Bombus (Alpinobombus) kluanensis Williams & Cannings, 2016
|
publication ID |
https://doi.org/ 10.1080/00222933.2016.1214294 |
|
persistent identifier |
https://treatment.plazi.org/id/9A68324A-FF07-417E-893C-FEA15F0A21CF |
|
treatment provided by |
Felipe |
|
scientific name |
Bombus (Alpinobombus) kluanensis Williams & Cannings |
| status |
sp. nov. |
Bombus (Alpinobombus) kluanensis Williams & Cannings View in CoL , sp. nov. Figures 1 ‒ 4 View Figure 1 View Figure 2 View Figure 3 View Figure 4
Material examined
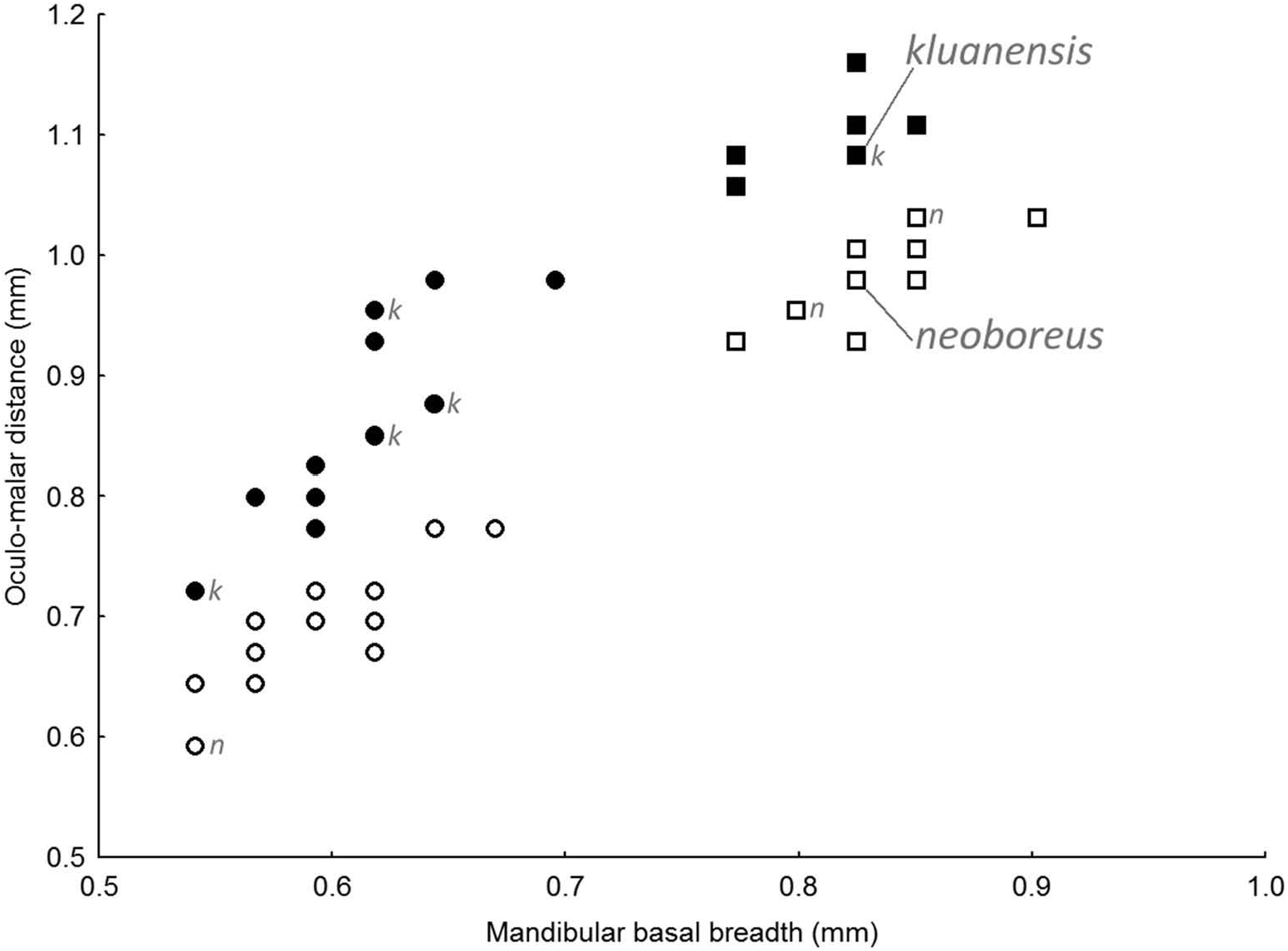
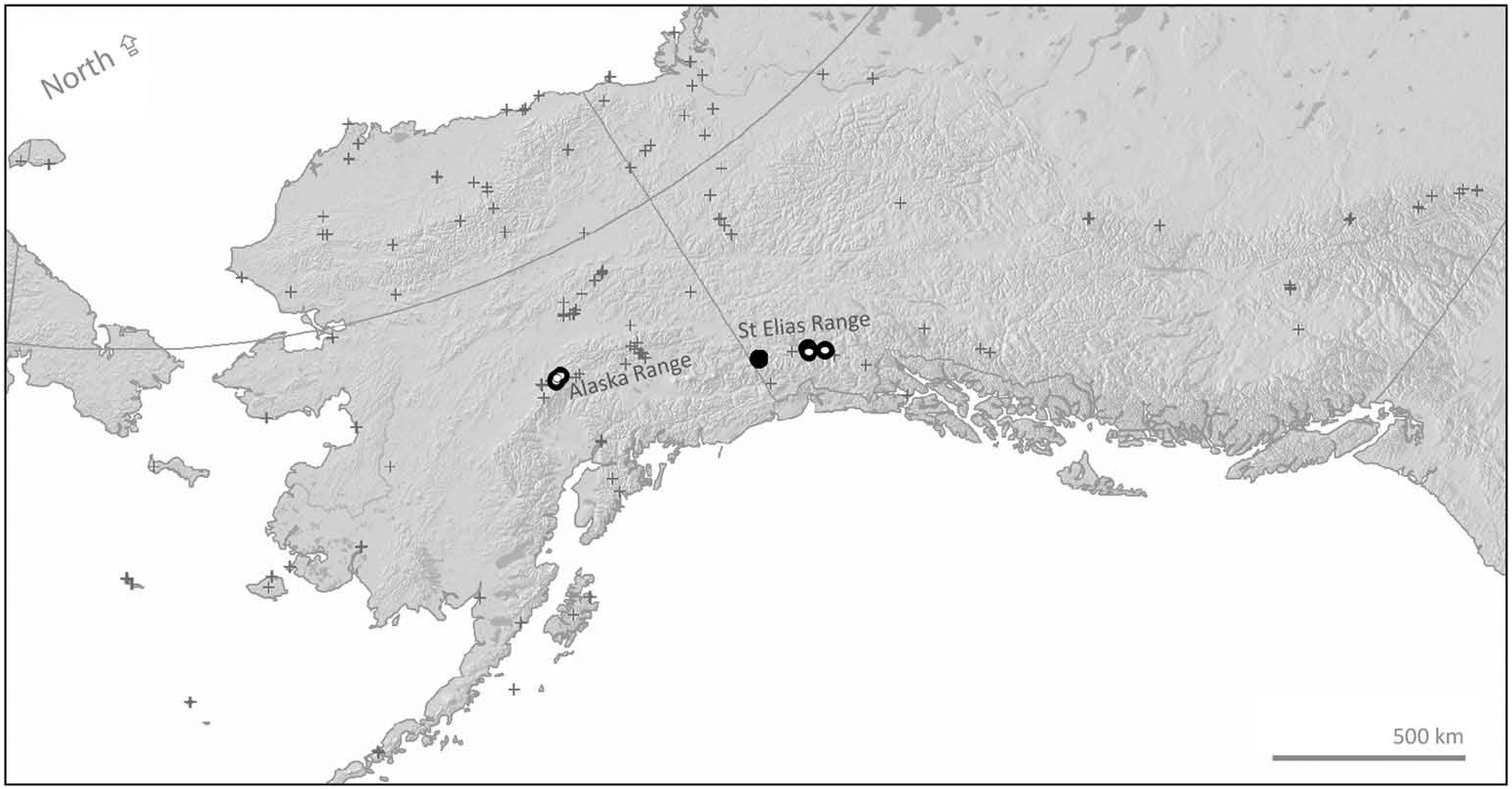
Records are mapped in Figure 2 View Figure 2 .
