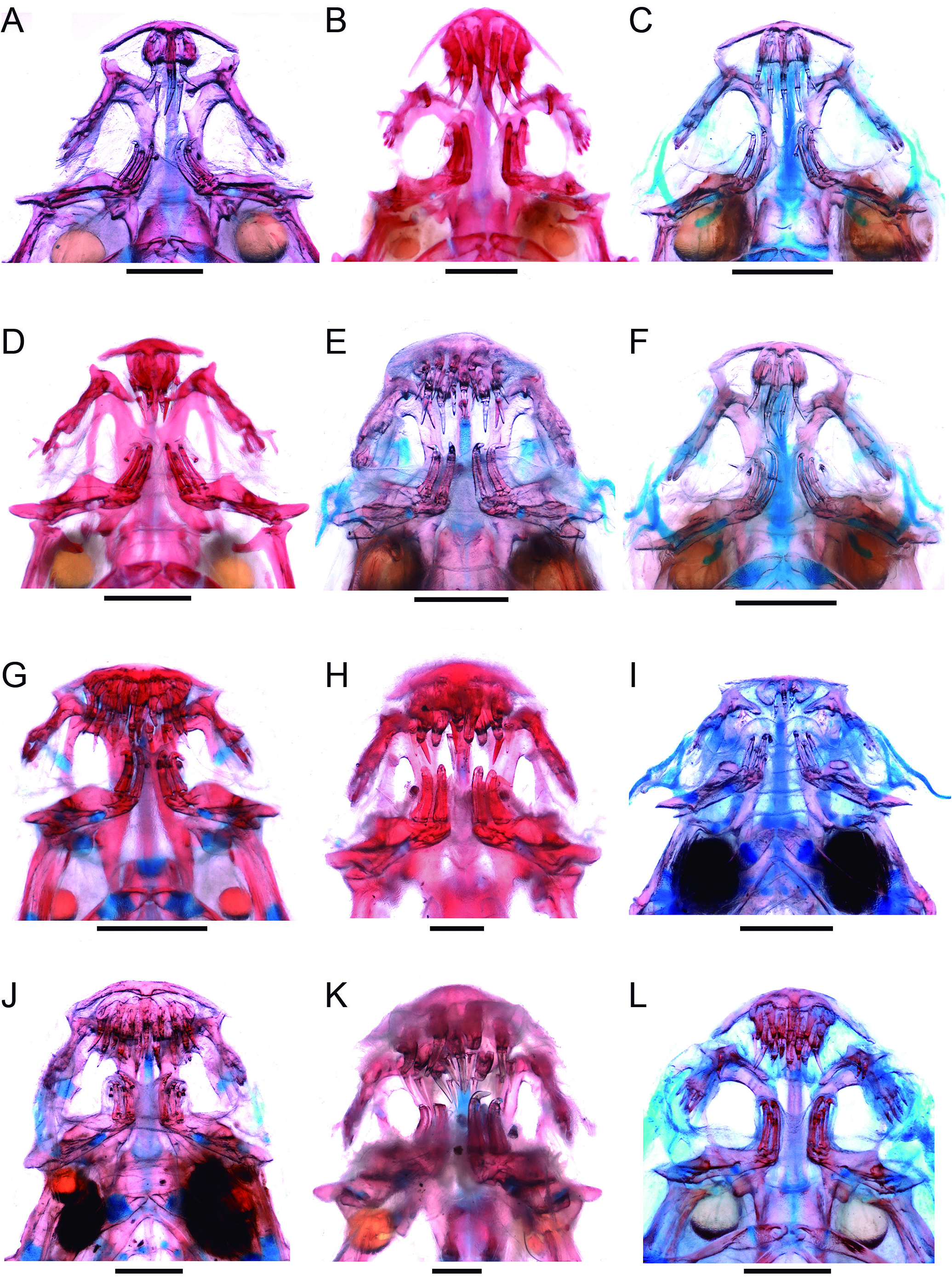
Description: Morphometric data for the holotype and paratypes are provided in Table 9 View Table9 . Body short (HL 18.0-22.1% SL) ( Fig. 28 View Figure 28 ). Cross-section of body slightly broader than deep at pectoral-fin insertion and increasingly compressed posterior to that point, tapering to caudal fin. Dorsal profile of body straight from head to origin of dorsal fin ( Fig. 28 View Figure 28 ), except in specimens preserved with curved vertebral column, often those with full guts, where it is convex. Dorsal and ventral profiles of caudal peduncle straight and converging towards midline along anterior half and straight or slightly convex and diverging along posterior half, sometimes angulate at beginning of procurrent caudal-fin rays. Caudal peduncle narrow, but expanded by procurrent rays along posterior third or half. Proportion of expansion greater in smaller individuals, with most of peduncle expanded, paddle-shaped, in very small specimens, ca. 11-14 mm SL ( e.g., ANSP 180020). Ventral profile of body gently convex until pelvic-fin origin ( Fig. 28 View Figure 28 ), with some specimens with greatly distented abdomens due to gut contents. Myotomes and longitudinal skeletogenous septum clearly visible along whole body. Axillary gland very large, elongate in shape, protruding markedly on surface of body and making anterior pat of trunk widest part of fish (except in those with distended abdomens). Anterior end of gland surrounding dorsal, ventral and posterior surfaces of muscular pectoral-fin, as thick corselet, extending posteriorly to beyond margin of adpressed pectoral fin (for at least 50% of fin length, sometimes 100%). Gland