Anolis subocularis Davis 1954
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3862.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:3FA375FE-E4E0-4509-BE02-EE5E786B07C6 |
|
DOI |
https://doi.org/10.5281/zenodo.7534612 |
|
persistent identifier |
https://treatment.plazi.org/id/8A288798-FFAE-E74D-7EC3-FB90FB3BC3B1 |
|
treatment provided by |
Felipe |
|
scientific name |
Anolis subocularis Davis 1954 |
| status |
|
Figs. 90–94 View FIGURE 90 View FIGURE 91 View FIGURE 92 View FIGURE 93 View FIGURE 94
Anolis subocularis Davis 1954: 3 ; type locality: “one mile southwest of Tierra Colorado, 900 ft., Guerrero ”. Holotype: TCWC 8675. Etheridge 1959, Cochran 1961, Davis & Dixon 1961, Smith et al. 1964, Smith & Taylor 1966, Liner & Dundee 1969, Webb & Baker 1969, Fitch & Henderson 1973, Fitch 1976, Fitch et al. 1976, Smith & Smith 1976, Fitch & Hillis 1984, Flores-Villela 1993, Flores-Villela & Gerez 1994, Lieb 1995, Pérez-Ramos et al. 2000, Lieb 2001, Poe 2004, Liner 2007, Urbina-Cardona & Flores-Villela 2010, Wilson & Townsend 2010, Wilson et al. 2013, Köhler 2014, Köhler et al. 2014
Anolis nebuloides: Mosauer 1936
Norops subocularis: Savage & Guyer 1989 , Nicholson 2002, Nicholson et al. 2012
Diagnosis. A small to moderate-sized species (SVL in largest male 55.0 mm, largest female 47.0 mm) of the genus Anolis (sensu Poe 2004) that differs from all Mexican and Central American anoles except A. boulengerianus , A. carlliebi , A. immaculogularis , A. quercorum , and A. sacamecatensis by having a combination of (1) strongly keeled ventral scales; (2) usually a patch of three greatly enlarged supraocular scales; (3) 13–18 rows of slightly to moderately enlarged dorsal scales that are smaller than ventral scales; (4) moderately long hind legs, longest toe of adpressed hind leg usually reaching to level of posterior border of eye or beyond, ratio shank length/SVL 0.25–0.31; and (5) a large pinkish red dewlap in males and a small red dewlap in females. Anolis subocularis differs from A. carlliebi , A. quercorum , and A. sacamecatensis by having longer legs with the longest toe of adpressed hind leg usually reaching to level of posterior border of eye or beyond, ratio shank length/SVL 0.25–0.31 (vs. to ear opening or to a point between ear opening and eye, ratio shank length/SVL 0.20–0.26). Anolis subocularis differs from A. boulengerianus and A. immaculogularis in male dewlap coloration in life (pinkish red with paler areas around gorgetals in A. subocularis vs. pinkish red without paler areas around gorgetals in A. immaculogularis and orange yellow with paler areas around gorgetals in A. boulengerianus ) and in hemipenial morphology (hemipenis unilobate with a single apical field void of ornamentation and without an asulcate processus or asulcate ridge in A. subocularis vs. hemipenis slightly bilobate with two apical fields void of ornamentation, one on each lobe and with a finger-like asulcate processus and an indistinct asulcate ridge in A. boulengerianus and A. immaculogularis ). Also, in A. subocularis the subocular scales separated from supralabials by one scale row or these scales in contact (vs. subocular scales are usually broadly in contact with supralabials in A. boulengerianus and A. immaculogularis ).