Holotype. One female (queen) pinned ( NHM). Labels: (1) white, printed with ‘ CANADA, YT, Outpost Mtn. / 3.5 km WNW. dry tundra//talus 60.9502N 138.4320W / 18.vi.2010 2000 m el. /S.G. Cannings’; (2) part green, printed with ‘BOLD /06717E06-YT’; (3) green printed ‘ Alpinobombus /AL# 16. det. PHW’; (4) red, printed with ‘ HOLOTYPE [female] / Bombus / kluanensis /Williams & Cannings, /2016 /det. PH Williams 2016’. Anthrenus damage on the right side of the metasoma and the left fore leg is missing.
Paratypes. Four females (workers) pinned . Labels : AL#17 ( RSKM) ‘ Dezadeash R .’ 600 m ‘ 61.7377N [an error: it should read 60.7377°N] 137.7633W’, Kluane National Park , Yukon, Canada, coll GoogleMaps . S GoogleMaps .G . Cannings 17 June 2010; AL#4392 ( UAM) ‘ Toklat R .’ ‘ 63.50352°N 150.04811°W’, Denali National Park , Alaska, USA, coll GoogleMaps . J GoogleMaps . Rykken 30 June 2012; AL#4393 ( PW) ‘ Stony Dome’ ‘ 63.45537°N 150.14747°W’, Denali National Park, Alaska, USA, coll GoogleMaps . J.
Rykken 1 July 2012; AL#4394 ( UAM) ‘above East Fork R .’ ‘ 63.54604°N 149.81896°W’, Denali National Park , Alaska, USA, coll GoogleMaps . J GoogleMaps . Rykken 9 July 2012 .
Additional material examined. Two queens pinned ( CNC), labels: AL#2305, 2306 ‘Kutlan [Klutlan] Glacier /Y. T. 9000 ft. ’, coll. A. Pattison, H.F.J. Lambart, 1 June 1913. Eighteen queens and 222 workers pinned ( USNM), labels: ‘Kluane, Y.T., Can.’, coll. L.W. Macior, 22 June‒2 August 1971 and 20 June‒25 July 1972.
Description of female
Morphology. Habitus illustrated in Figure 3 View Figure 3 . Body size large: queen body length 22‒ 25 mm, worker body length 12‒18 mm. Hair (pubescence) moderately long and even, wings clear. Mandible with a deep broad distal notch anterior to the posterior tooth. Oculo-malar area (‘cheek’ sensu Williams et al. 2014; not the gena) long, 1.27‒1.54 times longer (length measured between the ventral edge of the compound eye and the edge of the malar area at the articulation of the mandible midway between the mandibular condyles) than the breadth of the mandible at its base (breadth between and including the mandibular condyles), shown in Figures 1 View Figure 1 and 4 View Figure 4 . Clypeus moderately swollen and projecting, the central area with nearly uniformly scattered large, medium and small punctures, fewer along the midline. The area between the inner edge of the compound eye and the outer edge of the lateral ocellus occupied in its outer half by a broad band of mostly large punctures, spaced often by more than their own widths, the smaller punctures between the larger punctures much more abundant anteriorly. Midleg basitarsus with the distal posterior corner just acute but rounded; hindleg tibia outer surface with a corbicula, the surface sculpturing weak so that the surface appears shining and nearly smooth.
Colour pattern of the hair. Hair of the body predominantly black. Head with just a very few scattered yellow hairs posteriorly on the occiput. Thoracic dorsum with broad anterior and posterior yellow bands, sometimes with a minority of black hairs intermixed on the scutellum; side of the thorax (mesepisternum) predominantly black, in its dorsal two thirds varying from a minority to a majority of yellow hairs intermixed. Metasomal terga 1‒3 (T1‒3) yellow, but T3 varying from a minority to a majority of black hairs intermixed anteriorly; T4‒6 usually entirely black. Three workers (AL#17, 4652, 4689) have orange hair intermixed on T6 and on the posterior margin of T5. Two workers (AL#4473, 4686) have the hair on T5–6 predominantly orange, with more black hairs anteriorly on T5, resembling many B. neoboreus . Two workers tentatively associated with this species have T3 and the side of the thorax either predominantly black (AL#4466) or almost entirely black (AL#4700), resembling B. natvigi .