tapering to fine posterior tip, its large round or oval pore located at is midlength, approximately at vertical through beginning of last third of pectoral fin. Condition of gland posterior to pore evidently related to amount of secretion stored. In some specimens, postpore part of gland appearing as nearly absent, clearly due to empty condition of its lumen.
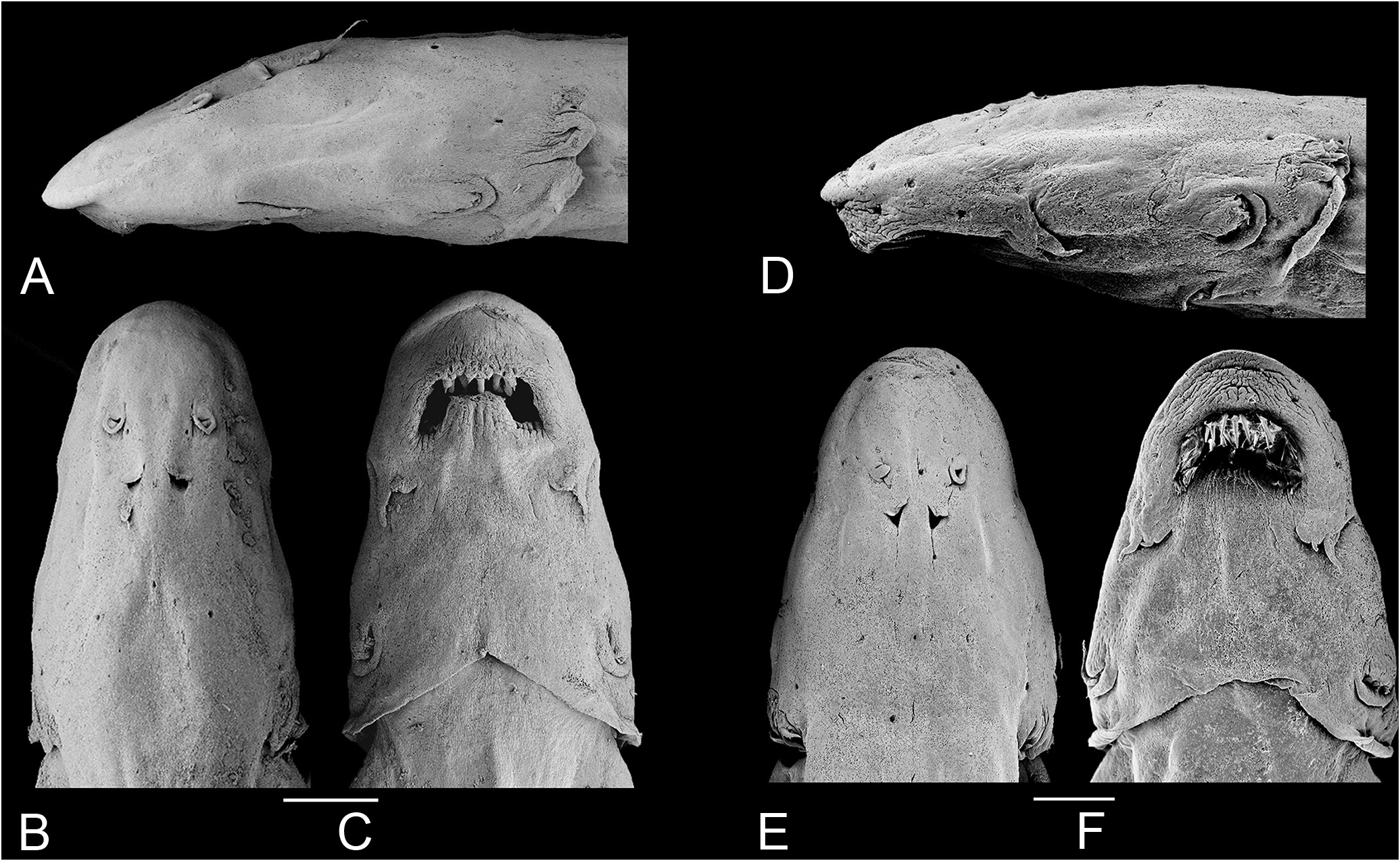
Dorsal profile of head continuous with that of dorsum, sometimes indicated by slight muscle constriction. Head longer than broad (head width 57.1-68.3% HL), snout broad, parabolic with a round tip, sometimes with central portion of snout slightly differentiated ( Figs. 28 View Figure 28 , 29 View Figure 29 ). Muscles covering most of dorsal part of head, with head width varying between 3 to 4 times the width of exposed skull roof in dorsal view. Exposed area proportionall larger in small specimens. Head deep for Paracanthopoma (head depth 32.3-42.9% HL), with dorsal profile straight and horizontal until eye in lateral view, then angled ventrally an straight to tip. Eye large (11.5-18.6% HL), without free orbital rim, located dorsolaterally on head and directed dorsolaterally, with pronounced dorsal component ( Fig. 28 View Figure 28 ). Integument over eye thin at middle, thick and opaque at margin. Middle of eye almost exactly at middle of HL, interorbital width approximately 75% of longitudinal diameter of eye. Eyelens very large, occupying most of lateral surface of eye and either entirely unconstricted by iris or constricted only marginally, with large round pupil, in specimens examined. Anterior nostril small, located in narrow teardrop-shaped slit on surface of skin and surrounded