Clytia elsaeoswaldae Stechow, 1914
|
publication ID |
https://doi.org/10.11646/zootaxa.4689.1.1 |
|
persistent identifier |
https://treatment.plazi.org/id/9E4CE23A-FFE6-F16D-FF03-671CFC172A50 |
|
treatment provided by |
Plazi (2019-10-25 12:55:46, last updated 2024-11-26 14:23:24) |
|
scientific name |
Clytia elsaeoswaldae Stechow, 1914 |
| status |
|
Clytia elsaeoswaldae Stechow, 1914 View in CoL
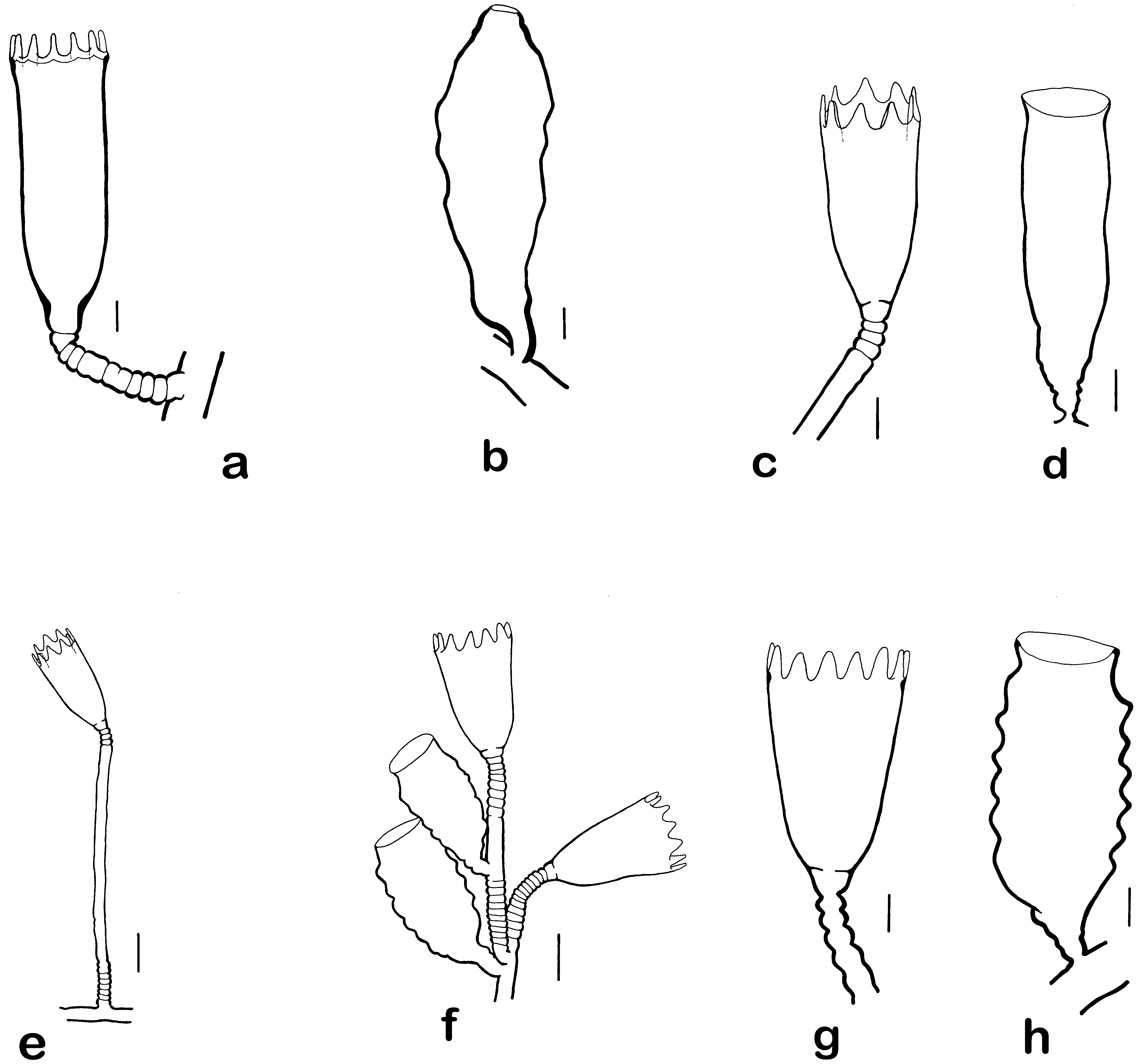
Figs. 10 View FIGURE 10 c–e, 11
Clytia elsae-oswaldae Stechow, 1914: 125 View in CoL , fig. 4.? Clytia coronata View in CoL .— Nutting, 1915: 51 [part].
? Clytia coronata View in CoL .— Fraser, 1944: 134 [part].
Gonothyraea gracilis View in CoL .— Fraser, 1944: 148 [part] [not Laomedea gracilis M. Sars, 1850 View in CoL ].
Clytia cylindrica View in CoL .— Joyce, 1961: 53, pl. 10, fig. 4, pl. 11, figs. 1, 2.— Shier, 1965: 34, pls. 17, 20 [not Clytia (Platypyxis) cylindrica L. Agassiz, 1862 View in CoL ].
Type locality. Virgin Islands of the United States: St. Thomas , port of Charlotte Amalie ( Stechow 1914: 125, as Clytia elsae-oswaldae ) .
Material examined. Sanibel Island, beach at Lighthouse Point, 26°26’57”N, 82°01’07”W, on a detached alga, in intertidal pool, 03 August 2014, two colonies or colony fragments, to 4 mm high, with gonothecae, coll. D. Calder, ROMIZ B4369.— Sanibel Island , beach at Lighthouse Point, 26°26’55”N, 82°01’08”W, on detached Syringodium in water along shore, 21° C, 34.5‰, 19 March 2018, several young colonies, 1 cm high, without gonothecae, coll. D. Calder, ROMIZ B4370 GoogleMaps .— Fort Myers Beach , 26°27’55”N, 81°58’04”W, on stranded Sargassum filipendula , 05 February 2018, two colonies, up to 6 mm high, with gonophores, coll. D. Calder, ROMIZ B4409 GoogleMaps .
Remarks. Hydroids of the genus Clytia Lamouroux, 1812 , as currently defined, are distinctive in overall colony form. However, identification of the various species is frequently a challenge. Given a preponderance of perfunctory original descriptions, a limited number of taxonomically useful morphological characters, and the likelihood of considerable intraspecific morphological variability, uncertainty and confusion exist about the identity and scope of several species. In view of the difficulties encountered by traditional taxonomists in dealing with this group, more molecular analyses are required to resolve the diversity, identities and relationships of clytiid species. Indeed, Clytia as presently constituted appears to be polyphyletic given the ambiguous placement of C. hummelincki (Leloup, 1935) and C. paulensis ( Vanhöffen, 1910) in phylograms of Proboscoida Broch, 1909 [1910] ( Cunha et al. 2017). Significant contributions to knowledge of C. hemisphaerica ( Linnaeus, 1767) , C. gracilis (M. Sars, 1850) , C. noliformis ( McCrady, 1859) , and C. elsaeoswaldae Stechow, 1914 , all of which occur along the east coast of the United States, have recently been made in papers such as those of Lindner and Migotto (2001, 2002), Lindner et al. (2011), and Cunha et al. (2017).
Hydroids of C. elsaeoswaldae are much like those of the boreal C. gracilis in morphology, and the two were considered conspecific for much of the 20 th century. In some works (e.g., Cornelius 1982), both were included in the synonymy of C. hemisphaerica , but recent molecular studies reveal that the three are distinct genetically ( Lindner et al. 2011; Cunha et al. 2017). According to Lindner et al., characters distinguishing C. elsaeoswaldae from C. gracilis include: (1) colonies are stolonal or mostly so rather than being branched, and (2) gonothecae arise from the hydrorhiza rather than from the branches. Morphological differences said to exist between C. elsaeoswaldae and C. hemisphaerica are summarized under the latter species below. Meanwhile, phylogenetic analyses by Lindner et al. and Cunha et al. indicate that the C. gracilis morphotype comprises several cryptic species.
Establishing the identities of several morphologically similar species of Clytia reported in 19 th and 20 th century literature of the Americas remains nearly unfathomable. Particular difficulties were encountered during this study in sorting out records that apply to C. elsaeoswaldae and C. hemisphaerica , both of which are reported here, and those that were based on specimens of the cool-temperate C. gracilis . Records in the Reported Distribution section below have been based for the most part on current ideas concerning synonymies of species, with consideration given also to probable biogeographic affinities of the species. Reports most likely to be sound are those in which gonothecae of specimens were described and illustrated. While most accounts of the cool-temperate C. gracilis (also reported as C. cylindrica and Gonothyraea gracilis ) from the tropical and warm-temperate western North Atlantic are likely based on the warm water C. elsaeoswaldae , those from pelagic Sargassum are here included under C. hemisphaerica unless gonothecae with smooth walls were described. The latter species is frequent on gulfweed in the western North Atlantic ( Rackley 1974; Calder 1995) while C. elsaeoswaldae is much less so. Records of C. gracilis to the north of Cape Hatteras are taken to have been correctly assigned to that species (or that species complex), and have been excluded from the list below on C. elsaeoswaldae . A few records of C. coronata Clark, 1879 from the warm western Atlantic have been included under C. hemisphaerica , following synonymy adopted in earlier work (Calder 1990 [1991a]: 60). However, Fraser’s (1912b, 1944, 1946 [ 1947 a]) concept of C. coronata more closely corresponds with that of C. elsaeoswaldae , especially in having gonothecae with smooth walls. His records of C. coronata , and those of several North American authors (e.g., Deevey 1950; Fincher 1955; Defenbaugh & Hopkins 1973) following him, remain highly uncertain. As records are interpreted here, C. elsaeoswaldae is believed to have a range in coastal waters from North Carolina to the southern Caribbean Sea, including Bermuda and the Gulf of Mexico, and southwards to Brazil in the western South Atlantic ( Lindner et al. 2011).