Description. Anolis subocularis is a small to moderate-sized anole (maximum recorded SVL 55.0 mm in males, 47.0 mm in females); dorsal head scales in internasal region keeled, uni- to tricarinate, other dorsal head scales mostly keeled in prefrontal and frontal region, smooth or keeled in parietal region; moderately deep prefrontal depression present, shallow parietal depression; 4–7 postrostrals; anterior nasal single or divided, the lower scale in contact with rostral and first supralabial; 6–8 internasals; canthal ridge sharply defined; scales comprising supraorbital semicircles well defined, weakly keeled, largest scale in semicircles subequal or larger than largest supraocular scale; supraorbital semicircles commonly in contact or separated by a complete row of scales, separated by two rows of scales in one specimen (KU 320871); 0–3 scales separating supraorbital semicircles and interparietal at narrowest point; interparietal well defined, greatly enlarged relative to adjacent scales, surrounded by scales of moderate size, longer than wide, larger than ear opening; enlarged supraoculars usually a patch of 3 greatly enlarged scales in a single row, separated from supraorbital semicircles by a complete row of small scales, or these scales narrowly in contact; 1–4 scales between enlarged supraoculars and superciliaries; 3 elongate superciliaries, anterior one longest, followed posteriorly by a series of 4–5 rounded or squarish scales of moderate size; usually 3 enlarged canthals, the second canthal largest; 5–9 scales between second canthals; 7–13 scales between posterior canthals; loreal region slightly concave, 21–36 strongly keeled loreal scales in a maximum of 5–6 horizontal rows; 6–8 supralabials to level below center of eye; suboculars keeled, completely separated from supralabials, or these scales narrowly to broadly in contact (1–3 suboculars in contact with 1–4 supralabials); ear opening vertically oval; scales anterior to ear opening keeled, juxtaposed, about four times larger than granulars posterior to ear opening; 5–7 infralabials to level below center of eye; 2–6 postmentals (commonly 4 or 5), outer pair slightly to distinctly larger than adjacent median postmental scales; 0–2 (commonly 0 or 1) enlarged sublabials in contact with infralabials on each side; keeled granular scales present on chin and throat; male dewlap large, extending from level below anterior margin of eye to level of chest; 7–8 horizontal gorgetal-sternal rows with 8–14 scales per row; modal number of marginal pairs 2–4; female dewlap very small; a nuchal crest and a dorsal ridge present in males; scales on middorsum strongly keeled, subimbricate with rounded posterior margins; 13–18 middorsal scale rows slightly to moderately enlarged, with a gradual transition to lateral scales; lateral scales keeled, granular, more or less homogeneous; 36–49 dorsal scales along vertebral midline between levels of axilla and groin in males, 36–53 in females; 22–34 dorsal scales along vertebral midline contained in one head length in males, 22–26 in females; ventral scales on midsection larger than largest dorsal scales; scales on midventer strongly keeled, imbricate, slightly mucronate; 35–49 ventral scales along midventral line between levels of axilla and groin in males, 32–42 in females; 24–40 ventral scales contained in one head length in males, 18–28 in females; 96–134 scales around midbody in males, 96–106 in females; tube-like axillary pocket absent; precloacal scales weakly to strongly keeled; males almost always with a pair of greatly enlarged postcloacal scales; tail moderately compressed in cross section, tail height/tail width 1.05–1.50 in males, 1.07–1.64 in females; all caudal scales strongly keeled, homogeneous, although an indistinct division in segments is discernible; dorsal medial caudal scale row hardly enlarged, strongly keeled, not forming a crest; scales on anterodorsal surface of brachium imbricate, strongly keeled, unicarinate, slightly mucronate; scales on dorsal surface of antebrachium subimbricate to imbricate, strongly keeled, unicarinate, slightly mucronate; 22–28 subdigital lamellae on Phalanges II–IV of Toe IV of hind limbs; 6–7 subdigital lamellae on distal phalanx of Toe IV of hind limbs; digital pads dilated, about twice the size of distal phalanx. In all specimens examined, the longest toe of the adpressed hind leg reaches to level of tympanum or to a point between shoulder and tympanum. For variation in selected scalation and morphometric characters see Table 10 View TABLE 10 .
The coloration in life of an adult male from Acapulco (SMF 96257; Fig. 90d View FIGURE 90 ) was recorded as follows: Dorsal ground color Raw Umber (22) grading laterally into Ground Cinnamon (270) and with Antique Brown (24) chevrons and lateral stripe edged above and below by broken Ferruginous (35) line; dorsal surface of hind limbs Brussels Brown (33) with Cinnamon-Rufous (31) bands; dorsal surface of forelimbs Ground Cinnamon (270) with faint Grayish Horn Color (268) bands; venter Pale Pinkish Buff (3) with Salmon Color (58) stipples in ventrolateral region; chin Cream White (52) with Glaucous (289) Speckles; ventral surface of limbs and tail Ground Cinnamon (270); dewlap Light Mauve (205) grading into Medium Rose (233) with Pale Buff (1) gorgetals; iris Verona Brown (37).
The coloration in life of an adult female from near Tierra Colorada (SMF 96262; Fig. 90g View FIGURE 90 ) was recorded as follows: Dorsal ground color Brownish Olive (276) with Sepia (279) indistinct chevrons; dorsal surface of limbs Olive Brown (278); ventral surfaces of body, limbs and tail Pale Buff (1); dewlap Pinkish Flesh Color (253); iris Yellow-Green (103).
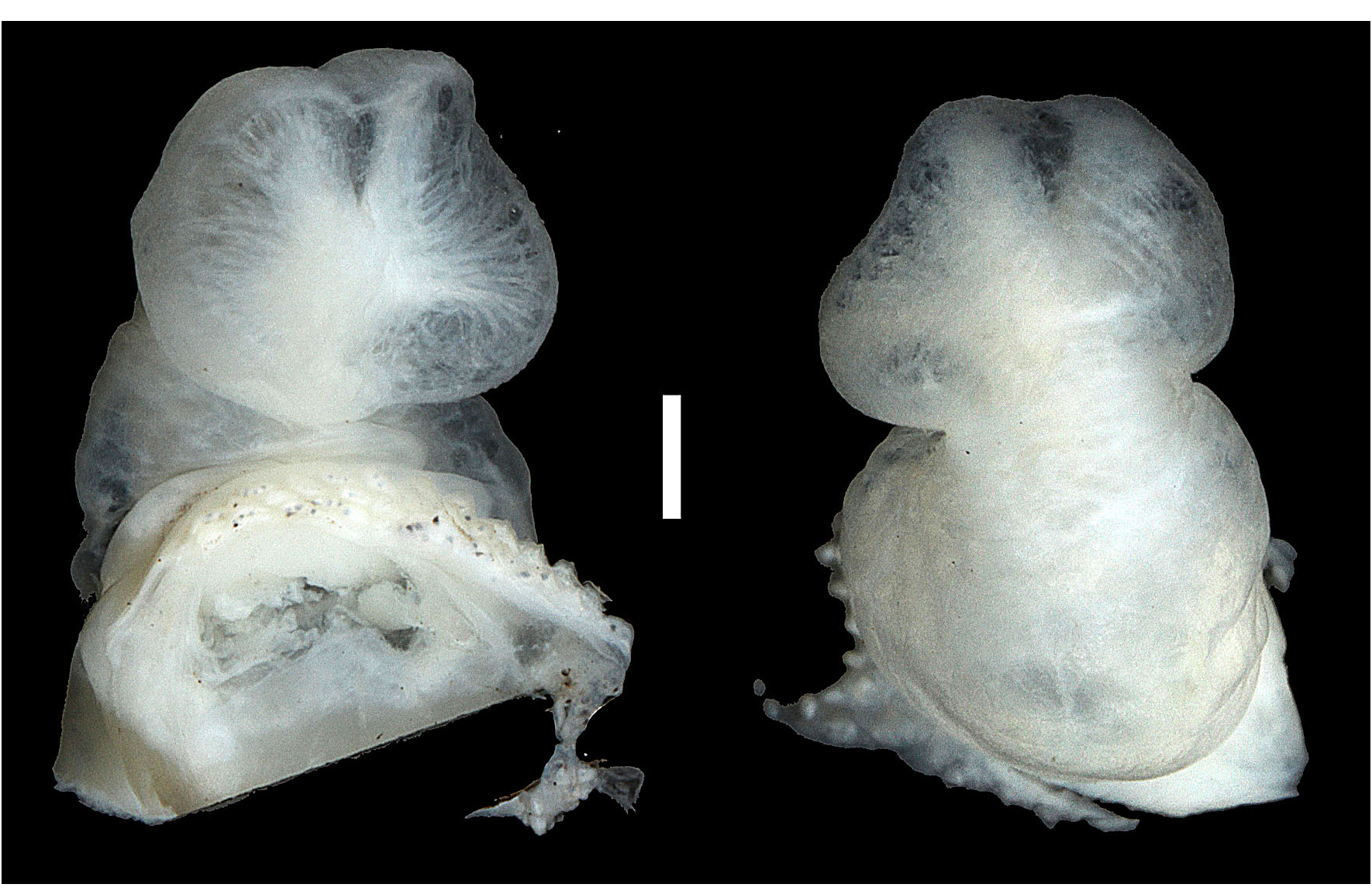
The completely everted hemipenis of SMF 96257 ( Fig. 94 View FIGURE 94 ) is a small, unilobate organ; sulcus spermaticus bordered by well developed sulcal lips and opening into a single apical field void of ornamentation; no asulcate processus or asulcate ridge present; no surface ornamentation discernible.
Natural History Notes: In the area of Acapulco and Tierra Colorada, the habitat of Anolis subocularis is mostly relatively open forest with rocks and boulders on hilly terrain. The lizards we observed were perching head down on up-standing tree trunks and on vertical surfaces of boulders.
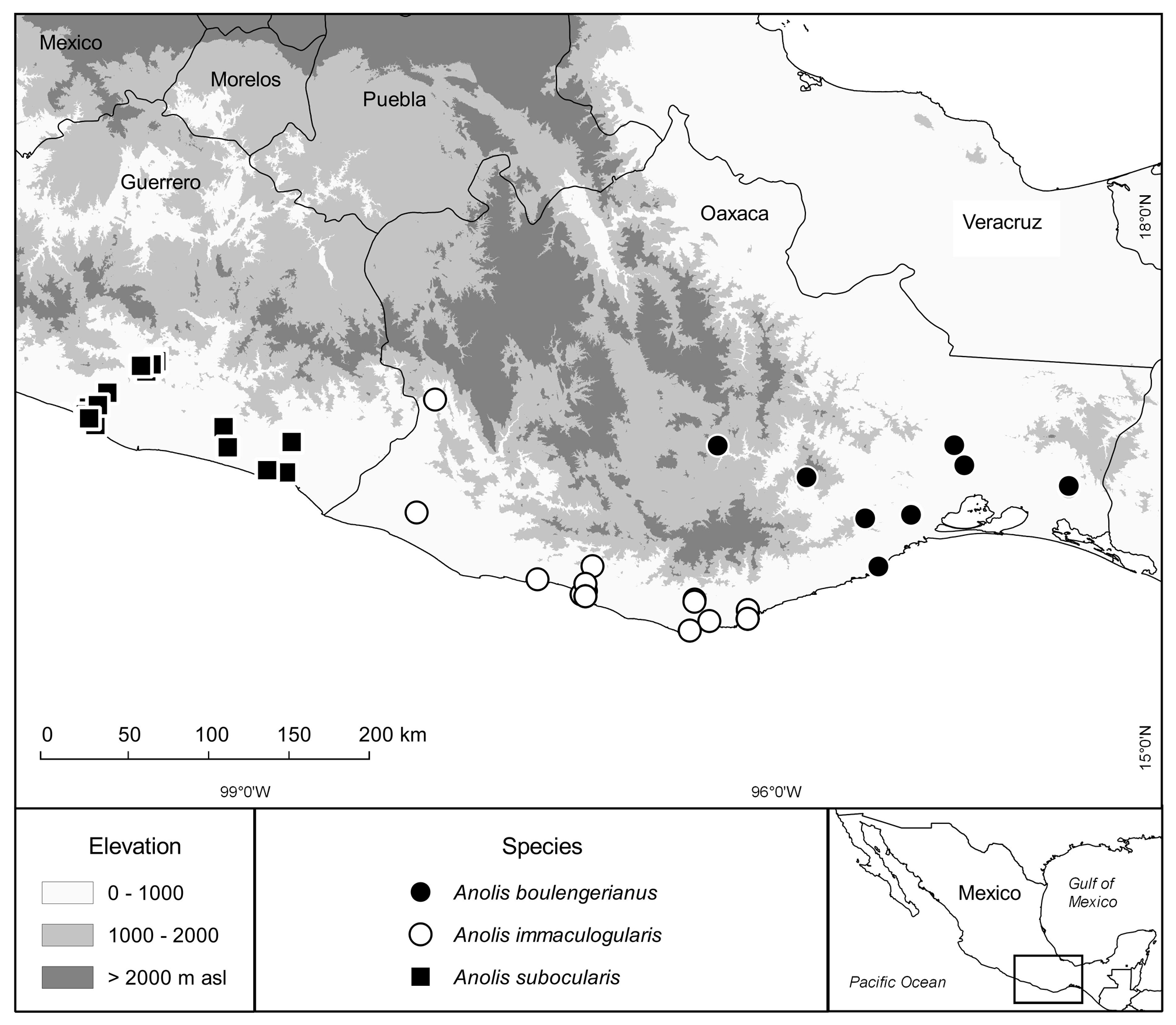
Geographic Distribution and Conservation. As currently known, Anolis subocularis is restricted to the Pacific versant in the south-central and southeastern portions of the Mexican State of Guerrero and adjacent southwestern Oaxaca at elevations between 10 and 993 masl ( Fig. 76 View FIGURE 76 ). Given its usual abundance wherever this species occurs, it seems justified to classify A. subocularis as Least Concern based on the IUCN Red List Categories and Criteria ( IUCN 2012).
Specimens examined ⎯ Mexico: Guerrero: 1 mi S El Treinta: FMNH 105055, 105086; 1.3 mi N El Treinta, 700 ft: UMMZ 119077; Acapulco: MCZ R-32080, USNM 46678; 1.5 mi S Acapulco, 10 m: MVZ 71866; 17.7 mi ESE San Marcos: KU 320878-79; 4 mi N Acapulco: FMNH 105063; Río Aguacatillo, 30 mi N Acapulco: TCWC 968; 1 mi SW Tierra Colorada: TCWC 8674-75; 4.4 mi (on road) S Tierra Colorada along branch of Río San Miguel: UMMZ 130946; 7.5 mi ESE Marquelia, 18.4 mi E Copala turnoff: KU 320883-85; 8.9 mi N Cruz Grande: KU 320870-72; Acapulco: USNM 46679, 46755; Acapulco, Jardin Botanico, 250 m: SMF 96257; Acapulco, Zona Arqueológica Palma Sola, 345 m: IBH 26566, SMF 96258; junction Mex Hwy 95 and Rio Papagayo, 183 m: UTA R-11512; near Palo Gordo, 290 m: IBH 26565, SMF 96261-62, 96469; near Tierra Colorada: FMNH 106126-30; road from Marquelia to San Luis Acatlán, near Jolotichan, 228 m: SMF 96460-66; road to Sitio Arqueológico Tehuacalco, 470 m: SMF 96259; Sitio Arqueológico Tehuacalco, 620 m: IBH 26568, SMF 96260; Tierra Colorada: USNM 133725-39; Mex Hwy 200, 11 km ESE Copala, 80 m: MCZ R-167239–41; 2 mi S Garrapates: MCZ R-56003; Oaxaca: 6 km S of Putla de Guerrero, 993 m: MVZ 106736.
Taxonomy of the Mexican anoles related to Anolis nebulosus ( Wiegmann 1834)
Anolis nebulosus ( Wiegmann 1834) was the first Mexican anole species described. No specific type locality was given in the original description. Smith & Taylor (1950b) restricted the type locality to Mazatlán, Sinaloa. Smith (1939) described Anolis schmidti from “Manzanillo, Colima,” Mexico (holotype FMNH 1667). Recently, Nieto Montes de Oca et al. 2013 relegated A. schmidti to the synonymy of A. nebulosus .
Since we cannot adequately address the geographic variation in external morphology and molecular genetic data in this species complex at this point, we assign the species name Anolis nebulosus to our samples. However, it is likely that what we call A. nebulosus at this point actually is a complex of several species. A comprehensive study of this species complex is needed in order to clarify the taxonomy of this nominal species.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.