Diagnostic characters. Most similar to those individuals of B. neoboreus with a light colour pattern, but distinguished by having the oculo-malar area (cheek) 1.27‒1.54 times longer than the breadth of the mandible at its base, longer than in B. neoboreus (1.08‒ 1.23 times longer than broad; Figure 1 View Figure 1 ). Colour pattern with the upper two-thirds of the side of the thorax yellow with many black hairs intermixed; T3 is yellow but with black hairs intermixed anteriorly (whereas both of these areas have few black hairs in many B. neoboreus if they have T3 with yellow hairs posteriorly); and T5‒6 are usually entirely black, rarely with orange hairs (T5‒6 usually have at least a few orange hairs in B. neoboreus ). Compared to the dark colour pattern of B. neoboreus , B. kluanensis sp. nov. always has the scutellum with a broad yellow band (whereas it may be black in dark B. neoboreus ). Keys and colour-pattern diagrams for the identification of all species of the subgenus Alpinobombus are in preparation.
Description of male
Unknown.
Remarks
North American bumblebee species are relatively well known compared to bumblebee species in other parts of the world ( Williams 1998, fig. 2). The last time a bumblebee species entirely unrecognised by science was described from the USA or Canada was by Frison (1927).
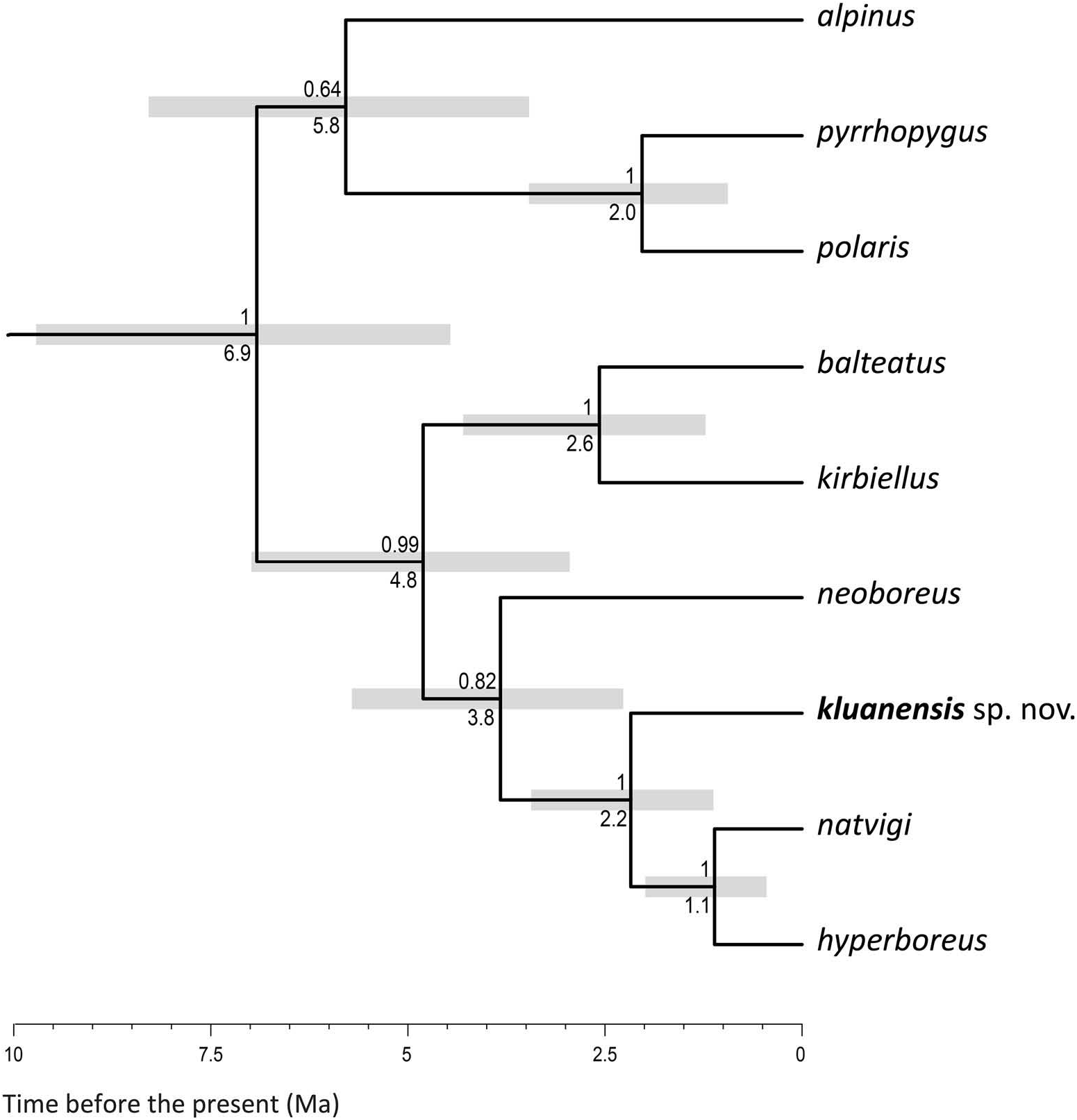
Bombus kluanensis sp. nov. is largely cryptic in morphology and colour pattern and was discovered using (1) coalescent-based analysis of a fast mitochondrial gene, which is corroborated by (2) divergence in a slow nuclear gene ( Williams et al. 2015). Here we show for the first time that there is also corroboration from (3) subtle morphological evidence. From the most closely related species, B. neoboreus , B. kluanensis sp. nov. differs slightly in the shape of the female head. de Queiroz (2007) described how in theory the unified species concept requires evidence minimally from just one practical criterion, providing that this criterion is applied appropriately. None of the factors most likely to compromise the interpretation of the genetic data in supporting a separate species (biased sampling, paralogy, introgression, heteroplasmy, incomplete lineage sorting) appears to be a substantial problem in this case ( Williams et al. 2015). Bombus kluanensis sp. nov. remains difficult to identify except from its COI barcode.