The name of this species was originally founded by Stechow (1914) as Clytia elsae-oswaldae . Following the code (ICZN Art. 32.5.2.3), the specific name has been corrected in earlier work ( Vervoort 1968: 15) to elsaeoswaldae . As noted below under C. hemisphaerica , Thaumantias elsaeoswaldae Stechow, 1914 was described as a different species, although it too is now assigned to Clytia . Of the two homonymous names, C. elsaeoswaldae Stechow, 1914 and C. elsaeoswaldae ( Stechow, 1914) , precedence was assigned to the former under the Principle of the First Reviser (ICZN Art. 24.2) because gonothecae were present in its type material, and the species has been recognized as valid a number of times (Calder 1990 [1991a]).
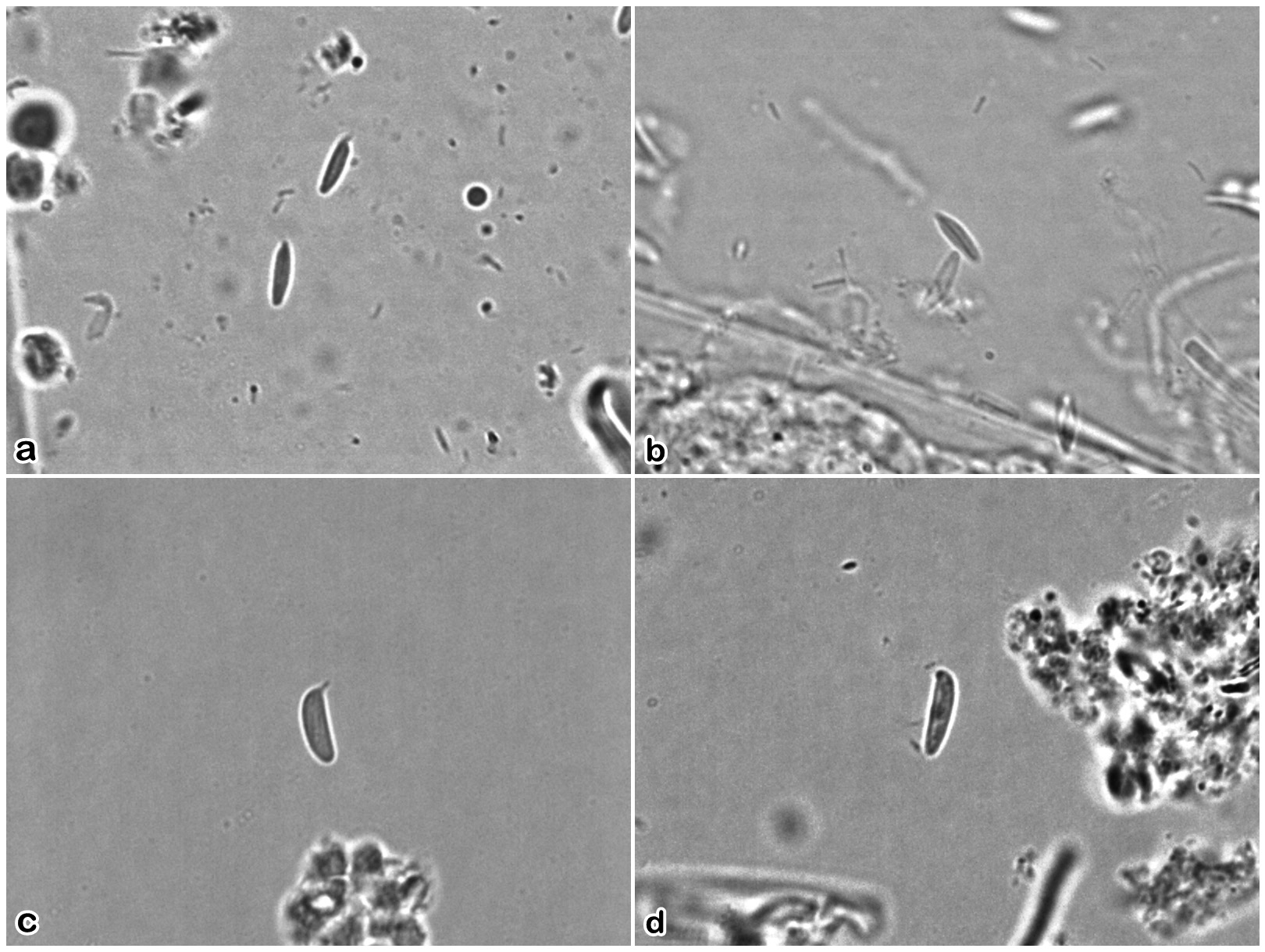
Two distinctly different categories of nematocysts were observed in hydroids of this species, with the larger of the two appearing to occur in two somewhat different forms ( Fig. 11 View FIGURE 11 ). They appear to correspond with A-type b-mastigophores (5.5–6.4 μm long x 1.2–1.5 μm wide, undischarged, n=10, ROMIZ B4369) and B-type b-mas- tigophores (7.0–8.0 long x 1.6–2.2 μm wide, undischarged, n=10, ROMIZ B4369).
