Elysia serca Er. Marcus, 1955
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4148.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:91353147-FDA8-45CC-A8F1-1DE801C835A6 |
|
DOI |
https://doi.org/10.5281/zenodo.5664193 |
|
persistent identifier |
https://treatment.plazi.org/id/A04A7E6D-9C71-FFC2-46C9-FED0FC0E1A64 |
|
treatment provided by |
Plazi |
|
scientific name |
Elysia serca Er. Marcus, 1955 |
| status |
|
Elysia serca Er. Marcus, 1955 View in CoL
( Figs. 6 View FIGURE 6 L, 33–35)
Elysia catulus [non Gould, 1870] — Espinosa et al. 2005: 56.
Elysia serca Er. Marcus 1955: 113 View in CoL –115, figs. 49–52, 59 (Type locality: Island of São Sebastião and Ubatuba, State of São Paulo) — Er. Marcus 1957: 415, fig. 45; Ev. Marcus 1970: 209 –210, fig. 8, non fig. 7 (see Ev. Marcus 1976a); Ev. Marcus 1976a: 6; Ev. Marcus 1980: 68, figs. 12, 28, 40, 50; Hosoe, 1956: 1 –6. pl. 1; Jensen 1982: 87 –93, figs. 2–5; Jensen & Clark 1983: 5; Clark 1994: 905; Valdés et al. 2006: 68 –69; Espinosa et al. 2007: 83; Händeler et al. 2009: fig. 7; Christa et al. 2014: fig. 3; Krug et al. 2015: 990 –991, figs. 3B, 4.
Elysia clena Er. Marcus & Ev. Marcus, 1970: 49 View in CoL , figs. 81, 89–90 (Type locality: Piscadera Bay, Curaçao and Barbados) — Ev. Marcus 1972a: 292 –293; Ev. Marcus & Hughes 1974: 507 –509, fig. 18–20; Ev. Marcus 1980: 72, figs. 18, 56.
Type material. Elysia serca— untraceable, not at MZSP ( Siqueira Dornellas & Simone 2011); Elysia clenauntraceable , not at USNM or MZSP ( Siqueira Dornellas & Simone 2011).
Material examined. Bahamas: Exuma, 15 December 2007, 1 specimen ( CPIC 00050 ), 9 March 2008, 1 specimen ( CPIC 00027 ), Stocking Island , 14 January 2005, 1 specimen ( LACM 172293 About LACM ) , Feb 2009, 1 specimen (CPIC 00075).