by short tubule of integument produced posteriorly into small pointed process ( Fig. 29 View Figure 29 ), with double elastin cores. Anterior internarial width slightly larger than interorbital. Posterior naris slightly larger than anterior ones, round or triangular in shape, partly occluded by anterior flap of integument. Posterior naris positioned anteromesially to eye, their middle at, or posterior to, transverse line through anterior margin of eyes. Posterior internarial width narrower than interorbital.
Opercular odontodophore medium-sized, dorsolaterally located on head, on dorsal half of head depth in lateral view. Opercular odontodes 4 or 5, closely positioned as two very large ones juxtaposed posteriorly and two or three smaller anterior ones. Main axis of opercular odontodes oriented horizontally in lateral view, with their distal portion curved dorsoposteriorly. Two or three caps of replacement odontodes posteriorly to mature ones. Interopercular odontodophore either similar-sized, slightly larger or slightly smaller than opercular one, located ventrolaterally on head, immediately ventral to horizontal through origin of pectoral fin, with 3 (rarely) to 5 (modally 4) odontodes closely positioned in single row of three or four near posterior edge of interopercle, plus single smaller one anteriorly (when 5). Interopercular odontodophore much closer to opercular one than to eye. Two or three replacement tooth caps located posteromesially to mature ones. Interopercular periodontodal fold of integument well-developed, nearly round and extending well-beyond tips of odontodes. Epiodontodeal velum thick, covering odontodes almost or quite to internal rim of periodontodal fold.
Mouth inferior (ventral), often filled with tightly bitten chunks of meat, supposedly from host fish, often entirely hiding internal mouth morphology. Each premaxilla with single scalpelloid teeth attached to its distal tip (visible only in skeletal preparations), but actually two adjacent tooth sockets, one of which normally vacant, corresponding to half-formed replacement tooth adjacent to mature one ( Figs. 4H View Figure 4 , 30 View Figure 30 ). Vacant socket position varying among specimens, being either lateral or mesial one. Normally two additional initial-stage replacement caps suspended in soft tissue dorsally to mature one and its incomplete neighbor. Mature scalpelloid tooth with distal portion disproportionately reduced and very strongly curved over rest of teeth, with pungent tip nearly adpressed to margin of basal plate. Scalpelloid tooth deeply hidden in labial tissue, but its distal surface easily emerging when premaxilla forcibly abducted. Conical teeth absent in premaxilla. Upper lip thick, ventral surface of its anterior region deeply plicate longitudinally ( Fig. 29 View Figure 29 ). Lateral portions of upper lip with less numerous oblique plicae, only on its parabucal surface. Median premaxilla very large, with 9 teeth disposed in one anterior row of four (convex anteriorly), one posterior row of four (convex posteriorly) plus single middle tooth ( Figs. 4H View Figure 4 , 30 View Figure 30 ). All teeth perpendicular to ventral surface of median premaxilla basally,but strongly curved posteriorly at distal pungent portion, those on anterior row taller than on posterior one. All median premaxillary teeth strongly laterally compressed basally. Median premaxillary dentition occupying almost all of upper jaw and most of interior of mouth. Many replacement tooth caps posterodorsally to mature dentition, creating confusing aspect to posterior limit of median premaxillary dentition. Median premaxillary velum absent. Hypodontal pad of median premaxilla thickly cushioning teeth. Lower jaw wide, occupied mostly with large dentary lobes nearly entirely fused to each other at midline, continuous with mental region posteriorly, and deeply plicate longitudinally. Jaw cleft deep and strongly directed posteriorly, approaching parallel to longitudinal axis and forming broad space separating lower jaw laterally from inner margin of upper jaw.Dentary diastema poorly differentiated, represented by small concave, sometimes angulate, area at midline, entirely absent in some specimens. Rami of mandible very close together at midline. Dentary teeth 4, closely packed at mesial end of dentary and disposed in two pairs, one dorsal and one ventral, with only latter visible in ventral view ( Figs. 4H View Figure 4 , 30 View Figure 30 ). Axis of dentary teeth anteriorly-directed at base, but curved dorsolaterally distally. Branchiostegal region with large, continuous, and posteriorly concave branchiostegal velum ( Fig. 29 View Figure 29 ), with small median notch at midline in few specimens. Dorsal portion of branchial membrane reaching anterior margin of pectoral-fin base. Branchial membrane broadly attached to isthmus, leaving small branchial opening anterodorsally to pectoral-fin base, spanning approximately for area between ventral margin of opercular odontodophore and middepth of interopercular odontodophore. Maxillary barbel very short and proximally broad, its base flap-like, only distal portion filamentous ( Fig. 29 View Figure 29 ). Posterior point of is base anterior to vertical through anterior margin of eye, its tip extending posteriorly aproximately to vertical through posterior margin of eyes in lateral view. Mesial (or ventral) part of maxillary-barbel base continuous with membranous outgrowth extending posteriorly from corner of mouth. Rictal barbel small, located mesially to base of maxillary one and approximately one-third or less of its size, its base immersed in membranous expansion at corner of mouth. Rictal barbel sometimes difficult to identify among irregularities of surrounding integument folds, but its homology with trichomycterid rictal barbel evident by well-developed internal core. In some specimens, no clear external component of rictal barbel. Nasal barbel vestigially represented by posterior elongated portion of fold around anterior naris described above, with double internal elastin core.
Lateral line short and straight, curved dorsally near posterior end in some populations ( e.g., MZUSP 53824) its terminal pore approximately at vertical through midlength of pectoral-fin,near dorsal margin of axillary pore. Short secondary branch splitting off ventrally from anterior portion of canal, with corresponding pore opening approximately at anterior third of main canal. Single lateral-line tubule extending for more than half of sector of canal posterior to bifurcation and dorsal bending.
Pectoral fin short (56.1-70.2% HL),with i +5 rays.Distal margin of pectoral fin gently convex,its base immediately ventral to midline of body in lateral view. Pelvic fins small, well-separated from each other at base, with i + 4 rays. Pelvic splint present. Origin of pelvics located at, or slightly anterior to, vertical through origin of dorsal-fin, entirely covering anus and extending posteriorly to almost reach origin of anal fin. Posterior margin of pelvic fin round. Dorsal fin small, triangular with broadly round apex, gently convex distal margin and ii + 6 fin rays, plus 3 to 5 procurrent ones. Few specimens with iii + 5 rays, apparently resulting from third unbranched ray failing to split, rather than actual meristic difference. Anal fin small, slightly more elongate in shape than dorsal one, with gently convex distal margin and ii + 5 fin rays, plus 3 to 5 procurrent ones. Origin of anal fin at, or slightly anterior to, vertical through end of dorsal-fin base. Anal fin normally slightly smaller than dorsal one, but opposite in some specimens. Caudal fin strongly bilobed, with equal lobes or lower lobe slightly larger than upper one. Bilobal condition of caudal fin less pronounced in small individuals, with fin practically truncate by 12 mm SL ( e.g., ANSP 180020). Specimens at 15 mm SL, however, already with adult bilobed condition ( e.g., INPA 8158). Principal caudal-fin rays 6 + 7. Procurrent caudal-fin rays 15 to 19 dorsally and 14 to 18 ventrally.