The medusa stage of C. elsaeoswaldae has been described in life cycle studies by Lindner & Migotto (2011).
Reported distribution. Gulf coast of Florida.? Cape Romano (as Cape Romanos) ( Nutting 1915: 52, as Campanularia coronata ). —? Cape Romano ( Fraser 1944: 134, as Clytia coronata ).—?West of Cape Romano, 2 miles (3 km) ( Fraser 1944: 149, as Gonothyraea gracilis ).—Seahorse Key area; gonothecae smooth ( Joyce 1961: 53, as Clytia cylindrica ).— Cape San Blas area, on ascidians, sponges, shells, the octocoral Leptogorgia virgulata , and the seagrasses Syringodium and Thalassia ; gonothecae smooth ( Shier 1965: 34, as Clytia cylindrica ).
Elsewhere in western North Atlantic. USA: North Carolina, Beaufort Harbor, on floating seaweed; gonothecae smooth ( Fraser 1912b: 358, as? Clytia coronata ).—? USA: North Carolina, near Beaufort, on Pennaria from piles of railroad bridge + seaward side of Bogue Bank, on gulfweed; gonothecae smooth ( Fraser 1912b: 361, as Gonothyraea gracilis ).— Virgin Islands of the United States: St. Thomas, Charlotte Amalie, on algae from an old wooden boat, surface ( Stechow 1914: 125, as Clytia elsae-oswaldae ).— Venezuela: near Islas Los Tortuguillos, 8–12 feet (2–4 m) ( Leloup 1937: 100, as Laomedea cylindrica ).—? USA: Louisiana, Grand Isle + Pass Christian, on floating seaweed ( Fraser 1944: 134, as Clytia coronata ).—? USA: Louisiana, East Bay ( Fraser 1944: 135, as Clytia cylindrica ).—? USA: Texas, Gulf coast ( Fraser 1944: 135, as Clytia cylindrica ).—? USA: North Carolina, off Cape Hatteras, 35°20’55”N, 75°20’55”W, 16 ftm (29 m) ( Fraser 1944: 149, as Gonothyraea gracilis ).—? USA: Louisiana, Bayou Pass + Grand Isle + East Bay ( Fraser 1944: 149, as Gonothyraea gracilis ).— Colombia: 1 mile (2 km) SW of Cabo de la Vela, 10–13 ftm (18–24 m) ( Fraser 1947b: 7, as Gonothyraea gracilis ).— Venezuela: 3 miles (5 km) N of Isla de Coche, 21–22 ftm (38–40 m) ( Fraser 1947b: 7, as Gonothyraea gracilis ).— USA: Texas, Buoy I-24, Sabine Pass ( Deevey 1950: 343, as Gonothyraea gracilis ).—? USA: Texas, Port Aransas, jetties, on rocks, shells, and occasionally stranded on beach ( Hedgpeth 1950: 73, as Gonothyraea gracilis ).—? USA: Louisiana, Grand Isle ( Deevey 1950: 339, as Clytia coronata ).—? USA: Louisiana, Grand Isle ( Deevey 1950: 341, as Clytia cylindrica ).—? USA: Louisiana, Grand Isle, on floating log ( Behre 1950: 7, as Clytia coronata ).—? USA: Mississippi, Mississippi Sound ( Fincher 1955: 92, as Clytia coronata ).—? Panama: Colón, on an alga on an experimental plate ( Vervoort 1968: 13, as Campanularia (Clytia) cylindrica ).—? Guatemala: Puerto Barríos, on a hydroid on jetty ( Vervoort 1968: 13, as Campanularia (Clytia) cylindrica ).— Virgin Islands of the United States: St. Thomas, sound; gonothecae smooth, on hydrorhiza ( Vervoort 1968: 15, as Laomedea (Phialidium) pelagica ).— USA: Texas, West Flower Garden Bank, on a float cable, 24 feet (7 m); gonothecae smooth ( Defenbaugh 1974: 97, as Clytia cylindrica ).— USA: Texas, West Flower Garden Bank, on a floating sea bean; gonothecae smooth ( Defenbaugh 1974: 99, as Gonothyraea gracilis ).—Gulf Stream, several stations between Florida and North Carolina, on Sargassum natans I, S. fluitans III, S. polyceratium , S. pteropleuron ; scarce; gonothecae smooth ( Rackley 1974: 24, as Clytia cylindrica ).—? USA: South Carolina, Charleston, in mariculture tanks ( Sandifer et al. 1974: 56, as Clytia gracilis ; Calder & Hester 1978: 90, as Clytia gracilis ).—? Colombia: Santa Marta area, on seagrasses, rocky littoral ( Wedler 1975: 332, 333, as Clytia pelagica ).—? Colombia: Santa Marta area, on algae, Thalassia , other hydroids ( Wedler 1975: 340, as Clytia cylindrica ).— Colombia: Bahía de Cartagena ( Flórez González 1983: 119, as Clytia cylindrica ).— USA: South Carolina, inner (17–18 m), middle (32–36 m), and outer (46–69 m) continental shelf + Georgia, inner (17–22 m), middle (23–29 m) and outer (59–67 m) continental shelf ( Wenner et al. 1984: 20, 39, as Clytia cylindrica ).— Bermuda: shallow inshore waters, common; gonothecae smooth ( Calder 1986: 136, as Clytia cylindrica ).—? USA: Louisiana, on a coastal petroleum platform ( Lewbel et al. 1987, as Clytia cylindrica ).— Bermuda: Whalebone Bay, on algae from ledge at entrance, 1 m + Flatts Inlet, on algae, 0.5–1.5 m + Castle Harbour, midway along causeway, on algae, 1.5 m (Calder 1990 [1991a]: 55, as Clytia gracilis ).— Colombia: Bahía de Chengue, on Rhizophora ( Reyes & Campos 1992: 108, as Clytia cylindrica ).— Bermuda: Argus (=Plantagenet) Bank + Challenger Bank ( Calder 2000: 1133, as Clytia gracilis ).— Panama: Mole Buoy, Atlantic entrance to canal + US Army Harbor Craft Ops. Pier #1, Atlantic side + Colón, Isla Margareta, Fort Randolph, shore, 09°23’15”N, 79°53’11”W, 0–1 m + Portobelo Harbor, dock, 09°33’14”N, 79°39’34”W, 0-1 m + Bocas del Toro area, Almirante pilings, 09°16.218’N, 82°23.382’W, 1–10 m + Bocas del Toro area, Hospital Point, 09°20’01.9”N, 82°13’07.7”W, 2–13 m + Bocas del Toro area, Cayo Solarte Sud, 09°18’45.3”N, 82°12’46.6”W, 2–3 m ( Calder & Kirkendale 2005: 486, as Clytia gracilis ).— Virgin Islands of the United States: St. Thomas, Charlotte Amalie, on algae from an old wooden boat (see Stechow 1914), surface ( Ruthensteiner et al. 2008: 13, as Clytia elsae-oswaldae ).—French Lesser Antilles: Guadeloupe, Grande-Terre, E of Saint François, 16°15’18.00”N, 61°14’37.00”W, on Thalassia + Basse-Terre, Petite Anse, 16°05’47.00”N, 61°46’17.00”W, on algae and concretions ( Galea 2008: 17, as Clytia gracilis ).—French Lesser Antilles: Les Saintes, Terre-de-Haut, Pompierre Bay, 15°52’25”N, 61°34’15”W, on Thalassia + Terre-de-Haut, Pain de Sucre, 15°51’45”N, 61°35’60”W, on Halimeda ( Galea 2008: 17, as Clytia gracilis ).—French Lesser Antilles: Guadeloupe, Grande-Terre, Pointe Plate, 16°27.220’N, 61°32.128’W, 15–20 m + Grande-Terre, Passe à Colas, 16°21.269’N, 61°34.193’W, 10–15 m ( Galea 2010: 3, 4, as Clytia gracilis ).—French Lesser Antilles: Les Saintes, Terre-de-Haut, Pointe Morel, 15°53.050’N, 61°34.410’W, 6–11 m ( Galea 2010: 3, 4, as Clytia gracilis ).— Cuba: Villa Clara, Marina Periquillo, 2 m, on marine phanerogams (Varela et al. 2010: 30, as Clytia gracilis ).— Virgin Islands of the United States: St. Thomas, Charlotte Amalie, on algae from an old wooden boat (see Stechow 1914) ( Lindner et al. 2011: 27).— USA: Florida, off St. Lucie Inlet, 27°10.7’N, 80°02.7’W, on Eudendrium carneum , 23 m ( Calder 2013: 54).—French Lesser Antilles: Martinique, Le Prêcheur, 14.780461, -61.211935, 15–18 m ( Galea 2013: 13, figs. 80A–C, as Clytia gracilis ).—Caribbean Sea ( Wedler 2017b: 89, figs. 80A–C, as Clytia gracilis ).— Mexico: Alacranes Reef, on seagrass, sponges ( Mendoza-Becerril et al. 2018b: 131, as Clytia cf. gracilis ).— Panama: Bocas del Toro area, San Cristóbal ( Miglietta et al. 2018b: 108, as Clytia gracilis ).