Live animal. Er. Marcus (1955) described the live animals as “nearly cylindrical.”
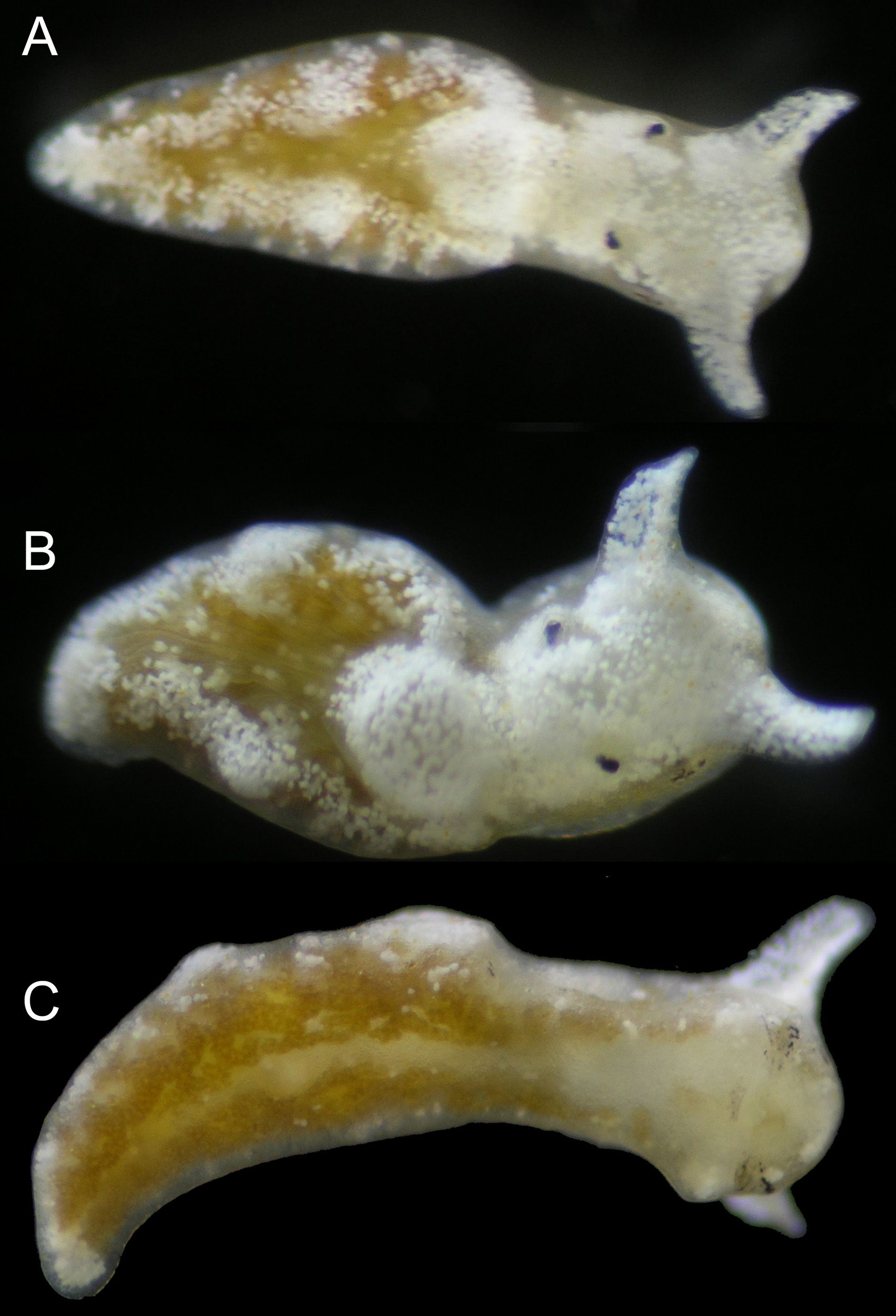
External anatomy. Body coloration green-brown to dark brown, with white patches scattered along parapodial flanks and margin. White patches concentrated on top and front of head, with head entirely white on some specimens ( Fig. 32 View FIGURE 32 ); on others, sides of head including area around eyespots having background green-brown color. Parapodia reduced, not covering rounded pericardium. Parapodial margin thickened. Rhinophores short, white; tips rounded or slightly pointed at one end ( Fig. 32 View FIGURE 32 A–B). Foot with same color as parapodial flanks, not clearly demarcated from sides of parapodia; clear medial line running down foot ( Fig. 32 View FIGURE 32 C). End of body forms elongated tail, narrowing to rounded tip.
Pericardium raised, rounded; color ranging from green-brown with white speckling, to all white ( Fig. 32 View FIGURE 32 B). Renopericardium elongated, gradually narrowing; running up to half of body length on larger specimens. Six to seven dorsal vessels emerging irregularly on either side of renopericardium, some branching once near margin of parapodium ( Fig. 33 View FIGURE 33 ).
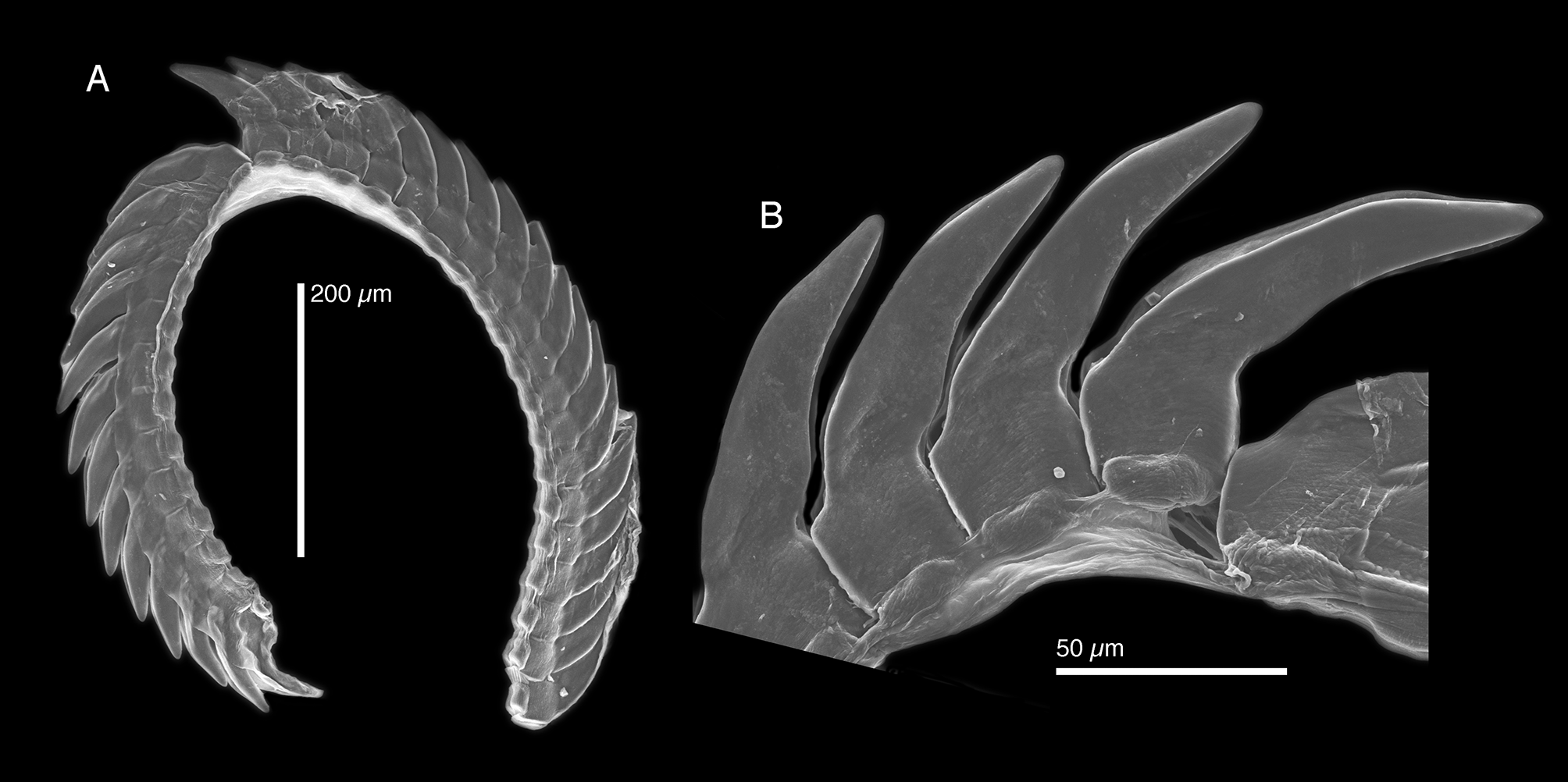
Internal anatomy. Radula with 31–35 teeth (CPIC 0 0 0 27, LACM 173228), 12–13 teeth in ascending limb and 19–22 in descending limb ( Fig. 35 View FIGURE 35 A) in a closely packed arrangement ( Fig. 35 View FIGURE 35 A–B). Leading tooth elongate, slightly curved, robust, and lacking denticles, with a subtle bend at distal ¼ of tooth. Tooth base tall, nearly cuboid ( Fig. 35 View FIGURE 35 B). Housing depression for interlocking teeth “V”-shaped and extending ¾ of tooth length. Base of tooth narrow, about ¼ or less total tooth length.
