Tropidopedia eliasi, Aguiar & Melo, 2007
|
publication ID |
https://doi.org/10.1111/j.1096-3642.2007.00328.x |
|
persistent identifier |
https://treatment.plazi.org/id/8523AF3A-FFA0-9C01-3A86-FF3AFE2AFAB0 |
|
treatment provided by |
Felipe (2021-08-31 13:31:05, last updated by Plazi 2023-11-06 09:36:59) |
|
scientific name |
Tropidopedia eliasi |
| status |
sp. nov. |
TROPIDOPEDIA ELIASI View in CoL SP. NOV.
Comments and diagnosis
Tropidopedia eliasi is similar to the species with carinated omaulus by having the integument mostly black, and the lateral portions of pronotal collar closed. Its omaulus, however, is only right angled, and lacks a carina. This species is known only from females from Vilhena, Rondônia, Brazil.
Distribution
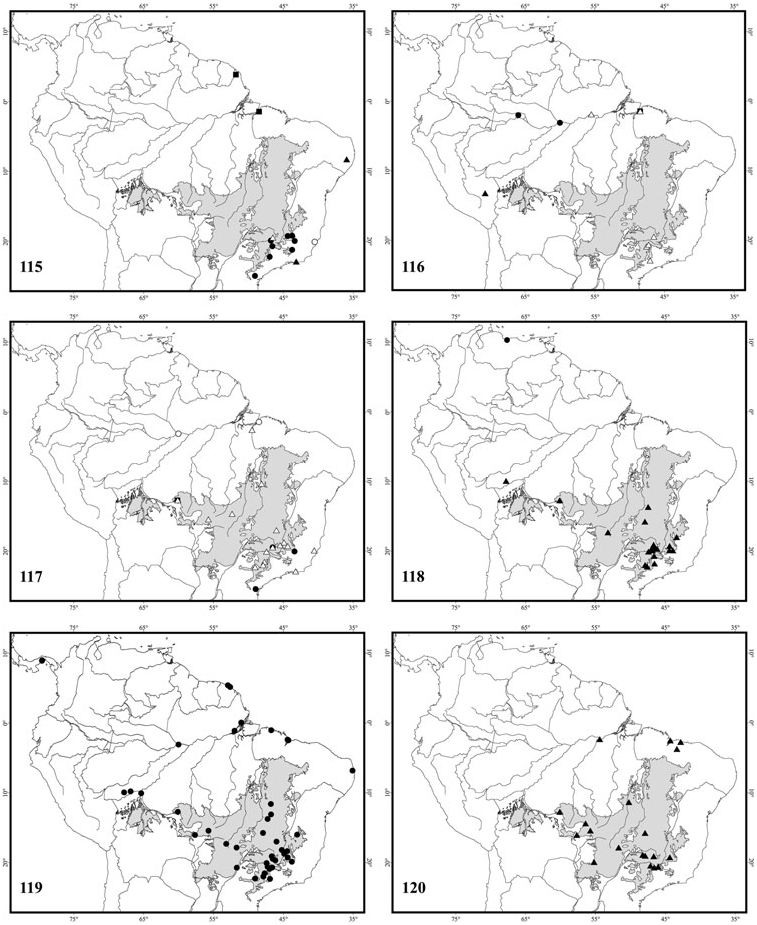
Brazil: Rondônia ( Fig. 117 View Figures 115–120 ).
Description
Holotype female – measurements (in mm): Body length, 6.5; maximum head width, 2.7; forewing length, with tegula, 6.9 (without tegula, 6.4); maximum T2 width, 2.5; head 1.2 times broader than long (2.7: 2.0); proportion of lower to upper interocullar distance, 0.8 (1.2: 1.5); clypeus 1.6 times wider than long (1.1: 0.67); scape length, 0.7, maximum width, 0.17; length of F1–F3, 0.26, 0.10, 0.17; F2 diameter, 0.2.
Colour: Integument mostly black. Mandible yellow with apex black; labrum yellow; clypeus with two yellow spots on lateral margins; supraclypeal area with a small yellow spot on lower margin. Disc of T1–T2 pale brown; sterna mostly yellow. Forewing membrane with proximal two thirds yellow infumated, with brownish yellow microtrichiae, and with distal third dark fuscous with black microtrichiae; veins and pterostigma light brown. Legs mostly black, tarsomeres 2–4 yellowish; hind basitarsus yellowish; tibial spurs pale yellow.
Pubescence: Face with short pale-yellowish plumose pubescence on parocular area and vertex; upper frons with erect reddish brown setae (c. 0.08–0.14-mm long). Mesoscutum and scutellum with very short plumose pale-yellow hairs; sparse erect simple black setae on mesoscutum c. 0.08-mm long; on scutellum, c. 0.08–0.39-mm long. Metapostnotum completely smooth. Complete hair band only on T5–T6. Legs mostly with black hairs; hind tibia and hind basitarsus with pale-yellow hairs.
Integumental surface: Clypeus with dense coarse punctures (0.5 pd); supraclypeal area mostly with contiguous punctures. Frons mostly with dense fine punctures (<0.5 pd), intermingled with dense coarse punctures adjacent to the midline and on the upper disc (c. 0.5–1 pd); scapal basin with dense fine punctures (c. 0.5 pd). Lateral mesepisternum with dense very coarse punctures (0.5–2 pd). Mesoscutum with dense fine punctures (0.5–1 pd), intermingled with sparse coarse punctures (> 2 pd). Scutellum disc mostly smooth, with sparse very coarse punctures (0.5–2 pd) and dense fine punctures on margin (c. 0.5– 1 pd). Metapostnotum smooth.
Structure: Pronotal collar with lateral portions closed, deeply concave as a closed gutter. Omaular area right angled, carina absent. Scutellum weakly convex, almost flat.
Type material
Holotype female, ‘ DZUP \ 023227’ ‘VILHENA, RO\ 5/ X/1986 \ C. ELIAS LEG.\ POLONOROESTE’ . Paratypes, one female, ‘ DZUP \ 023228’ ‘VILHENA, RO\ 12/XI/1986 \ C. ELIAS, LEG.’ ‘POLONO- ROESTE’ , one female, same except ‘023286’; two females, ‘ DZUP \ 023285’ ‘VILHENA, RO\ 17/12/ 1986 \ C. ELIAS, LEG.\ POLONOROESTE’ , one female, same except ‘023221’.
Etymology
The species name is in honor of Claudionor Elias, a retired technician from the DZUP, who during more than 25 years service contributed enormous numbers of specimens to the insect collection.
TROPIDOPEDIA FLAVOLINEATA SP. NOV.
( FIGS 13 View Figures 8–18 , 35 View Figures 29–35 , 46 View Figures 36–49 , 58 View Figures 50–61 , 78, 79 View Figures 74–89 , 102 View Figures 99–107 )
Comments and diagnosis
This species is easily distinguished by its elongated metasoma (more than 2.0 times longer than wide), the terga of which are yellow and brown striped. As already mentioned by Silveira et al. (2002: 136, referred to as an undescribed species of Amphipedia ), T. flavolineata can be confused with some species of Paratetrapedia , e.g. P. lineata (Spinola, 1851) , by having a similar pattern of body shape and colour, and milky wing membranes. In T. flavolineata , the pubescence on male sterna is much reduced, with S2–S3 almost devoid of hairs, and S7–S8 completely glabrous. It exhibits some variation in body size and colour pattern in specimens from scattered localities, in which the yellow stripe on the gena can be complete or interrupted, the lateral mesepisternum and metasoma are almost completely yellow, and the body size is smaller.
Tropidopedia flavolineata presents a wide distribution, from south-eastern Brazil to Panama. Its distribution seems to follow an ancient eastern corridor of savanna from central Brazil to northern areas in Venezuela and Panama, going through Amapá, in Brazil, and coastal areas of the Guianas.
Distribution
Brazil: Acre, Amapá, Amazonas, Distrito Federal , Goiás, Maranhão, Mato Grosso, Mato Grosso do Sul,
Minas Gerais, Pará, Paraíba, Rondônia, São Paulo; French Guiana; Panama ( Fig. 119 View Figures 115–120 ).
Description
Holotype male – measurements (in mm): Body length, 6.6; maximum head width, 2.3; forewing length, with tegula, 6.3 (without tegula, 5.8); maximum T2 width, 1.4; head 1.2 times broader than long (2.3: 1.8); proportion between lower and upper interocullar distance, 0.76 (1.0: 1.3); clypeus about 1.5 times wider than long (1.0: 0.64); scape length, 0.5, maximum diameter, 0.17; length of F1–F3, 0.16, 0.10, 0.19; F2 diameter, 0.2.
Colour: Integument painted yellow and black. Mandible yellow with apex black; labrum yellow; clypeus mostly yellow with a transverse reddish brown stripe on upper margin; parocular area with a long yellow stripe on inner eye margin. Scape yellow; pedicel and F1–F2 pale yellow; F3–F11 reddish brown. Gena mostly black, with a narrow yellow stripe along the eye margin (c. 0.8 times F2 diameter). Pronotum mostly yellow. Lateral mesepisternum mostly black; upper portion yellow. Mesoscutum black with yellow stripes on disc and lateral margins; scutellum yellow, with posterior portion of disc reddish brown; scutellar axilla yellow dorsally and black laterally. Disc of metapostnotum yellow, with lower margins narrowly black; propodeum yellow. Terga yellow, with anterior and posterior margin reddish brown; sterna yellow. Legs mostly yellow; mid and hind leg with tibia and basitarsus black infuscated; tibial spurs pale yellow. Forewing membrane white hyaline; veins and pterostigma yellow; microtrichiae milkly white.
Pubescence: Pubescence mostly yellow. Parocular area with dense fine short plumose pale-yellow hairs; upper parocular area and vertex with long simple yellow setae (c. 0.17-mm long). Scape with whitish setae (c. 0.08-mm long). Mesoscutum and scutellum with dense short plumose pale-yellow hairs; sparse erect simple setae on mesoscutum c. 0.05-mm long; on scutellum, c. 0.17-mm long. Disc of metapostnotum with a few sparse short yellowish hairs. Margins of T5–T6 with dense bands of plumose yellow hairs with brownish apices. Medial tuft on S2 inconspicuous, with a few short hairs; S3 with a few sparse short hairs on margins of depressed area; margin of S4 with dense long plumose decumbent hairs, mid portion with a broad glabrous interval along one third of the margin; S5 with short plumose hairs on margins; apical portion of S6 with long simple setae on the apical portion ( Fig. 35 View Figures 29–35 ). Leg pubescence with black and yellow hairs intermingled.
Integumental surface: Clypeus and supraclypeal area with dense coarse punctures (0.5–2 pd). Frons with coarse punctures (c. 1–2 pd), intermingled with fine punctures (1–2 pd) on the central disc. Scapal basin fine punctured (c. 0.5–1 pd), with a small smooth area above the antennal socket. Lateral mesepisternum with dense coarse punctures on upper portion (1 pd) and sparse and finer punctures on the lower portion (2 pd). Mesoscutum with dense fine punctures (0.5– 1 pd), intermingled with sparse coarse punctures (> 1 pd). Metapostnotum with sparse fine punctures on central disc (1–2 pd).
Structure: Pronotal collar with lateral portions confluent with remainder of pronotum. Scutellum weakly convex, almost flat. Metasoma elongated, more than twice longer than broad.
Female – measurements (in mm): Body length, 7.2; maximum head width, 2.6; forewing length, with tegula, 6.6 (without tegula, 6.0); maximum T2 width, 1.9; head 1.3 times broader than long (2.6: 2.0); proportion of lower to upper interocullar distance, 0.8 (1.2: 1.5); clypeus about 1.4 times broader than long (1.0: 0.7); scape length, 0.7, maximum diameter, 0.2; length of F1–F3: 0.25, 0.10, 0.14; F2 diameter, 0.2.
Colour: Similar to male except clypeus is completely yellow with two reddish brown oval spots on the upper portion; supraclypeal area completely yellow ( Fig. 102 View Figures 99–107 ). Wing membrane mostly hyaline; basal portion weakly yellow infumated; milkly white microtrichiae on most of membrane, with a few black microtrichiae on apical portion; veins and pterostigma orange yellow.
Integumental surface: Similar to male, except for clypeus with dense coarse punctures (0.5–1 pd), slightly sparser on upper portion (2 pd); supraclypeal area with dense coarse punctures (<0.5 pd). Frons with dense homogeneous fine punctures (0.5–1 pd), intermingled with coarse punctures on central disc (c. 2 pd); scapal basin with a small smooth area. Lateral mesepisternum with dense coarse punctures on upper portion (0.5 pd), and fine punctures on lower portion (2 pd). Mesoscutum and scutellum with dense minute punctures (0.5–1 pd), intermingled with sparse coarse punctures on central disc (2 pd); metapostnotum with sparse minute punctures on central disc (2 pd).
Pubescence: Similar to male; margins of T5–T6 with complete hair band of orange yellow hairs. Hind leg mostly with blackish hairs intermingled with yellow hairs on anterior portion.
Structure: Similar to male. Supraclypeal with trapezoidal appearance.
Type material
Holotype male, ‘DZUP\ 023438’ ‘ Brazil \ Distrito Federal,\ Brasília, MSPW\ 24.xii. 2004 AJCAguiar’. Paratypes: Brazil, Acre, one male, ‘DZUP\ 023162’ ‘R. Branco\ BR 15–20-XI 61\ F.M. Oliveira’, one male and one female, same except ‘023163’, and ‘023160’; three females (INPA), ‘ Brazil, Acre \ Acrelândia\ 10°04′S, 67°25′W’ 02-04/XI/2001 \ Oliveira, Morato\ & Cunha leg.’; Amapá, one female (IEPA), ‘Brazil-AP\ Ressaca do Coração\ Escola Agrícola\ 10/V/2002 \ (10:30 às 12 h)\ Charton’; one male (IEPA), ‘Brazil- Amapá\ Laranjal do Jari\ Santa Rosa\ 00:35S/ 52:19W\ 18/IX/2001 \ G.A.Melo’; Amazonas: one male, ‘Coleção\ Campos Seabra’ ‘DZUP\ 023159’ ‘Manaus\ Amazonas Brazil \ Setembro 1959\ C. Elias’; Distrito Federal: one female, ‘DZUP\ 023174’ ‘ Brazil, Brasília, DF\ MSPW\ 7.i. 2003 \ AJCAguiar leg’, two females, same except ‘023175–023177’; Goiás, one male, ‘DZUP\ 023164’ ‘ Brazil, Goiás, 11 Km SE de\ Campos Belos, 13°07′32″S, 46°44′29″W, 650m. 04.iv.2003,\ Melo, Aguiar, Marchi e Gonçalves, em cerrado sobre\ massapé, em Hyptis ′, one male and one female, same except ‘023165’ and ‘023166’; one female, ‘DZUP\ 023167’ ‘ Brazil, Goiás,\ 2 Km W de Teresina de Goiás,\ Fazenda Santa Tereza\ 13°47′43″S, 47°17′39″W, 800m,\ 03.iv.2003, Melo, Aguiar,\ Marchi e Gonçalves′, four females, same except ‘023168– 023171’; one female, ‘DZUP\ 023172’ ‘Dianópolis-GO\ Brazil 2-III-62\ FM Oliveira’; one female, ‘Coleção\ Campos Seabra’ ‘DZUP\ 023173’ ‘Jataí Goiás \ Brazil I-1955 \ F. Pereira’; Maranhão, one female (LEA), ‘Alcantará-MA-Br\ 20/VIII/1992 \ Araújo & Gonçalves’ ‘Pl. n°. 022\ Hr. 9–10’ ‘ Paratetrapedia \ sp1 (21)’; one female (ZMB), ‘ Brazil \ Maranhão \ 9. 1903\ Ducke’ ‘ Tetrapedia \ elongata \ F 1900 Friese det. Fr.’ ‘Zool. Mus.\ Berlin’; Mato Grosso, one male, ‘DZUP\ 026574’ ‘Cáceres, MT\ 27.III. 1984 \ C. Elias leg.\ Polonoroeste’, seven males, same except ‘023138–023144’; one male, ‘DZUP\ 023145’ ‘Chap. Guimarães-MT\ 30.III. 1983 \ C. Elias col.’, one female, same except ‘023146’; one male, ‘DZUP\ 023148’ ‘Chap. Guimarães- MT\ 28.III-IV-1983\ Exc. Det. Zool-UFPR\ (Polonoroeste)’, one female, same except ‘023147’; one female, ‘DZUP\ 023149’ ‘Chapada dos\ Guimarães, MT\ 8-1- 1987 \ C. Elias, leg.’; one female, ‘DZUP\ 023150’ ‘Mato Grosso-MT\ Brazil-2-XI-61\ F.M. Oliveira’; one female, ‘DZUP\ 023147’ ‘Chap. Guimarães-MT\ 28.III.-IV. 1983 \ Exc. Department Zool -UFPR\ (Polonoroeste)’, one female, same except ‘023148’; one female (MPEG), ‘Chapada dos\ Guimarães\ 27-I-1961 ’ ‘ Brazil, MT\ J. & B. Bechyné’; Mato Grosso do Sul, one female, ‘DZUP\ 023158’ ‘ Brazil, Três Lagoas,\ MS; 18– 20.iv.2003 \ AJCAguiar leg’; Minas Gerais, one male (DZMG), ‘Monitor, V & M\ Volchysia rufa \ Faz. Brejão\ 7874–23931’ ‘Brazilândia de Minas MG\ Brazil 23/05/2002 \ A.A.A. Azevedo’, one male, same except ‘8494–25910’ ‘ 23/05/2003 ’; one female, ‘DZUP\ 023189’ ‘Campos Altos-MG\ Brazil- 28/8/1965 \ C. Elias leg’; one female, ‘DZUP\ 023191’ ‘ Brazil, Minas Gerais,\ Corinto, 1–15.viii.1979, C. Elias leg’; one male, ‘DZUP\ 023180’ ‘Ibiá-Brazil\ MG- 10/12/1965 \ C. Elias leg.’, one male and one female, same except ‘023181’ and ‘023182’; one female (DZMG), ‘Abelhas-Cerrado\ Mannesmann\ Faz. Santa Cruz\ 5807–15870’ ‘Felixlândia MG\ Brazil 17/11/1999 \ A.A. Azevedo’ ‘ Paratetrapedia F\ ( Lophopedia ) sp.7\ F.A. Silveira, det. 2000’; one female, ‘DZUP\ 023192’ ‘89/240’ ‘Paraopeba, MG, Brazil \ Data 05/11/1986 \ F.A. Silveira’ ‘ Paratetrap.\ cfr. lineata ’; one female, same except ‘DZUP\ 023193’ ‘152/ 412’ ‘ 04/12/1987 ’; one female, ‘DZUP\ 023186’ ‘Passos- MG\ Brazil-13–18 XI-61\ Claudionor Elias’, one female, same except ‘24–31-XII 62’ and ‘023188’; one female, ‘DZUP\ 023187’ ‘Passos MG Brazil \ 5–10 XI 1961 \ C. Elias leg.’; one male, ‘DZUP\ 023179’ ‘ Brazil, Minas Gerais,\ 15 km SE Riacho dos\ Machados, área de\ cerrado, 12.iv.1998 \ G.A.R. Melo’; one female (DZMG), ‘Abelhas da Zona\ Metalurgica MG\ Clube A. Scharlé\ 0057–0218’ ‘Sabará MG\ Brazil 14/01/1996 \ F.A. Silveira’ ‘ Paratetrapedia F\ ( Lophopedia )\ sp.7\ F.A. Silveira, det. 1996’; one female, ‘DZUP\ 023190’ ‘S. Seb. Paraíso\ MG- Brazil-VI 61\ C. Elias leg’; one male, ‘DZUP\ 023178’ ‘ Brazil, MG, Serra do\ Salitre, RPPN Cachoeira\ do Campo, 24–30.xii.2003,\ AJCAguiar leg’; one female, ‘DZUP\ 023184’ ‘ Brazil, Serra do Salitre, MG\ Fazenda Goiabeira\ 9– 13.i.2003 \ AJCAguiar leg’; one female (DZMG), ‘ Ilha Três Marias\ 3369–9923’ ‘Três Marias MG\ Brazil 22/ 11/1997 /D. A. Yanega’; Pará, one female (MPEG), ‘ Pará \ 29.11.99\ Ducke’; one female (MPEG), ‘PA Bragança\ 26.v. 1978 \ Brazil Pará \ FF Ramos’; Paraíba, one female (DSEC), ‘1839’ ‘ Brazil; PB; Mamanguape; \ Res. Biol. Guaribas\ 6°41′S, 35°07′W \ 12/XII/1999 \ AJCAguiar leg’, two females, same except ‘1859’ and ‘2728’ ‘ 16/II/2000 ’ ‘ Byrsonima \ sericea ’ ‘ Amphipedia ’; Rondônia, one female, ‘DZUP\ 023152’ ‘Vilhena, RO\ 29/X/1986 \ C. Elias, leg.\ POLONO- ROESTE’, six females, same except ‘023153’, ‘023151’ ‘ 9/X/1986 ’, ‘023154’ ‘ 17/12/1986 ’, ‘023155’ ‘ 13/XI/1986 ’, ‘023156’ ‘ 12/XI/1986 ’ ‘H. 8 às 10\ P. 4 Cerrado’ and ‘023157’ ‘H. 14 às 16\ P. 4 Cerrado’; one female (INPA), ‘ Brazil, Rondônia \ próx. Guajará Mirim\ 10°48′S, 65°22′W ‘ 12–14/X/2001 \ Oliveira, Morato\ & Cunha leg.’; São Paulo, one female (UNESP), ‘UNESP, Bauru, SP\ Brazil 11.11. 1997 \ Col. F. Knoll P73\ 7:55 CU’; one female (UNESP), same except ‘ 25.11. 1996 \ Col. F. Knoll 27\ 11: 40 CU’, one female (DZMG), ‘UNESP, Bauru, SP\ Brazil 11.8.1993 \ Col. F. Knoll 4\ 12:25 CU’; one female (SM), ‘Cerrado-Est. Ecol. Jataí-SP.\ BR 27 ix 1992 \ no. 2492\ h: 10–12, Mateus, S. Leg.’; 1 female (SM), ‘Cerrado-Est. Ecol. Jataí-SP.\ BR 11 viii 1993 \ no. 3580\ h: 14–16, Mateus, S. Leg.’, one female (SM), same except ‘3579’; one female (SM), ‘Cerrado- Est. Ecol. Jataí-SP.\ BR 05 v 1993 \ no. 3590\ h: 8–10, Mateus, S. Leg.’, 12 females (SM), same except ‘3091’, ‘3092’ ‘h. 10–12’, ‘20 × 1992\ no. 2280\ h: 10–12’, ‘ 22 xii 1992 \ no. 2640\ h: 8–10’, ‘ 28 vii 1993 \ no. 3541\ h: 8– 10’, ‘ 8 ix 1992 \ no. 1906\ h: 14–16’, ‘ 8 ix 1992 \ no. 1886\ h: 12–14’, ‘ 3 xi 1992 \ no. 2322\ h: 8–10’, ‘ 09 iv 1992 \ no. 0537\ h:12–14’, ‘ 07 v 1992 \ no. 0678\ h: 14– 16’, ‘ 07 xi 1991 \ no. 0288\ h: 14–16’ and ‘06 × 1992\ no. 2191\ h: 14–16, Mateus, S. Leg.’; one female, ‘DZUP\ 023194’ ‘Mogiguaçu\ SP.\ 18.29967’; one female (UFU), ‘16’ ‘ Brazil, SP, Patrocínio\ Paulista\ 13/10/ 1999 \ Augusto, S.C. col.’, one female (UFU), same except ‘24’; one female (UFU), ‘ Brazil, SP, Patrocínio\ Paulista\ 24/XI/1999 \ Augusto, S.C. col.’ ‘123’ ‘ Paratetrapedia lineata \ (Spinola, 1851)\ Gaglianone,\ M.C. det. 2000’, one female (UFU), same except ‘DZUP\ 26521’ ‘ 13/X/1999 \ Augusto, S.C. col.’ ‘24’; one female (UFU), ‘ Brazil, SP, Patrocínio\ Paulista\ 29/10/ 1999 \ Augusto, S.C. col.’ ‘ Paratetrapedia lineata \ (Spinola, 1851)\ Gaglianone,\ M.C. det. 2000’ ‘80’, eight females (UFU), same except ‘85’, ‘30’, ‘59’, ‘86’, ‘256’, ‘257’, ‘292’, and ‘291’; one female, ‘DZUP\ 026522’ ‘ Brazil, SP, Patrocínio\ Paulista\ 10/XII/1999 \ Augusto, S.C. col.’ ‘ Paratetrapedia lineata \ (Spinola, 1851)\ Gaglianone,\ M.C. det. 2000’ ‘207’, two females, same except ‘026520’ ‘ 9/I/2000 ’ ‘402’, and ‘026520’ ‘ 25/I/ 2000 ’ ‘464’; one female, ‘DZUP\ 023185’ ‘Rifaina-Brazil\ SP- 28/10/1965 /C. Elias leg.’, one female, same except ‘023183’; one female, ‘DZUP\ 023195’ ‘São Carlos\ S.P.- 23/XII/71\ Pe. J.S. Moure’; one female (SEMC), ‘ BRAZIL São Paulo \ São Carlos, 23 December \ 1971 C. D. Michener’; Tocantins, one female, ‘DZUP\ 023162’ ‘S. Rita Araguaia\ GO Brazil XII-63\ M. Alvarenga, leg’; PANAMA: one male and two females (SEMC), ‘ PANAMÁ Panamá Prov.\ 21 Km N. Curundu\ March 31, 1981 on\ Byrsonima crassif -\ olia Robt. W. Brooks’; one male and two females (SEMC), ‘Ft. Kobbe\ Panama C.Z.\ IX-1946 ’ ‘N.L.H. Krauss’; FRENCH GUIANA: one female (SEMC), ‘ FRENCH GUIANA \ 11 km. SW. Kourou\ 18 July 1977 \ C.D. Michener’; onefemale (SEMC), ‘ FRENCH GUIANA \ Sinamari, 10 km S\ 9 October “76\ D. Roubik, No.”; one male (SEMC), ‘ FRENCH GUIANA \ 14 km SE Sinnamary\ X-8-76, Winston,\ Otis & Michener’.
Etymology
The species name refers to the pattern of yellow and brownish stripes on the terga.
TROPIDOPEDIA NIGROCARINATA SP. NOV.
( FIGS 34 View Figures 29–35 , 47 View Figures 36–49 , 59 View Figures 50–61 , 82, 83 View Figures 74–89 , 103, 104 View Figures 99–107 )
Comments and diagnosis
Tropidopedia nigrocarinata is quite similar to T. caracicola in having the wing membrane black infu- mated with dense black microtrichiae and the hind leg with mostly pale-yellow hairs, but is distinct mainly by the omaular carina extending below the middle of the lateral mesepisternum. The males can be easily differentiated by the T4–T6 with complete marginal bands of short black hairs, S4 with complete band of long plumose hairs on margin, and S6 with dense plumose hairs on apical portion. There is some variation, with some specimens lacking the parocular yellow stripe and the yellow spot on the supraclypeal area. The density of the fine punctution can vary on clypeus, supraclypeal area, and frons, from densely contiguous to sparse (> 2 pd). One male from Bauru (São Paulo, Brazil) presents the wing membrane yellow infumated and the hind legs with yellow pubescence, a condition similar to that of T. carinata , being identical to other males of T. nigrocarinata in the structural features.
Distribution
Brazil: Espirito Santo, Goiás, Mato Grosso, Minas Gerais, Pará, Rondônia, São Paulo ( Fig. 117 View Figures 115–120 ) .
Description
Holotype male – measurements (in mm): Body length, 8.0; maximum head width, 2.7; forewing length, with tegula, 8.0 (without tegula, 7.7); maximum T2 width, 2.4; head about 1.3 times broader than long (2.7: 2.0); proportion between lower and upper interocullar distances, 0.8 (1.2: 1.5); clypeus about 1.7 times broader than long (1.2: 0.7); scape length, 0.6, maximum width, 0.2; length of F1–F3, 0.17, 0.14, 0.23; F2 diameter, 0.17.
Colour: Integument mostly black; mandible yellow with apex black; labrum yellow; lower and lateral margin of clypeus with a broad transverse yellow stripe; supraclypeal area with a small yellow spot on the lower margin; malar area with a small yellow spot ( Fig. 103 View Figures 99–107 ). Scape mostly yellow; inner surface reddish brown; pedicel and flagellomeres reddish brown. Legs mostly black; tarsomeres 2–4 yellowish; tibial spurs pale white. Wing membrane entirely black infumated; veins and pterostigma dark reddish brown; microtrichiae black on membrane and veins.
Pubescence: Lower parocular area with dense, fine, short plumose white hairs; upper frons with erect, black setae c. 0.17-mm long. Scape with simple yellowish setae (c. 0.08-mm long). Mesoscutum and scutellum with dense short plumose yellowish brown hairs; sparse simple erect setae on mesoscutum c. 0.03-mm long; on scutellum, c. 0.17-mm long. Metapostnotum with sparse short plumose white hairs. Margin of T4 with hair band of short black hairs almost complete, with a short glabrous interval on mid portion; margin of T5–T6 with complete band of plumose black hairs. Medial tuft on S2 with short plumose white hairs; S3 with sparse short black hairs on margin of depressed area; S4 with dense long plumose decumbent hairs on entire margin; S5 with dense plumose hairs on margin; apical portion of S6 with few simple hairs among dense plumose hairs ( Fig. 34 View Figures 29–35 ). Leg pubescence mostly black; distal half of hind tibia and hind basitarsus with pale-yellow hairs.
Integumental surface: Clypeus and supraclypeal area with dense coarse punctures (<0.5–1 pd). Scapal basin with dense fine punctures (<0.5 pd); frons with midline narrowly sulcated, sulcus extending to ocellus. Frons mostly with fine dense punctures (<1 pd), intermingled with sparse coarse punctures on central disc (1– 2 pd). Lateral mesepisternum with dense very coarse punctures (<0.5–2 pd). Mesoscutum with dense fine punctures (c. 1 pd), intermingled with sparse coarse punctures (c. 2 pd). Scutellum with sparse very coarse punctures on disc (1–2 pd), with smooth integument between the punctures on disc; margins with dense fine punctures (c. 1 pd). Metapostnotum with fine punctures on the central disc (1–2 pd).
Structure: Pronotal collar with lateral portions conspicuously closed. Scutellum weakly convex.
Female – measurements (in mm): Body length, 9.0; maximum head width, 2.9; forewing length, with tegula, 7.9 (without tegula, 7.1); maximum T2 width, 2.7; head 1.2 times broader than long (2.9: 2.3); proportion of lower to upper interocullar distance, 0.76 (1.3: 1.7); clypeus 1.6 times wider than long (1.3: 0.7); scape length, 0.8, maximum width, 0.2; length of F1–F3: 0.23, 0.10, 0.21; F2 diameter, 0.2.
Colour: Similar to male, except lacking yellow spot on supraclypeal area ( Fig. 104 View Figures 99–107 ); scape entirely reddish brown; disc of T1–T2 brownish; legs completely black.
Pubescence: Similar to male; upper frons with reddish brown stout setae (c. 0.14–0.2-mm long); metapostnotum glabrous; margins of terga with bands of short hairs only on T5–T6.
Integumental surface: Similar to male, except for clypeus with dense coarse punctures (0.5–1 pd); frons with dense fine punctures (0.5 pd), intermingled with coarse punctures on central disc (0.5–2 pd); mesoscutum with dense fine punctures (0.5 pd), intermingled with sparse coarse punctures (2 pd); metapostnotum completely smooth.
Structure: Similar to male.
Type material
Holotype male, ‘DZUP\ 023292’ ‘Chavantina-MT\ Brazil-VII-1962\ Alvarenga-Oliveira’. Paratypes: Bra- zil, Espirito Santo, one female, ‘DZUP\ 023076’ ‘Sta. Teresa-ES\ Brazil 22-V-64\ C.Elias leg’, one female, same except ‘023074’; one female, ‘DZUP\ 023075’ ‘Sta. Teresa-ES\ Brazil 28-III-64\ C.Elias leg’; one female, ‘DZUP\ 023080’ ‘Sta. Teresa-ES\ Brazil 27-V- 64\ C.Elias leg’; Mato Grosso: one female, ‘DZUP\ 023094’ ‘Chapada MT\ Brazil 27-x-61\ F.M. Oliveira leg’; one male, ‘DZUP\ 023293’ ‘Chavantina-MT\ Brazil-VII-1962\ Alvarenga-Oliveira’; Minas Gerais, one male (DZMG), ‘Monitor V & M\ H. canum \ Faz. Galheiros\ 8141–24715’ ‘Abaeté MG\ Brazil 23/08/2002 \ A.A. Azevedo’; one male (DZMG), ‘Monitor. V & M\ T. emarginatus \ Faz. Brejão\ 7630–22639’ ‘Brazilândia de Minas MG\ BRAZIL 10/10/2001 \ R. Loyola’; one male (DZMG), ‘Monitor V & M\ T. emarginatus \ Faz. Brejão\ 7520–22224’ ‘Brazilândia de Minas MG\ Brazil 11/10/2001 /J. Damasceno’; one male (DZMG), ‘Projeto Abelhas de\ Brazilândia de Minas\ A. macrocarpum ’ ‘Brazilândia de Minas MG\ Brazil 19/10/1996 \ A.G. Damasceno’ ‘ Paratetrapedia M\ ( Lophopedia ) sp.1\ F.A. Silveira, det. 1996’; one female, ‘DZUP\ 023286’ ‘Ibiá-Brazil\ MG- 10/12/ 1965 \ C. Elias leg.’, one female, same except ‘023088’; one female (DZMG), ‘Abelhas-Cerrado\ Mannesmann\ Faz. Santa Cruz\ 5807–15874’ ‘Felixlândia MG\ Brazil 17/11/1999 \ A.A. Azevedo’ ‘ Paratetrapedia F\ ( Lophopedia ) sp26\ F.A. Silveira det. 2000’; one female, ‘DZUP\ 023064’ ‘202/491’ ‘Paraopeba, MG, Brazil\ Data 04/02/1987 \ F.A. Silveira’ ‘ Paratetr.\ sp.8 F’; Pará, one male, ‘DZUP\ 023084’ ‘Coleção\ Campos Seabra’ ‘Mangabeira\ Mocajuba Pará \ Brazil IV-1953 \ Orlando Rego’; Rio de Janeiro, one male (ZMB), ‘ Rio de Janeiro \ 19.Dez. 1926 \ A. Seitz leg.’ ‘ Tetrapedia \ bunchosiae \ Friese\ Alfken det. 1927’ ‘Zool Mus.\ Berlin’; Rondônia, one female, ‘DZUP\ 023085’ ‘Vilhena, RO\ 11/XII/1986 \ C.Elias, leg.\ Polonoroeste’; São Paulo, one male (UNESP), ‘UNESP, Bauru, SP\ Brazil 18.11. 1996 \ Col: F. Knoll 55\ 10:12 CU’ ‘ Paratetrapedia M\ ( Paratetrapedia ) sp.01\ F.A. Silveira, det. 2000’; one male (UNESP), ‘UNESP, Bauru, SP\ Brazil 3.11. 1997 \ Col. F. Knoll P73\ 13:02 CU’ ‘ Paratetrapedia M\ ( Lophopedia ) sp. 04\ F.A. Silveira, det. 2000; one female, ‘DZUP\ 026564’ ‘Brazil, São Paulo,\ Corumbataí, 22°15′S, 47°00′W, 800m,\ M.J.O. Campos leg.’ ‘cor 16\ 19.10.82\ 10.00L\ CL’, one male, same except ‘026565’ ‘COR39\ 28.11.84\ 11.25L P40\ CL’; one female (SM), ‘Cerrado- Est. Ecol. Jataí-SP.\ BR 30 × 1992\ no. 2281\ h: 10– 12, Mateus, S. LEG.’ ‘P. sp.3′ “ Paratetrap.\ Paratetra ”, 13 females (SM), same except ‘no 2241’, ‘no 2250\ h: 8– 10’, ‘no 2248\ h: 14–16’, ‘ 09 xii 1992 \ no. 2524\ h: 8– 10’, ‘ 09 xii 1992 \ no. 2525\ h: 8–10’, ‘ 03 xii 1991 \ no. 0385\ h: 10–12’, ‘ 03 xii 1992 \ no. 2333\ h: 10–12’, ‘20 × 1992\ no. 2282\ h: 10–12’, ‘20 × 1992\ no. 2251\ h: 16–18’, ‘20 × 1992\ no. 2252\ h: 16–18’, ‘06 × 1992\ no. 2190\ h: 14–16’, ‘30 × 1992\ no. 1962\ h: 14–16’, and ‘30 × 1992\ no. 2058\ h: 8–10’; one female, ‘DZUP\ 023092’ ‘Rifaina-Brazil\ 28/10/1965 \ C.Elias leg.’; one female, ‘DZUP\ 023299’ ‘Mogi Guaçu-\ SP.\ 18–29967’; two females (SEMC), ‘Brazil São Paulo \ São Carlos, 23 December \ 1971 C. D. Michener’; Tocantins, one female (RPSP), ‘Taquaruçu do Porto\ TO- Brazil- 24-VII- 1994 \ SC.22– 10°22′S, 48° 8′W \ Camargo leg. 940776’ ‘RPSP’.
Etymology
The species name refers to the black infumated wing membrane and the omaular carina.
TROPIDOPEDIA PUNCTIFRONS ( SMITH, 1879) View in CoL ( FIGS 5 View Figures 1–7 , 17 View Figures 8–18 , 21, 28 View Figures 19–28 , 48 View Figures 36–49 , 60 View Figures 50–61 , 84, 85 View Figures 74–89 , 105, 106 View Figures 99–107 , 112 View Figures 108–113 )
Tetrapedia punctifrons Smith (1879: 130) . Holotype female, Brazil, Pará, Santarém (BMNH). Dalla-Torre (1896: 300), Cockerell (1905: 325), and Cockerell (1909: 399).
Tetrapaedia [sic] punctifrons ; Schrottky (1902: 558).
Paratetrapedia (Amphipedia) haeckeli View in CoL (misidentification); Michener & Moure (1957: 413, figs 13–15), Vogel (1974: 207), Neff & Simpson (1981: 110), and Silveira et al. (2002: 136).
Paratetrapedia (Amphipedia) sp.; Vogel (1974: 190, fig. 67c) (fore basitarsus).
Paratetrapedia (Tropidopedia) punctifrons View in CoL ; Aguiar & Melo (2005: 32).
Paratetrapedia (Amphipedia) haeckli [sic]; Silveira & Campos (1995: 375) (misidentification, based on distribution).
Paratetrapedia (Tropidopedia) duckei View in CoL ; Albuquerque & Mendonça (1996: 49) (misidentification).
Paratetrapedia duckei View in CoL ; Rebêlo, Rêgo & Albuquerque (2003: 273) (misidentification).
Comments and diagnosis
This species is distinct mainly by the presence of contiguous coarse punctures on the clypeus and supraclypeal area ( Figs 5 View Figures 1–7 , 105, 106 View Figures 99–107 ); fore basitarsus of females with lower margin projected beyond apex of 2nd tarsomere ( Fig. 21 View Figures 19–28 ); lateral mesepisternum with dense coarse punctures (<0.5 pd); frons and vertex with dense short plumose yellow pubescence, intermingled with long, erect simple setae (c. 0.21–0.26-mm long); mesoscutum with dense short plumose pale-yellow pubescence, intermingled with sparse erect simple long setae (c. 0.07–0.10-mm long); margins of T5–T6 with hair bands of long orange yellow hairs; metapostnotum with fine punctures (c. 2 pd). The male presents marginal hair bands on T5–T7 with long plumose hairs, hairs orange yellow with brown apices; S4 with dense long plumose decumbent hairs on margin, with a broad interval on the mid one third.
The colour of the integument and of the pubescence in T. punctifrons varies from orange yellow to brownish black. The holotype presents the integument mostly orange yellow, with the mesoscutum orange, and the pubescence yellow. Most of the specimens examined present the integument with orange yellow areas intermingled with dark brownish areas, as follows: mandible yellow with apex black; labrum, clypeus, and supraclypeal area mostly yellow; parocular area with a narrow yellow stripe on inner eye margin; frons mostly black ( Fig. 105 View Figures 99–107 ); gena black, sometimes presenting a narrow yellow stripe along most of eye margin; pronotum with upper portion yellowish; mesoscutum black with narrow yellow stripes ( Fig. 112 View Figures 108–113 ); lateral mesepisternum, scutellum, metanotum, and propodeum yellow; terga orange yellow and marginal zone brownish; sterna mostly yellow. Even the darker specimens present a yellow stripe on the lower parocular area, pronotum with the upper portion yellowish, metanotum pale yellow, and posterior lateral margins of mesoscutum with narrow yellow stripes.
Dark brown and orange yellow specimens have been found in the same locality, but most dark specimens come from higher areas (c. 1100 meters a.s.l) of southeastern Brazil (Passos, Paraopeba , and Serra do Salitre , in Minas Gerais), whereas orange yellow specimens are from central and northern Brazil ( Barreirinhas and São Luís , in Maranhão; Cáceres and Chapada , in Mato Grosso). T. punctifrons seems to be restricted to the open areas of cerrado in Brazil, including more isolated areas in the states of Maranhão and Pará. Indeed , Santarém , where the holotype was collected, is known to present isolated patches of savanna ( Silva, 1995) .
Michener & Moure (1957: 412) described and presented drawings of S7–S8 and the genital capsule of T. punctifrons under the name Paratetrapedia haeckeli (Friese, 1910) . As shown in Aguiar & Melo (2005), those authors misidentified Friese’s species.
Measurements (in mm): Body length, male, 8.2, female, 8.5; maximum head width, male, 2.7, female, 2.75; forewing length (with tegula), male, 7.5, female, 7.5 (without tegula, male, 6.8, female, 6.8); maximum T2 width, male, 2.4, female, 2.4; proportion between head width and length, male, 1.3 (2.7: 2.0), female, 1.3 (2.7: 2.0); proportion between lower and upper interocullar distances, male, 0.8 (1.2: 1.5), female, 1.3 (1.37: 1.02); proportion between clypeus width and length, male, 1.4 (1.1: 0.8), female, 2.0 (1.2: 0.6); scape length, male, 0.8, female, 0.8; scape maximum width, male, 0.21, female, 0.2; length of F1–F3, male, 0.21, 0.12, 0.17, female, 0.26, 0.12, 0.17; F2 diameter, male, 0.2; female, 0.2.
Distribution
Brazil: Distrito Federal, Goiás, Minas Gerais, Maranhão, Mato Grosso, São Paulo, Pará ( Fig. 120 View Figures 115–120 ) .
Type material
Holotype female (BMNH), ‘Type\ H.T.’ ‘B.M. Type\ Hym\ 17B.885’ ‘Santar\ Am\\ 53\ 72’ ‘ Tetrapedia \ punctifrons \ (Type) Sm.’.
Additional examined material
Brazil, Distrito Federal, one female, ‘DZUP\ 023213’ ‘Brazil C. Sujo\ Loc. J. Botânico\ Data 25-09-95\ Col. Marcelino’ ‘1330’; one female, ‘DZUP\ 023213’ ‘Brazil Cerrado\ Loc. J. Botânico\ Data 12-11-96\ Col. M Araújo’ ‘1480’; Goiás, one male (SEMC), ‘ BRAZIL Goiás, Ilha\ do Bananal, Santa\ Izabel do Moro June\ 1961 (M. Alvarenga)’; one female, ‘DZUP\ 023220’ ‘Ilha do Bananal- GO\ Brazil 1-3/IX/74\ F. Giacomel leg.’; one female (AMNH), ‘Brazil, Goiáz:\ Jataí, November 1972 \ F.M. Oliveira’; one male, ‘DZUP\ 23206’ ‘JATAI GO\ Brazil-XI-63\ M. Alvarenga’; Minas Gerais, one female (ZAN), ‘Brazil, MG, Capitólio\ Rio Turvo, 15.V. 1999 \ 20°38′S, 46°13′W,\ 950 m. F. Zanella leg.’; one female, ‘DZUP\ 023204’ ‘Ibiraci-MG\ Brazil-X-61\ C. Elias leg.’, one female, same except ‘023203’ ‘15-X-62\ Claudionor Elias’; one female (GOTT), ‘Fundort: Brazil, Esp. Minas Gerais \ Faz. Bela Tanda, Veget. Cerrado\ Município Indianápolis\ Datum: 16-14983\ Leg. G. Gottsberger\ Pfl. Art. Pterodon pubescens ’ ‘ Paratetrapedia \ sp.1\ Det. Moure. 87’ ‘25318’; six females (GOTT), same except ‘ Paratetrapedia \ sp.1\ Moure. 1987’ ‘ Paratetrapedia \ sp.1\ det. Moure. 1987’, ‘ Paratetrapedia \ cf. lineata \ (Spinola)\ Det. Moure 1987’ ‘ Paratetrapedia \ cf. lineata \ (Spinola)\ Det. Moure 1987’ ‘25018’, ‘ Paratetrapedia \ sp.1\ det. Moure. 1987’ ‘25518’, ‘Datum: 11- 11083\ Pfl. Art. Andira ’ ‘ Paratetrapedia \ sp.1\ det. Moure 1987’ ‘25718’, ‘Datum: 17-17983\ Pfl. Art. Byrsonima gelb’ ‘25918’, and ‘Datum: 12-9983\ Pfl. Art. Styrax \ cf. ferruginea ’ ‘ Paratetrapedia \ sp.1\ det. Moure. 1987’ ‘25818’; one female, ‘DZUP\ 023211’ ‘375/976’ ‘Paraopeba, MG, Brazil\ Data 04/07/1987 \ F.A. Silveira’ ‘ Paratetr.\ F n.sp.5’; one male and three females, same except ‘023223’ ‘322/828’ ‘Data 07/05/ 1987 ’ ‘ Paratetrap.\ M cfr. lineata ’, ‘023283’ ‘37/128’ ‘Data 30/09/1986 ’, ‘023284’ ‘53/166’ ‘Data 01/10/1986 ’ ‘ Paratetr. F\ sp. n° 2’, and ‘026556’ ‘436/1186’ ‘Data 28/ 08/1987 ’ ‘ Amphipedia \ cfr. haeckeli M\ Friese, 1910\ Pe. J. S. Moure det. 1988’; one male, ‘DZUP\ 023202’ ‘Passos-MG\ Brazil 13-18 XI 61\ Claudionor Elias’, two females, same except ‘023197’, and ‘023199’; one female, ‘DZUP\ 023198’ ‘Passos-MG\ BR- 2-11-VIII- 1962 \ Claudionor Elias’; one female, ‘DZUP\ 023196’ ‘Passos-MG Brazil\ 5-10-XI-1961 \ C. Elias leg.’, one female, same except ‘023201’; one male, ‘DZUP\ 023200’ ‘Passos-MG\ Brazil 8-15 IX 62\ Claudionor Elias’; one female, ‘DZUP\ 023210’ ‘Brazil, Minas Gerais,\ Serra do Salitre, RPPN Cachoeira\ do Campo; 11.ix.2004 \ AJCAguiar’; one female (MZSP), ‘Uberlândia\ M. Gerais-Brazil\ X.1962 \ Exp. Department Zool.’; Maranhão, one female (LEA), ‘Barreirinhas-MA\ Brazil 27/XII/91\ Brito & Mendonça’ ‘Pl. N° 014\ Hr 16-17’ ‘ Paratetrapedia \ sp2’, one female (LEA), same except ‘Pl. N° 006.\ Hr 15-16’ ‘ Paratetrapedia \ sp1’; one female (LEA), ‘Barreirinhas MA\ Brazil 28 XII\ 1991\ Brito & Mendonça’ ‘ Paratetrapedia \ ( Amphipedia )\ sp.n.\ Det. Camargo, 1992’ ‘Pl. N° 014\ Hr 7-8’; one female (LEA), ‘Barreirinhas-MA\ Brazil 18/VII/92\ Brito & Mendonça’ ‘Pl. N° 006.\ Hr 11-12’ ‘ Paratetrapedia \ sp1’; one male (LEA), ‘Chapadinha-MA\ Brazil 13/X/94\ Brito & Rego leg\ 1091’ ‘Pl. N° 036H\ Hr 10:00–12:00’ ‘ Paratetrapedia \ duckei ’, one male and one female, same except ‘DZUP\ 026563’ ‘28/XII/93’ ‘0113’ ‘Pl N° 001–D\ Hr. 16:00– 18:00’, and ‘DZUP\ 026544’ ‘13/I/194\ 1332’ ‘Pl No 012 E\ Hr 6–8’; one female (LEA), ‘Chapadinha-MA\ Brazil 12/XII/194\ Brito & Rego Leg\ 1093’ ‘Pl. No 012C\ Hr. 12:00–14:00 14’ ‘ Paratetrapedia sp’; one female (LEA), ‘Turu, S. Luís Ma, BR\ 23/XI/02\ Aragão-leg’ ‘ Paratetrapedia sp.1 \ (F)\ 13:00–14:00\ 0761’, one female (LEA), same except ‘18/I/03’ ‘08:00–09:00\ 1035’; one female (LEA), ‘Hr 6:00–7:00’ ‘São Luis MA\ Brazil 21-X 1994 \ Albuquerque leg.’ ‘ P. nasuta ’; one female (LEA), ‘São Luís, MA, Br\ 2/X/1999 \ Cruz & Sodré leg’ ‘PL. N° 27\ Hr 7–8’ ‘ Paratetrapedia sp. \ 523’, two females (LEA), same except ‘PL 18\ Hr: 15– 16’ ‘ Paratetrapedia sp. \ 351’, and ‘Hr 7–8’ ‘ Paratetrapedia sp. \ 519’; one female (LEA), ‘São Luís, MA, Br\ 18/IX/1999 \ Cruz & Sodré leg’ ‘PL. N° 27\ Hr 10–11’ ‘ Paratetrapedia sp. \ 307’; two females (LEA), same except, ‘Hr. 12–13’ ‘ Paratetrapedia sp. \ 266’, and ‘Hr 9–10’ ‘ Paratetrapedia sp. \ 313’; one female (LEA), ‘São Luís-MA, Brazil\ 16-X-1999 \ Cruz e Sodré leg’ ‘Pl N° 27\ Hr. 13–14’ ‘ Paratetrapedia sp. \ 359’; one female (LEA), ‘São Luís, MA, Br\ 15/I/2000 \ Cruz & Sodré leg’ ‘PL. N° 27\ Hr 8–9’ ‘ Paratetrapedia sp. \ 549’; one female (LEA), ‘Hr. 7:00–8:00’ ‘São Luis. MA\ Brazil 4- VIII-1984 \ Albuquerque leg.’ ‘ Paratetrapedia \ sp1’; one male (LEA), ‘São Luís-MA, Brazil\ 10-VII-2000 \ Cruz e Sodré leg’ ‘Saco\ Hr. 8–9’ ‘ Paratetrapedia sp. \ 881’, one male (LEA), same except, ‘ Paratetrapedia sp. \ 888’; one male (LEA), ‘Pl. N° 07\ Hr 13:00 as 14:00 h’ ‘São Luís MA Brazil\ 04-XII-1983 \ Brenha- Maracaio\ F.0937’ ‘ Paratetrapedia sp. ’; Mato Grosso: one male, ‘DZUP\ 023219’ ‘Cáceres, MT\ 12.18. X. 1984 \ C. Elias leg\ Polonoroeste’; one male, ‘DZUP\ 023205’ ‘Chapada-MT\ Brazil XI-63\ M. Alvarenga’, one female, same except ‘023207’; one female, ‘DZUP\ 023208’ ‘Chapada-MT\ Brazil 27-X-61\ F.M. Oliveira leg’, one female, same except ‘023209’; one male, ‘DZUP\ 023224’ ‘Diamantino (259 m)\ Mato Grosso- Brazil\ 21.VII.961\ K. Lenko col.’ ‘ Paratetrapedia \ lineata M\ (Spinola, 1853)\ Pe. J.S. Moure det. 1990’; one female, ‘DZUP\ 023222’ ‘São Felix M. Grosso\ Brazil 21–28/VII/68\ C. Elias leg.’; São Paulo: one male (DZUP), ‘Brazil\ Batatais’ ‘ Amphipedia \ haeckeli (Fr.) \ Det. J.S. Moure 1957’; one female (DZUP), ‘Brazil-São Pa\ Batatais XII. 1943 \ Pe. Pereira col.’ ‘ Amphipedia \ haeckeli (Fr.) \ Det. J.S. Moure 1957’; uncertain locality, one male (DZUP), ‘BRAZIL’ ‘01’ ‘ Tropidopedia ’, dissected specimen probably used to produce the terminalia drawings presented in Michener & Moure (1957: figs 13–15).
TROPIDOPEDIA VENEZUELANA SP. NOV.
( FIGS 49 View Figures 36–49 , 61 View Figures 50–61 , 86, 87 View Figures 74–89 , 107 View Figures 99–107 )
Comments and diagnosis
Tropidopedia venezuelana is very similar to T. carinata , differing by the wing membrane dark brown infumated, and the extensions of yellow marks on labrum and clypeus, and details of S7–S8 and genital capsule. These two species present widely disjunct distributions.
Distribution
Venezuela: Aragua ( Fig. 118 View Figures 115–120 ).
Description
Holotype male – measurements (in mm): Body length, 7.0; maximum head width, 2.4; forewing length, with tegula, 7.4 (without tegula, 7.0); maximum T2 width, 2.7; head about 1.2 times broader than long (2.4: 2.0); proportion between lower and upper interocullar distances, 0.8 (1.12: 1.4); clypeus 1.5 times wider than long (1.1: 0.7); scape length, 0.6, maximum width, 0.16; length of F1–F3, 0.18, 0.1, 0.2; F2 diameter, 0.2.
Colour: Integument mostly black. Mandible mostly black, with mid portion yellow; labrum yellow with a transverse black stripe on upper margin; lateral margins of clypeus with two narrow yellow stripes. Scape, pedicel, and flagellomeres reddish brown. Legs mostly black; tarsomeres 2–4 and hind basitarsus yellowish; tibial spurs pale white. Forewing membrane dark brown infumated, with dark brown microtrichiae; veins dark brown basally, and gradually becoming lighter towards apex of wing; pterostigma brownish yellow.
Pubescence: Lower parocular area and scapal basin with dense short plumose whitish hairs; upper frons with erect black setae (c. 0.18-mm long). Scape with yellowish setae (c. 0.1-mm long). Mesoscutum and scutellum with dense short plumose yellowish brown hairs; sparse simple erect black setae on mesoscutum, c. 0.06-mm long; on scutellum, c. 0.26-mm long. Terga mostly glabrous, with short lateral bands of white hairs on less than one fifth of T3–T4 width; T6 with a complete band of long black plumose hairs on the premarginal zone. Medial hair tuft on S2 with white plumose hairs (c. 0.3-mm long); S3 with sparse short blackish hairs on margins of depressed area; S4 with dense long plumose decumbent hairs on margin, mid portion with a wide glabrous interval; S5 with dense plumose hairs along the margin; S6 with mostly simple setae intermingled with a few plumose hairs on apical portion (c. 0.12-mm long). Leg pubescence mostly black.
Integumental surface: Clypeus and supraclypeal area with dense coarse punctures (<0.5–2 pd). Frons heterogeneously punctured, with dense coarse and fine punctures adjacent to midline (<0.5–1 pd); lateral portions of disc mostly smooth; scapal basin with dense fine punctures (c. 0.5–1 pd), lateral mesepisternum with dense very coarse punctures (c. 0.5–2 pd). Mesoscutum with dense fine punctures (0.5–1 pd), intermingled with sparse coarse punctures (> 2 pd). Disc of scutellum mostly smooth with dense fine punctures on the midline, and on margins intermingled with very coarse punctures (0.5–2 pd). Metapostnotum smooth.
Structure: Pronotal collar lamella with lateral portions closed. Carina on omaulus, occupying about one third of upper lateral mesepisternum. Scutellum conspicuous convex.
Female – measurements (in mm): Body length, 7.3; maximum head width, 2.5; forewing length, with tegula, 7.3 (without tegula, 6.8); maximum T2 width, 2.5; head 1.2 times broader than long (2.5: 2.0); proportion between lower and upper interocullar distance, 0.78 (1.1: 1.4); clypeus 1.6 times wider than long (1.06: 0.66); scape length, 0.7, maximum width, 0.16; length of F1–F3, 0.22, 0.1, 0.16; F2 diameter, 0.2.
Colour: Similar to male; except for mandible mostly yellow with apex black; labrum completely black; clypeus with two much-reduced yellow stripes on lateral margins. Tarsomeres completely black.
Pubescence: Similar to male; upper frons with short erect dark yellow setae (c. 0.14-mm long). Mesoscutum and scutellum with short plumose brownish yellow hairs; sparse simple erect setae on mesoscutum c. 0.04-mm long; on scutellum, c. 0.14–0.3-mm long. Legs mostly with black pubescence; hind leg with few plumose pale-yellow hairs intermingled with the black pubescence. T1–T4 with margins glabrous; T5–T6 with complete hair band on margin.
Integumental surface: Similar to male, except disc of frons mostly with dense fine punctures (0.5–1 pd), intermingled with sparse coarse punctures on central disc (1–2 pd).
Structure: Similar to male, except pronotal collar only slightly closed on lateral portions.
Type material
Holotype male ( SEMC), ‘ Venezuela: Aragua \ Rancho Grande Biol. Stn., Portachuelo \ Pass , 10°21′0″N, 67°41′0″W, 1100 m \ 4 JUN 1998; J. Ashe, R. Brooks, R. Hanley \ VEN1ABH98 186 ex: insects moving thru\ pass against wind-migration’ ‘ SM0334050 \ KUNHM-ENT’ GoogleMaps . Paratypes, one male ( SEMC), ‘ Venezuela: Aragua \ Rancho Grande Biol. Stn., Portachuelo \ Pass , 10°21′0″N, 67°41′0″W, 1100 m \ 4 JUN 1998; J. Ashe, R. Brooks, R. Hanley \ VEN1ABH98 186 ex: insects moving thru\ pass against wind-migration’ ‘ SM0334049 GoogleMaps \ KUNHM-ENT’, two males and one female ( SEMC), same except ‘ SM0334048 ’, ‘ SM0334062 ’, and ‘ SM0334066 ’.
Etymology
The species name refers to the country where the type material was collected.
Aguiar AJC, Melo GAR. 2005. Notes on the type species of the subgenera Paratetrapedia (Lophopedia) and P. (Amphipedia) (Hymenoptera, Apidae, Tapinotaspidini). Zootaxa 1084: 31 - 42.
Albuquerque PMC, Mendonca JAC. 1996. Anthophoridae (Hymenoptera; Apoidea) e flora associada em uma formacao de cerrado no municipio de Barreirinhas, MA, Brasil. Acta Amazonica 26: 45 - 54.
Camargo JMF, Pedro SRM. 2003. Meliponini neotropicais: o genero Partamona Schwarz, 1939 (Hymenoptera, Apidae, Apinae) - bionomia e biogeoografia. Revista Brasileira de Entomologia 47: 311 - 372.
Cockerell TDA. 1905. Notes on some bees in the British Museum. Transactions of the American Entomological Society 31: 309 - 364.
Cockerell TDA. 1909. Descriptions and records of bees-XXIII. Annals and Magazine of Natural History 8: 393 - 404.
Friese H. 1899. Monographie der Bienengattungen Exomalopsis, Ptilotrix, Melitoma und Tetrapedia. Annalen des kaiserlich-koniglichen Naturhistorischen Hofmuseums in Wien 14: 247 - 304.
Michener CD, Moure JS. 1957. A study of the classification of the more primitive non-parasitic anthophorine bees (Hymenoptera, Apoidea). Bulletin of the American Museum of Natural History 112: 395 - 452.
Neff JL, Simpson BB. 1981. Oil-collecting structures in the Anthophoridae (Hymenoptera): morphology, function, and use in systematics. Journal of the Kansas Entomological Society 54: 95 - 123.
Rebelo JMM, Rego MMC, Albuquerque MCA. 2003. Abelhas (Hymenoptera, Apoidea) da regiao setentrional do Estado do Maranhao, Brasil. In: Melo GAR, Alves-dos- Santos I, eds. Apoidea Neotropica: Homenagem aos 90 anos de Jesus Santiago Moure. Criciuma (SC): Editora Unesc, 265 - 278.
Schrottky C. 1902. Ensaio sobre as abelhas solitarias do Brazil. Revista do Museu Paulista 5: 330 - 613.
Silva JMC. 1995. Biogeographic analysis of the South American Cerrado avifauna. Steenstrupia 21: 49 - 67.
Silveira FA, Campos MJO. 1995. A melissofauna de Corumbatai (SP) e Paraopeba (MG) e uma analise da biogeografia das abelhas do cerrado brasileiro (Hymenoptera, Apoidea). Revista Brasileira Entomologia 39: 371 - 401.
Silveira FA, Melo GAR, Almeida EAB. 2002. Abelhas Brasileiras: Sistematica e Identificacao. Belo Horizonte: Fernando Silveira.
Smith F. 1879. Descriptions of new species of hymenoptera in the collection of the British Museum. London: British Museum.
Vachal J. 1909. Especes nouvelles ou litigieuses d'Apidae du haut bassin du Parana et des regions contigues et delimitation d'une nouvelle sous-famille Diphaglossinae. Revue d'Entomologie 28: 5 - 64.
Vogel S. 1974. Olblumen und olsammelnde Bienen. Tropische und Subtropische Pflanzenwelt 7: 285 - 547.
Figures 115–120. Distribution records for species of Tropidopedia. Figure 115: Δ, Tropidopedia danunciae sp. nov.;, Tropidopedia friesei sp. nov.; •, Tropidopedia nigrita sp. nov.; O, Tropidopedia pallidipennis (Friese, 1899); A, Tropidopedia seabrai (Michener & Moure, 1957). Figure 116: Δ, Tropidopedia arcuatilis (Vachal, 1909);, Tropidopedia ornata sp. nov.; •, Tropidopedia japuraensis sp. nov.; A, Tropidopedia peruana sp. nov. Figure 117: •, Tropidopedia caracicola sp. nov.; O, Tropidopedia duckeana sp. nov.;, Tropidopedia eliasi sp. nov.; Δ, Tropidopedia nigrocarinata sp. nov. Figure 118: A, Tropidopedia carinata sp. nov.; •, Tropidopedia venezuelana sp. nov. Figure 119: •, Tropidopedia flavolineata sp. nov. Figure 120: A, Tropidopedia punctifrons (Smith, 1879).
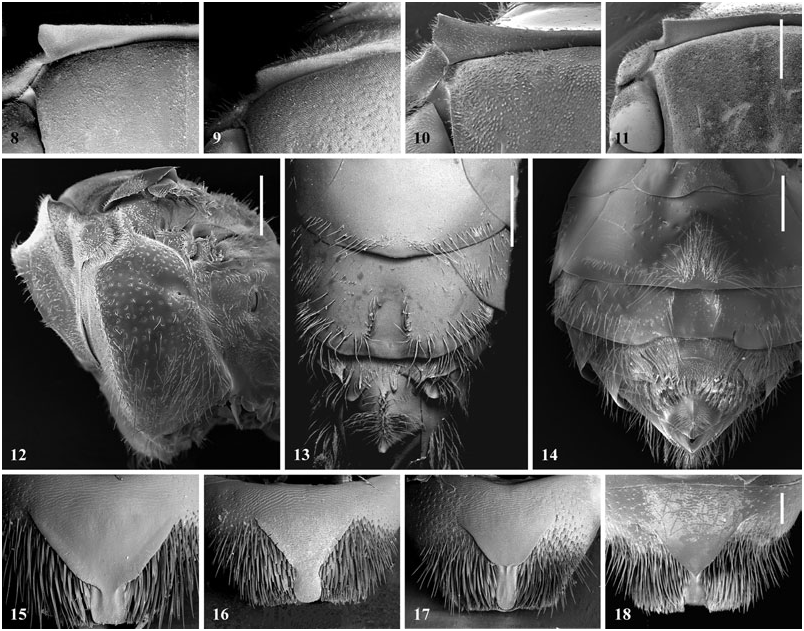
Figures 8–18. Figures 8–11: Lateral portion of pronotal colar; scale = 0.5 mm. Figure 8: Female of Tropidopedia nigrita sp. nov. Figure 9: Female of Paratetrapedia lineata (Spinola, 1851). Figure 10: Male of Lophopedia pygmaea. Figure 11: Female of Tropidopedia carinata sp. nov. Figure 12: Mesosoma of male of Tropidopedia carinata sp. nov. showing the right-angled omaulus; scale = 0.2 mm. Figures 13, 14: Sterna of male showing the tuft of hairs on medial portion of S2, and the ‘U’ sinuosity on margin of S3; scale = 0.5 mm. Figure 13: Tropidopedia flavolineata sp. nov. Figure 14: Tropidopedia carinata sp. nov. Figures 15–18: Pygidial plate of female; scale = 0.2 mm. Figure 15: Tropidopedia nigrita sp. nov. Figure 16: Arhysoceble dichroopoda Moure, 1948. Figure 17: Tropidopedia punctifrons (Smith, 1879). Figure 18: Paratetrapedia fervida (Smith, 1879).
Figures 29–35. Sternum 6 of male; scale = 0.2 mm. Figure 29: Tropidopedia nigrita sp. nov. Figure 30: Tropidopedia japuraensis sp. nov. Figure 31: T. seabrai (Michener & Moure, 1957). Figure 32: Tropidopedia friesei sp. nov. Figure 33: Tropidopedia carinata. sp. nov. Figure 34: Tropidopedia nigrocarinata sp. nov. Figure 35: Tropidopedia flavolineata sp. nov. Scale bar for Figs 29–34 shown to right of Fig. 32.
Figures 36–49. Sternum 7 of male; scale = 0.5 mm. Figure 36: Tropidopedia arcuatilis (Vachal, 1909). Figure 37: Tropidopedia danunciae sp. nov. Figure 38: Tropidopedia friesei sp. nov. Figure 39: Tropidopedia japuraensis sp. nov. Figure 40: Tropidopedia nigrita sp. nov. Figure 41: Tropidopedia ornata sp. nov. Figure 42: Tropidopedia seabrai (Michener & Moure, 1957). Figure 43: Tropidopedia caracicola sp. nov. Figure 44: Tropidopedia carinata sp. nov. Figure 45: Tropidopedia duckeana sp. nov. Figure 46: Tropidopedia flavolineata sp. nov. Figure 47: Tropidopedia nigrocarinata sp. nov. Figure 48: Tropidopedia punctifrons (Smith, 1879). Figure 49: Tropidopedia venezuelana sp. nov.
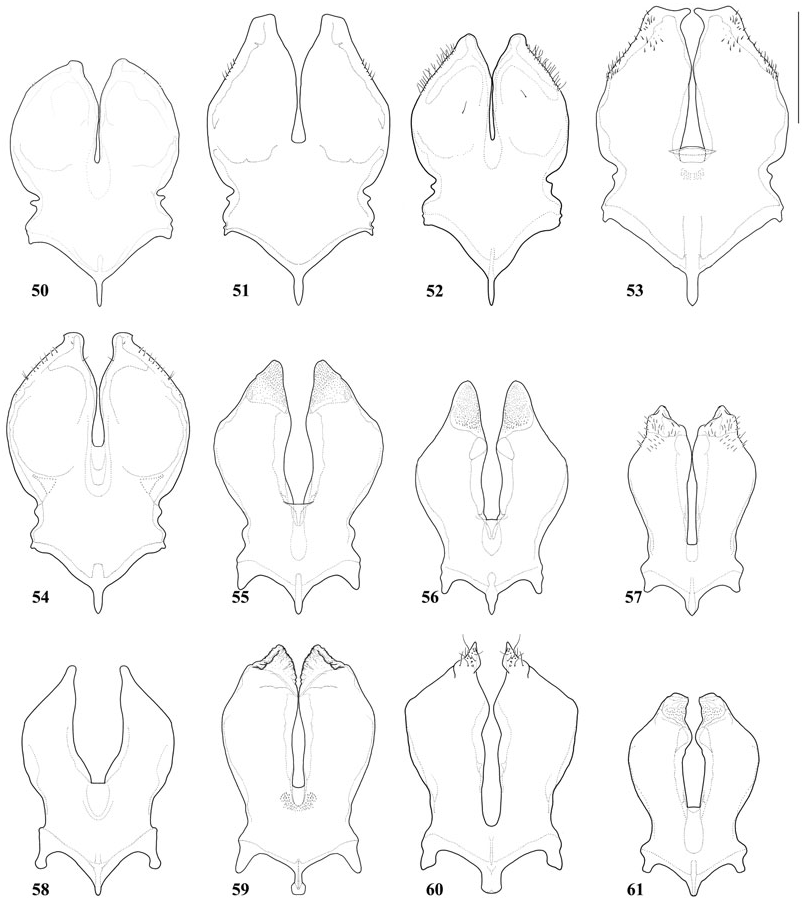
Figures 50–61. Sternum 8 of male; scale = 0.5 mm. Figure 50: Tropidopedia arcuatilis (Vachal, 1909). Figure 51: Tropidopedia danunciae sp. nov. Figure 52: Tropidopedia japuraensis sp. nov. Figure 53: Tropidopedia nigrita sp. nov. Figure 54: Tropidopedia ornata sp. nov. Figure 55: Tropidopedia caracicola sp. nov. Figure 56: Tropidopedia carinata sp. nov. Figure 57: Tropidopedia duckeana sp. nov. Figure 58: Tropidopedia flavolineata sp. nov. Figure 59: Tropidopedia nigrocarinata sp. nov. Figure 60: Tropidopedia punctifrons (Smith, 1879). Figure 61: Tropidopedia venezuelana sp. nov.
Figures 74–89. Figures 74–87: Genital capsule, male, dorsal and ventral view; scale = 0.5 mm. Figures 74, 75: Tropidopedia carinata sp. nov. Figures 76, 77: Tropidopedia duckeana sp. nov. Figures 78, 79: Tropidopedia flavolineata sp. nov. Figures 80, 81: Tropidopedia caracicola sp. nov. Figures 82, 83: Tropidopedia nigrocarinata sp. nov. Figures 84, 85: Tropidopedia punctifrons (Smith, 1879). Figures 86, 87: Tropidopedia venezuelana sp. nov. Figures 88, 89: Hind tarsomeres, outer view; scale = 0.5 mm. Figure 88: Tropidopedia friesei sp. nov. Figure 89: Tropidopedia peruana sp. nov.
Figures 99–107. Head; scale = 0.5 mm. Figures 99, 100: Tropidopedia carinata sp. nov., holotype male and paratype female. Figure 101: Tropidopedia duckeana sp. nov., holotype male. Figure 102: Tropidopedia flavolineata sp. nov., paratype female. Figures 103, 104: Tropidopedia nigrocarinata sp. nov., holotype male and paratype female. Figures 105, 106: Tropidopedia punctifrons (Smith, 1879), males. Figure 105: Dark brown specimen. Figure 106: Orange yellow specimen. Figure 107: Tropidopedia venezuelana sp. nov., female.
Figures 1–7. Figures 1–4: Punctation patterns; scale = 0.1 mm. Figure 1: Finely minute punctures, 1 dp (mesoscutum of Tropidopedia nigrita sp. nov., female). Figure 2: Fine punctures, 2 dp (mesoscutum of Lophopedia pygmaea (Schrottky, 1902), female). Figure 3: Coarse punctures, 1–2 dp (frons of Paratetrapedia sp.; female). Figure 4: Very coarse punctures, 0.5−2 dp (lateral mesepisternum of Tropidopedia carinata sp. nov., female). Figures 5–7: Head, females; scale = 0.5 mm. Figure 5: Tropidopedia punctifrons (Smith, 1879). Figure 6: Tropidopedia carinata sp. nov. Figure 7: Tropidopedia arcuatilis (Vachal, 1909).
Figures 19–28. Figures 19–22: Foretarsus; scale = 0.25 mm. Figure 19: Tropidopedia carinata sp. nov., female, outer surface. Figure 20: Paratetrapedia connexa (Vachal, 1909), female, outer surface. Figure 21: Tropidopedia punctifrons (Smith, 1879), female, outer surface. Scale = 0.25 mm. Figure 22: Tropidopedia arcuatilis (Vachal, 1909), male, lateral view showing the long plumose pubescence; scale = 0.25 mm. Figures 23, 24: Ventral surface of middle tibia, showing the comb with stout simple setae; scale = 0.01 mm. Figure 23: Lophopedia pygmaea (Schrottky, 1902). Figure 24: Xanthopedia larocai (Moure, 1995). Figures 25–28: Basitibial plate of female; scale = 0.25 mm. Figure 25: Tropidopedia nigrita sp. nov. Figure 26: P. connexa. Figure 27: L. pygmaea. Figure 28: T. punctifrons.
Figures 108–113. Figures 108–112: Mesosoma in dorsal view; scale = 0.5 mm. Figure 108: Tropidopedia arcuatilis (Vachal, 1909), female. Figure 109: Tropidopedia danunciae sp. nov., holotype male. Figure 110: Tropidopedia seabrai (Michener & Moure, 1957), female. Figure 111: Tropidopedia peruana sp. nov., holotype female. Figure 112: Tropidopedia punctifrons (Smith, 1879), female. Figure 113: Tropidopedia danunciae sp. nov., lateral portion of disc of T3 showing the microreticulation (anterior to the left); scale = 0.10 mm.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Tropidopedia eliasi
| Aguiar, Antonio J. C. & Melo, Gabriel A. R. 2007 |
Paratetrapedia (Tropidopedia) punctifrons
| Aguiar AJC & Melo GAR 2005: 32 |
Paratetrapedia duckei
| Rebelo JMM & Rego MMC & Albuquerque MCA 2003: 273 |
Paratetrapedia (Tropidopedia) duckei
| Albuquerque PMC & Mendonca JAC 1996: 49 |
Paratetrapedia (Amphipedia) haeckli
| Silveira FA & Campos MJO 1995: 375 |
Paratetrapedia (Amphipedia)
| Vogel S 1974: 190 |
Paratetrapedia (Amphipedia) haeckeli
| Silveira FA & Melo GAR & Almeida EAB 2002: 136 |
| Neff JL & Simpson BB 1981: 110 |
| Vogel S 1974: 207 |
| Michener CD & Moure JS 1957: 413 |
Tetrapaedia [sic] punctifrons
| Schrottky C 1902: 558 |
Tetrapedia punctifrons
| Cockerell TDA 1909: 399 |
| Cockerell TDA 1905: 325 |
| Smith F 1879: ) |