Agassiz, L. (1862) Contributions to the natural history of the United States of America. Vol. IV. Little, Brown, Boston, 380 pp.
Behre, E. H. (1950) Annotated list of the fauna of the Grand Isle region 1928 - 1946. Occasional Papers of the Marine Laboratory, Louisiana State University, 6, 1 - 66.
Broch, H. (1909 [1910]) Die Hydroiden der Arktischen Meere. Fauna Arctica, 5, 127 - 248. [dating of this publication, sometimes given as 1909, follows evidence from an original wrapper provided by Cornelius (1995 b)]
Calder, D. R. & Hester, B. S. (1978) Phylum Cnidaria. In: Zingmark, R. G. (Ed.), An annotated checklist of the biota of the coastal zone of South Carolina. University of South Carolina Press, Columbia, pp. 87 - 93.
Calder, D. R. (1986) Class Hydrozoa. In: Sterrer, W. (Ed.), Marine fauna and flora of Bermuda: a systematic guide to the identification of marine organisms. Wiley-Interscience, New York, pp. 127 - 155.
Calder, D. R. (1995) Hydroid assemblages on holopelagic Sargassum from the Sargasso Sea at Bermuda. Bulletin of Marine Science, 56, 537 - 546.
Calder, D. R. (2000) Assemblages of hydroids (Cnidaria) from three seamounts near Bermuda in the western North Atlantic. Deep-Sea Research, Part I, 47, 1125 - 1139. https: // doi. org / 10.1016 / S 0967 - 0637 (99) 00093 - X
Calder, D. R. & Kirkendale, L. (2005) Hydroids (Cnidaria, Hydrozoa) from shallow-water environments along the Caribbean Coast of Panama. Caribbean Journal of Science, 41, 476 - 491.
Calder, D. R. (2013) Some shallow-water hydroids (Cnidaria: Hydrozoa) from the central east coast of Florida, USA. Zootaxa, 3648 (1), 1 - 72. https: // doi. org / 10.11646 / zootaxa. 3648.1.1
Cornelius, P. F. S. (1982) Hydroids and medusae of the family Campanulariidae recorded from the eastern North Atlantic, with a world synopsis of genera. Bulletin of the British Museum (Natural History), Zoology, 42, 37 - 148.
Cunha, A. F., Collins, A. G. & Marques, A. C. (2017) Phylogenetic relationships of Proboscoida Broch, 1910 (Cnidaria, Hydrozoa): are traditional morphological diagnostic characters relevant for the delimitation of lineages at the species, genus, and family levels? Molecular Phylogenetics and Evolution, 106, 118 - 135. https: // doi. org / 10.1016 / j. ympev. 2016.09.012
Deevey, E. S. Jr. (1950) Hydroids from Louisiana and Texas, with remarks on the Pleistocene biogeography of the western Gulf of Mexico. Ecology, 31, 334 - 367. https: // doi. org / 10.2307 / 1931490
Defenbaugh, R. E. & Hopkins, S. H. (1973) The occurrence and distribution of the hydroids of the Galveston Bay, Texas, area. TAMU-SG- 73 - 210. Texas A & M University, College Station, Texas, 202 pp.
Defenbaugh, R. E. (1974) Hydroids. In: Bright, T. J. & Pequegnat, L. H. (Eds.), Biota of the West Flower Garden Bank. Gulf Publishing Company, Houston, pp. 94 - 112.
Fincher, J. A. (1955) Notes on the hydroids of the Mississippi Sound. Journal of the Alabama Academy of Science, 27, 91 - 92. [abstract]
Florez Gonzalez, L. (1983) Inventario preliminar de la fauna hydroide de la Bahia de Cartagena y areas adyacentes. Boletin del Museo del Mar, Bogota, 11, 112 - 140.
Fraser, C. M. (1912 b) Some hydroids of Beaufort, North Carolina. Bulletin of the United States Bureau of Fisheries, 30, 339 - 387.
Fraser, C. M. (1944) Hydroids of the Atlantic coast of North America. University of Toronto Press, Toronto, 451 pp.
Fraser, C. M. (1946 [1947 a]) Distribution and relationship in American hydroids. University of Toronto Press, Toronto, 464 pp. [dating of this work, as February 1947, follows Calder & Choong 2018: 77]
Fraser, C. M. (1947 b) Hydroids of the 1939 Allan Hancock Caribbean Sea Expedition. Allan Hancock Atlantic Expedition, 4, 1 - 24.
Galea, H. R. (2008) On a collection of shallow-water hydroids (Cnidaria: Hydrozoa) from Guadeloupe and Les Saintes, French Lesser Antilles. Zootaxa, 1878 (1), 1 - 54. https: // doi. org / 10.11646 / zootaxa. 1878.1.1
Galea, H. R. (2010) Additional shallow-water thecate hydroids (Cnidaria: Hydrozoa) from Guadeloupe and Les Saintes, French Lesser Antilles. Zootaxa, 2570 (1), 1 - 40. https: // doi. org / 10.11646 / zootaxa. 2570.1.1
Galea, H. R. (2013) New additions to the shallow-water hydroids (Cnidaria: Hydrozoa) of the French Lesser Antilles: Martinique. Zootaxa, 3686 (1), 1 - 50. https: // doi. org / 10.11646 / zootaxa. 3686.1.1
Hedgpeth, J. W. (1950) Annotated list of certain marine invertebrates found on Texas jetties. In: Whitten, H. L., Rosene, H. F. & Hedgpeth, J. W. The invertebrate fauna of Texas coast jetties; a preliminary survey. Publications of the Institute of Marine Science, the University of Texas, 1 (2), 72 - 85.
Joyce, E. A. Jr. (1961) The Hydroida of the Seahorse Key area. M. S. Thesis, University of Florida, Gainesville, 116 pp.
Lamouroux, J. V. F. (1812) Extrait d'un memoire sur la classification des polypiers coralligenes non entierement pierreux. Nouveau Bulletin des Sciences, par la Societe Philomatique de Paris, 3, 181 - 188.
Leloup, E. (1937) Resultats scientifiques des croisieres du navire-ecole belge Mercator. VI. Hydroidea, Siphonophora, Ceriantharia. I. - Hydropolypes. Memoires du Musee Royal d'Histoire Naturelle de Belgique, Serie 2, 9, 91 - 121.
Lewbel, G. S., Howard, R. L. & Gallaway, B. J. (1987) Zonation of dominant fouling organisms on northern Gulf of Mexico petroleum platforms. Marine Environmental Research, 21, 199 - 224. https: // doi. org / 10.1016 / 0141 - 1136 (87) 90066 - 3
Lindner, A. & Migotto, A. E. (2001) Merotrichous isorhiza, a nematocyst new to the Campanulariidae (Cnidaria: Hydrozoa), and its relevance for the classification of cnidae. Proceedings of the Biological Society of Washington, 114, 825 - 832.
Lindner, A. & Migotto, A. E. (2002) The life cycle of Clytia linearis and Clytia noliformis: metagenic campanulariids (Cnidaria: Hydrozoa) with contrasting polyp and medusa stages. Journal of the Marine Biological Association of the United Kingdom, 82, 541 - 553. https: // doi. org / 10.1017 / S 0025315402005866
Lindner, A., Govindarajan, A. F. & Migotto, A. E. (2011) Cryptic species, life cycles, and the phylogeny of Clytia (Cnidaria: Hydrozoa: Campanulariidae). Zootaxa, 2980, 23 - 36. https: // doi. org / 10.11646 / zootaxa. 2980.1.2
Linnaeus, C. (1767) Systema naturae per regna tria naturae, secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis. Tomus I. Pars II. Editio duodecima, reformata. Laurentii Salvii, Holmiae, 785 pp. [pp. 533 - 1317] https: // doi. org / 10.5962 / bhl. title. 156765
McCrady, J. (1859) Gymnopthalmata of Charleston Harbor. Proceedings of the Elliott Society of Natural History, 1, 103 - 221.
Mendoza-Becerril, M. A., Simoes, N. & Genzano, G. (2018 b) Benthic hydroids (Cnidaria, Hydrozoa) from Alacranes Reef, Gulf of Mexico, Mexico. Bulletin of Marine Science, 94, 125 - 142. https: // doi. org / 10.5343 / bms. 2017.1072
Miglietta, M. P., Piraino, S., Pruski, S., Alpizar Gonzalez, M., Castellanos-Iglesias, S., Jeronimo-Aguilar, S., Lawley, J. W., Maggioni, D., Martell, L., Matsumoto, Y., Moncada, A., Nagale, P., Phongphattarawat, S., Sheridan, C., Soto Angel, J. J., Sukhoputova, A. & Collin, R. (2018 b) An integrative identification guide to the Hydrozoa (Cnidaria) of Bocas del Toro, Panama. Neotropical Biodiversity, 4, 102 - 112. https: // doi. org / 10.1080 / 23766808.2018.1488656
Nutting, C. C. (1915) American hydroids. Part III. The Campanularidae and the Bonneviellidae. Smithsonian Institution, United States National Museum Special Bulletin, 4 (3), 1 - 126.
Rackley, D. H. (1974) Hydroids of the pelagic Sargassum community of the Gulf Stream and Sargasso Sea. M. A. Thesis, College of William and Mary, Williamsburg, Virginia, 94 pp.
Reyes, R. & Campos, N. H. (1992) Macroinvertebrados colonizadores de raices de Rhizophora mangle en la Bahia de Chengue, Caribe Colombiano. Anales del Instituto de Investigaciones Marinas de Punta Betin, 21, 101 - 116. https: // doi. org / 10.25268 / bimc. invemar. 1992.21.0.422
Ruthensteiner, B., Reinicke, G. - B. & Straube, N. (2008) The type material of Hydrozoa described by Eberhard Stechow in the Zoologische Staatssammlung Munchen. Spixiana, 31, 3 - 27.
Sandifer, P. A., Smith, T. I. J. & Calder, D. R. (1974) Hydrozoans as pests in closed-system culture of larval decapod crustaceans. Aquaculture, 4, 55 - 59. https: // doi. org / 10.1016 / 0044 - 8486 (74) 90018 - 0
Sars, M. (1850) Beretning om en i Sommeren 1849 foretagen zoologisk Reise i Lofoten og Finmarken. Nyt Magazin for Naturvidenskaberne, 6, 121 - 211.
Shier, C. F. (1965) A taxonomic and ecological study of shallow water hydroids of the northeastern Gulf of Mexico. M. S. Thesis, Florida State University, Tallahassee, 128 pp.
Stechow, E. (1914) Zur Kenntnis neuer oder seltener Hydroidpolypen, meist Campanulariden, aus Amerika und Norwegen. Zoologischer Anzeiger, 45, 120 - 136.
Vanhoffen, E. (1910) Die Hydroiden der Deutschen Sudpolar-Expedition 1901 - 1903. Deutsche Sudpolar-Expedition 1901 - 1903, 11, Zoologie, 3, 269 - 340.
Vervoort, W. (1968) Report on a collection of Hydroida from the Caribbean region, including an annotated checklist of Caribbean hydroids. Zoologische Verhandelingen, 92, 1 - 124.
Wedler, E. (1975) Okologische Untersuchungen an Hydroiden des Felslitorals von Santa Marta (Kolumbien). Helgolander Wissenschaftliche Meeresuntersuchungen, 27, 324 - 363. https: // doi. org / 10.1007 / BF 01611700
Wedler, E. (2017 b) Hidroides del Mar Caribe con enfasis en la region de Santa Marta, Colombia. Instituto de Investigaciones Marinas y Costeras-INVEMAR. Serie de Publicaciones Generales del INVEMAR # 94, Santa Marta, Colombia, 200 pp.
Wenner, E. L., Hinde, P., Knott, D. M. & Van Dolah, R. F. (1984) A temporal and spatial study of invertebrate communities associated with hard-bottom habitats in the South Atlantic Bight. United States Department of Commerce, National Oceanic and Atmospheric Administration, National Marine Fisheries Service, NOAA Technical Report NMFS, 18, 1 - 104.
FIGURE 10. a, Campanularia colombiana: hydrotheca, pedicel and stolon, Sanibel Island, ROMIZ B4356. Scale equals 0.1 mm. b, Campanularia colombiana: gonotheca and stolon, Sanibel Island, ROMIZ B4356. Scale equals 0.1 mm. c, Clytia elsaeoswaldae: hydrotheca and part of pedicel, Sanibel Island, ROMIZ B4369. Scale equals 0.1 mm. d, Clytia elsaeoswaldae: gonotheca, Sanibel Island, ROMIZ B4369. Scale equals 0.1 mm. e, Clytia elsaeoswaldae: pedicel and hydrotheca, Sanibel Island, ROMIZ B4369. Scale equals 0.2 mm. f, Clytia hemisphaerica: part of colony with hydrothecae and gonothecae, Sanibel Island, ROMIZ B4371. Scale equals 0.2 mm. g, Clytia hemisphaerica: hydrotheca and part of pedicel, Sanibel Island, ROMIZ B4372. Scale equals 0.1 mm. h, Clytia hemisphaerica: gonotheca, Sanibel Island, ROMIZ B4372. Scale equals 0.1 mm.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
SubClass |
Hydroidolina |
|
Order |
|
|
Family |
|
|
Genus |
Clytia elsaeoswaldae Stechow, 1914
| Calder, Dale R. 2019 |
Clytia coronata
| Fraser, C. M. 1944: 134 |
Gonothyraea gracilis
| Fraser, C. M. 1944: 148 |
Clytia elsae-oswaldae
| Nutting, C. C. 1915: 51 |
| Stechow, E. 1914: 125 |
1 (by plazi, 2019-10-25 12:55:46)
2 (by ExternalLinkService, 2019-10-25 15:56:46)
3 (by veselin, 2019-10-30 15:08:16)
4 (by angel, 2020-01-08 13:00:57)
5 (by ExternalLinkService, 2020-06-27 02:34:44)
6 (by ExternalLinkService, 2021-03-04 16:31:35)
7 (by ExternalLinkService, 2022-01-29 12:13:50)
8 (by GgImagineBatch, 2022-04-30 05:07:27)
9 (by plazi, 2023-10-30 20:30:32)
10 (by ExternalLinkService, 2023-10-31 14:06:23)