Penis narrow with a long, curved tip tapering from a wide base (CPIC 0 0 0 27, CPIC 0 0 0 50, CPIC 00075) and devoid of armature ( Fig. 6 View FIGURE 6 L). Deferent duct long, narrow, and highly convoluted.
Reproduction and development. Larval development is planktotrophic, with a mean egg diameter of 61 µm and no ECY ( Clark & Jensen 1981).
Host ecology. Er. Marcus (1955) described E. serca from Brazil, collected from “Phaeophyceae” ( Sargassum or Padina ) and Ulva , which were later asserted to be the host algae (Er. Marcus 1957). Later work indicated this was a mistaken assumption that the algae on which animals were found also constituted their diet. Studies by Jensen (1982, 1983a) clearly demonstrated that E. serca specializes on seagrasses in at least three genera: Halophila engelmanni , Halodule wrightii , and Thalassia testudinum . Slugs prefer young, fully developed leaves free from epiphytes ( Jensen 1982). Epithelial cells are pierced by the radula to allow suctorial feeding on larger mesophyll cells. Slugs grew fastest on, and preferentially associated with, Halophila engelmanni , which has large but thin epidermal cells easily pierced by the radula overlying large mesophyll cells ( Jensen 1983a). In H. wrightii , epidermal cells are comparably thin, but small relative to the underlying mesophyll cells, necessitating a zigzag feeding behavior to avoid repeatedly penetrating the epidermis above an already-emptied mesophyll cell. In T. testudinum , the epidermal cells are thicker than the length of the radular tooth, slowing feeding on this least preferred host.
Phylogenetic relationships. A member of subclade 2, Elysia serca was recovered as sister to E. chlorotica ( Fig. 4 View FIGURE 4 ); however, the seagrass-feeding species E. catulus was not available for inclusion in our phylogeny. Based on their derived seagrass diet, we predict that E. catulus is the true sister species of E. serca .
Range. Bahamas ( Valdés et al. 2006), Barbados ( Er. Marcus & Ev. Marcus 1970; Ev. Marcus & Hughes 1974), Belize ( Clark and DeFreese 1987), Brazil ( Er. Marcus 1955, 1957; Ev. Marcus 1970), Curaçao ( Er. Marcus & Ev. Marcus 1970), Cuba (Espinosa et al. 2005; Espinosa et al. 2007), Florida ( Jensen 1982; Jensen & Clark 1983; Clark 1994; Valdés et al. 2006) , U.S. Virgin Islands ( Jensen 1983b).
Remarks. The pharynx of E. serca is exceptionally large for the body size, relative to other small Elysia spp. (Er. Marcus 1957). Maximum body length reported for the collection of slugs that yielded the type material for E. serca was 8 mm alive and 3.5 mm preserved. In the original description, freshly collected specimens of E. serca were noted to differ in external coloration depending on the substrate. The darker morph from brown algae was described as “brownish with a reddish violet area between the parapodia behind the region of the heart ... There are three large white spots, one in front of the heart and two in the middle of the free border of the parapodia.” A lighter morph from Ulva was “light green with darker green alimentary organs. They have the same three large white spots and white stipples as the brownish slugs and a black line along the margin of the parapodia that may also occur in the brownish animals.”
Both forms from Brazil had a roughly serrated cutting edge on the radular tooth, which was figured as having the tooth cusp bent at a right angle to the base ( Er. Marcus 1955: fig. 52). Er. Marcus & Ev. Marcus (1970) later described E. clena as having similarly shaped, but smooth, radular teeth, and a slightly different pattern of dorsal vessel venation. Jensen (1982, 1983b) synonymized E. clena Er. Marcus & Ev. Marcus 1970 with E. serca , based on population-level variation in radular denticulation and ontogenetic changes in dorsal venation pattern. Four of five populations from Florida had smooth radular teeth ( clena - type), but in a fifth population, teeth were coarsely denticulate ( serca - type). In specimens from St. Thomas, teeth were predominantly smooth but some were faintly denticulate ( Jensen 1983b). There was also inter-population variation in the number of teeth, and in the ratio of outer-to-inner cusp length for teeth. Jensen hypothesized tooth morphology varied depending on the species of seagrass included in the diet of an individual. Further, juvenile slugs had the pattern of dorsal vessels described for E. clena , but upon maturation in the lab, developed the pattern described for E. serca ( Jensen 1982) .
Jensen (1982, 1983b) further hypothesized that E. serca was itself a junior synonym of E. catulus ( Gould, 1870) . The poorly studied E. catulus was the most common sacoglossan in Connecticut, U.S. ( Clark 1975), but is restricted to colder waters, ranging from northern New England to North Carolina, U.S. ( Ev. Marcus 1980). Both E. serca and E. catulus share an ascus displaced to the right side of the pharynx, weakly developed parapodia, and bent radular teeth, but E. catulus is typically black whereas dark specimens are rare in E. serca ( Ev. Marcus 1972b, Jensen 1982). Diet distinguishes the species, but is a covariate of range: E. catulus feeds on the seagrass Zostera , restricted to temperate waters in the western Atlantic, whereas E. serca feeds on tropical seagrasses not found in the range of E. catulus . We do not consider the synonymy of E. serca and E. catulus here, as typical E. catulus are not found in the Caribbean, but we consider it unlikely that one sacoglossan species could span such different biogeographical provinces as the tropical Caribbean and northeastern U.S.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Elysia serca Er. Marcus, 1955
| Krug, Patrick J., Vendetti, Jann E. & Valdés, Ángel 2016 |
Elysia clena
| Ev 1980: 72 |
| Ev 1974: 507 |
| Ev 1972: 292 |
| Er 1970: 49 |
Elysia serca
| Krug 2015: 990 |
| Espinosa 2007: 83 |
| Valdes 2006: 68 |
| Clark 1994: 905 |
| Jensen 1983: 5 |
| Jensen 1982: 87 |
| Ev 1980: 68 |
| Ev 1976: 6 |
| Ev 1970: 209 |
| Hosoe 1956: 1 |
| Er 1955: 113 |