Vertebrae 35 (n = 2), 36 (n = 10), 37 (n = 31), 38 (n = 19), 39 (n = 8) or 40 (n = 1). First dorsal-fin pterygiophore subsequent to neural spine of vertebra 18 (n = 3), 19 (n = 8, holotype), or 20 (n = 5). First anal-fin pterygiophore subsequent to haemal spine of vertebra 20 (n = 2), 21 (n = 6, holotype), 22 (n = 6) or 23 (n = 2). Dorsal-fin pterygiophores 7 (n = 15). Anal-fin pterygiophores 6 (n = 15). Branchiostegal rays 3 (n = 15; 4 rays on one side of one specimen in MZUSP 30400 View Materials ).Type specimens have 37 and 38 vertebrae.
Pigmentation in preservative: Most specimens nearly entirely white ( Fig. 28 View Figure 28 ). Neurocranium dark with brain pigment seen by transparency. Faint dark field along lateral surface of snout, anterodorsally to maxillary barbel base. Thin streaks of dark, sometimes reduced to small spot, also along anterodorsal margin of opercular odontodophore. Irregular dark cloud vertically crossing bases of principal caudal-fin rays, sometimes extending shortly horizontally along proximal portions of middle rays. Specimens in MZUSP 109848 ( Fig. 32A View Figure 32 ) have a comparatively heavy dark pigmentation unlike any other samples of the species, including (in addition to traits described above) paired series of dark spots along each side of dorsal midline (this detail is also seen in a single juvenile specimen, MZUSP 114443), intense dark fields at bases of dorsal, anal and caudal fins and distal portion of hypural plate (latter two separated by narrow white band). Because those specimens have no additional co-varying characteristics which might indicate specific differentiation, they are considered as an unusual color morph of Pc. parva .
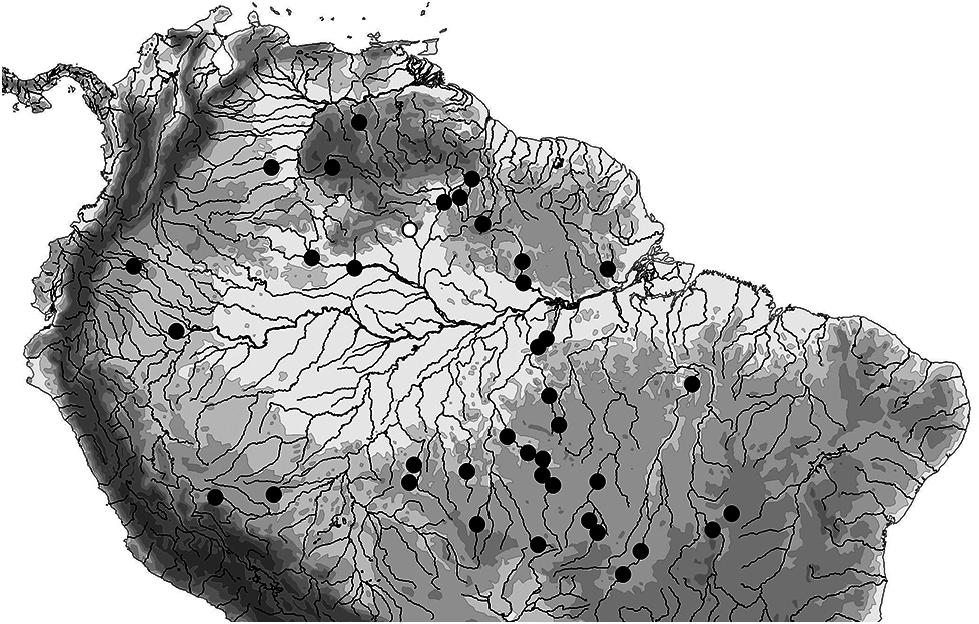
Geographical distribution: Paracanthopoma parva is widely distributed in the uplands of the Amazon, Essequibo and Orinoco drainages in Brazil, Colombia, Ecuador, Guyana and Peru ( Fig. 31 View Figure 31 ). In the Amazon, it occurs in upland sectors of most major northern and southern tributaries, but is conspicuously absent from lowland areas.
Remarks: Paracanthopoma parva is a variable species, in both details of body and head shape, and in pigmentation ( Figs. 29 View Figure 29 , 32 View Figure 32 ). A preliminary examination of the material available initially suggested the existence of various different species across the ample geographical range of what is here considered as Pc. parva . However, potentially distinguishing characteristics are of degree or proportion, rather than qualitative, and tend to vary continuously across populations, when enough representative material is available. The few discontinuities encountered are usually associated with lack of samples from intervening areas, generating suspicions about the significance of such apparent discontinuity. Obviously there is some degree of differentiation among some of the various disjunct populations of Pc.parva , but the level of clinal morphological variation indicates that they do not warrant recognition as separate species according to the criteria and data utilized in this work. But we do not rule out the possibility that more detailed studies, perhaps including genetic markers will find grounds for the recognition of additional species within what is here considered as Pc. parva .
Small specimens of Paracanthopoma parva (until ca. 14 mm SL; e.g., ANSP 180020, MZUSP 95662) have a spatulate caudal peduncle, expanded dorsally and ventrally by large procurrent rays, markedly different from the narrow caudal peduncle of larger specimens. This juvenile morphology resembles the situation in species with small adult sizes,such as Pc.irritans , suggesting that the spatulate caudal peduncle in the latter is a paedomorphic feature. The smallest Pc. parva with evidence of ingested blood is a 10.5 mm SL specimen in ANSP 180020 (which also happens to be the smallest individual known of the species).
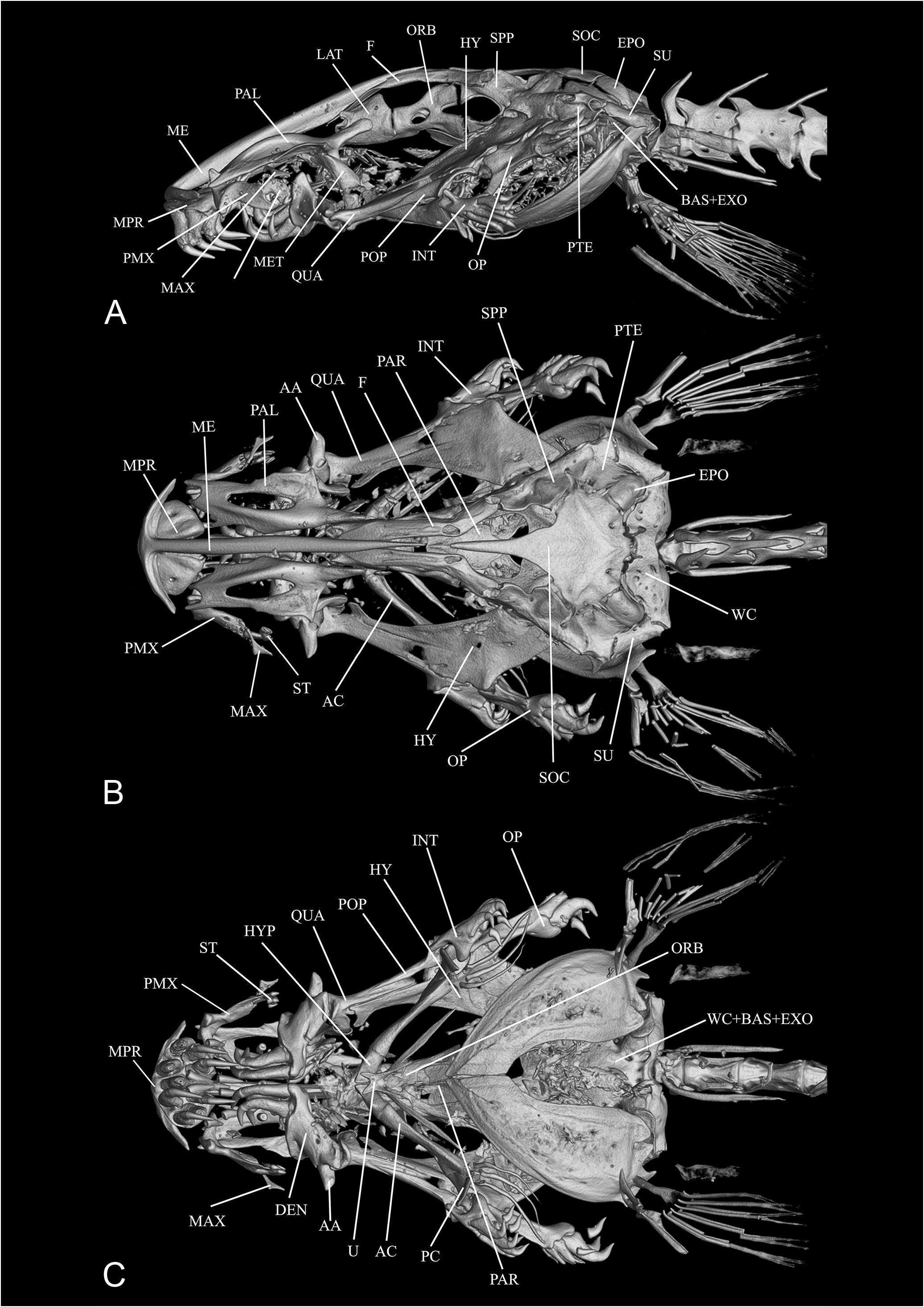
There are additional non-type specimens of Pc. parva collected from the same locality ( upper rio Catrimany), approximate dates and collectors ( Carlos Lako) as the types, namely MNRJ 4227 View Materials , 3 View Materials ex (listed in Miranda-Ribeiro, 1947: 1) and CAS-SU 118205 View Materials , the latter used for CT-scan imaging here ( Fig. 30 View Figure 30 ) ; the collector of the latter is listed on records as C. Laks, probably a misprint.
Henschel et al. (2021b) provide a redescription of Paracanthopoma parva based on the types and non-type specimens, along with the description of Pc. alleynei . The information on the types is most valuable and includes osteological data obtained with CT scan images. A lectotype designation proposed in that paper ( Henschel et al., 2021b) is invalid. Giltay (1935: 1) had already designated one of the specimens as"Type″ and the other as"Cotype″ (and not jointly as "cotypes″ as alleged in Henschel et al., 2021b: 11) and this is sufficient discrimination as a holotype designation. In any event, the specimen chosen as the lectotype corresponds to the one originally labelled as "Type″ by Giltay.
| MZUSP |
Museu de Zoologia da Universidade de Sao Paulo |
| CAS |
California Academy of Sciences |
| INPA |
Instituto Nacional de Pesquisas da Amazonia |
| R |
Departamento de Geologia, Universidad de Chile |
| MNRJ |
Museu Nacional/Universidade Federal de Rio de Janeiro |
| MPEG |
Museu Paraense Emilio Goeldi |
| LIRP |
Laboratorio de Ictiologia, Faculdade de Filosofia |
| PCH |
Prestwich and Pilkington Botanical Society |
| T |
Tavera, Department of Geology and Geophysics |
| FMNH |
Field Museum of Natural History |
| ANSP |
Academy of Natural Sciences of Philadelphia |
| MHNLS |
Coleccion de Mastozoologia, Museo de Historia Natural de La Salle |
| INHS |
Illinois Natural History Survey |
| USNM |
Smithsonian Institution, National Museum of Natural History |
| MCNG |
Museo de Ciencias Naturales de la UNELLEZ en Guanare |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Paracanthopoma parva Giltay, 1935
| Pinna, Mário de & Dagosta, Fernando Cesar Paiva 2022 |
Paracanthopoma parva
| Ferreira, E. & Zuanon, J. & Forsberg, B. & Goulding, M. & Briglia-Ferreira, S. R. 2007: 190 |
| Ferraris, C. J. 2007: 410 |
| Wosiacki, W. B. & de Pinna, M. C. C. 2007: 73 |
| de Pinna, M. C. C. & Wosiacki, W. B. 2003: 276 |
| Spotte, S. 2002: 97 |
| Eschmeyer, W. N. & Ferraris, C. J. & Hoang, M. & Long, D. 1998: 1292 |
| Burgess, W. E. 1989: 324 |
| Walschaerts, L. 1987: 16 |
| Gosline, W. A. 1945: 66 |
| Giltay, L. 1935: 1 |