Following the Bayesian estimate of phylogeny based on COI and PEPCK for the species of the subgenus Alpinobombus by Williams et al. (2015), we can substitute the name ‘ kluanensis sp. nov. ’ for ‘unnamed’ in the tree in Figure 5 View Figure 5 . It is still possible that the list of species for the subgenus Alpinobombus ( Figure 5 View Figure 5 ; Table 1) may change as genetic data become available from other less accessible Arctic areas, especially from the more remote islands. Figure 5 View Figure 5 shows that the crown diversification of the subgenus Alpinobombus occurred after the northern hemisphere ice sheets started forming in the late Miocene after c. 8 Ma ( Zachos et al. 2001).
The habitat of B. kluanensis sp. nov. at higher elevations (six known records with data, 600 ‒ 2000 m, median 1098 m above sea level) in the St Elias Mountains near Kluane in the Yukon and in the Denali National Park in the Alaska Range of Alaska is alpine tundra and dwarf shrub heath. The habitat at lower elevations is flower-rich dry grassland. Its food plants are currently unknown.
Diversification of the neoboreus-kluanensis-natvigi group may have taken place within the Beringian refuge, which has remained relatively ice-free ( Abbott and Brochmann 2003). The biotic history of the Arctic and of the Beringian refuge may be complex ( Abbott and Brochmann 2003; Elias and Brigham-Grette 2013; Pringle 2014). It is possible that B. kluanensis sp. nov. and B. neoboreus could represent relict populations remaining from successive waves of range expansion and contraction following the cycles of climate change with associated glaciations. High elevations of the Yukon’ s Kluane mountains in the St Elias Range are unusual for having outlying disjunct and genetically divergent populations of otherwise Arctic species: for example, the moth, Gynaephora groenlandica (Wocke) (Erebidae) , the locoweed, Oxytropis arctica R. Br. (Fabaceae) , and the grass Puccinellia vahliana (Liebm.) Scrib. and Merr. (Poaceae) ( Barrio et al. 2013).
Etymology
The name is proposed to convey the idea that much of the current knowledge of this species comes from the longest series of specimens from Kluane. The formation of the name adopted here follows the form used for plant taxa named after Kluane (www.efloras.org).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |