Mimosticus Sharp, 1884
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3893.1.2 |
|
publication LSID |
lsid:zoobank.org:pub:E5EC4E8B-A59E-478D-8A7A-21626F312564 |
|
DOI |
https://doi.org/10.5281/zenodo.6126382 |
|
persistent identifier |
https://treatment.plazi.org/id/826487FB-E872-FFCA-60FC-FA6D305BFA85 |
|
treatment provided by |
Plazi |
|
scientific name |
Mimosticus Sharp, 1884 |
| status |
|
Genus Mimosticus Sharp, 1884 View in CoL
(Type species: Mimosticus viridipennis Sharp, 1884 , by monotypy)
Diagnosis. The following combination of characters in both sexes is unique for Mimosticus within the tribe Staphylinini : neck completely or at least dorsally without nuchal ridge, and without dorsal basal ridge; posterior area of ventral head capsule anteriad to postgenal ridge with depression margined anteriorly by more or less pronounced ridge medially confluent with wide gula ( Fig. 2 View FIGURE 2 E); pronotal hypomera strongly deflexed, not visible in lateral view, relatively wide posteriad of anterior coxae, without translucent post coxal process; elytra without subbasal ridge or spines on humeri; anterior tarsi wide with thick adhesive setation ventrally; all tarsi with one short empodial seta; mesoscutellum with single scutellar ridge; abdominal tergites III–IV with two basal carinae, tergites V–VIII with one carina. In the tribe Staphylinini , Mimosticus is the only genus with males possessing a single black iridescent comb on the mesotrochanter, that continues onto the base of the mesofemur ( Fig. 2 View FIGURE 2 F); it is also one of a few genera in this tribe with a weakly to non-emarginate male sternite VIII ( Fig. 4 View FIGURE 4 ) and a nearly symmetrical sternite IX ( Fig. 6 View FIGURE 6 ).
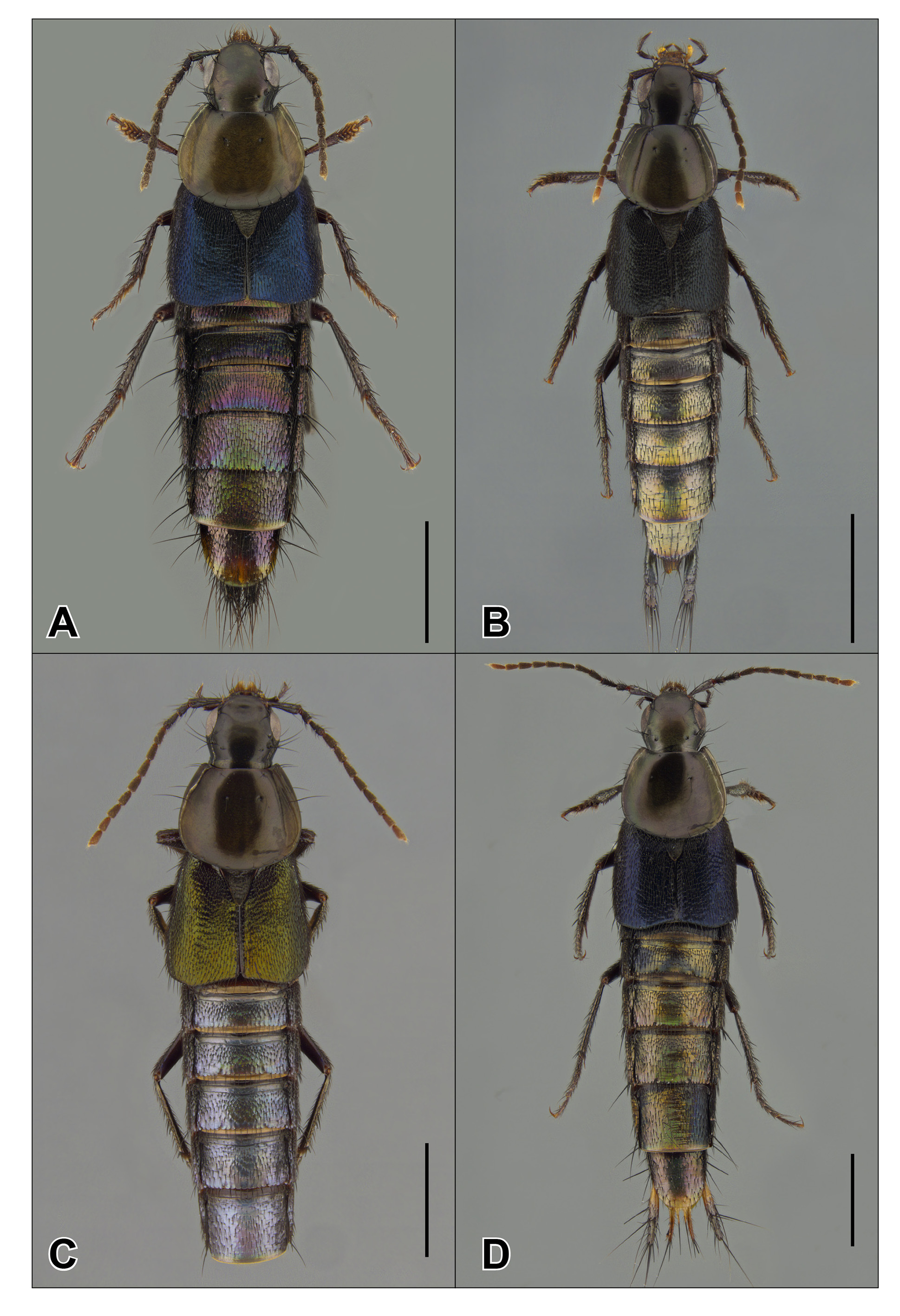
Redescription. Medium-sized (body length 11–13 mm, generally with very slight intra- and interspecific variation), dark with distinct metallic lustre, sometimes with yellowish tip of abdomen and pale apical antennomeres; with head distinctly shorter and narrower than pronotum, pronotum strongly narrowed anteriad, elytra slightly wider and longer than pronotum; in habitus somewhat similar to some metallic Neotropical species of Philonthus Stephens or Chroaptomus Sharp , but with a ‘ Quedius -like’ pronotum shape and with only 2 punctures each in the dorsal pronotal rows, although an additional puncture may occur in one of the rows.
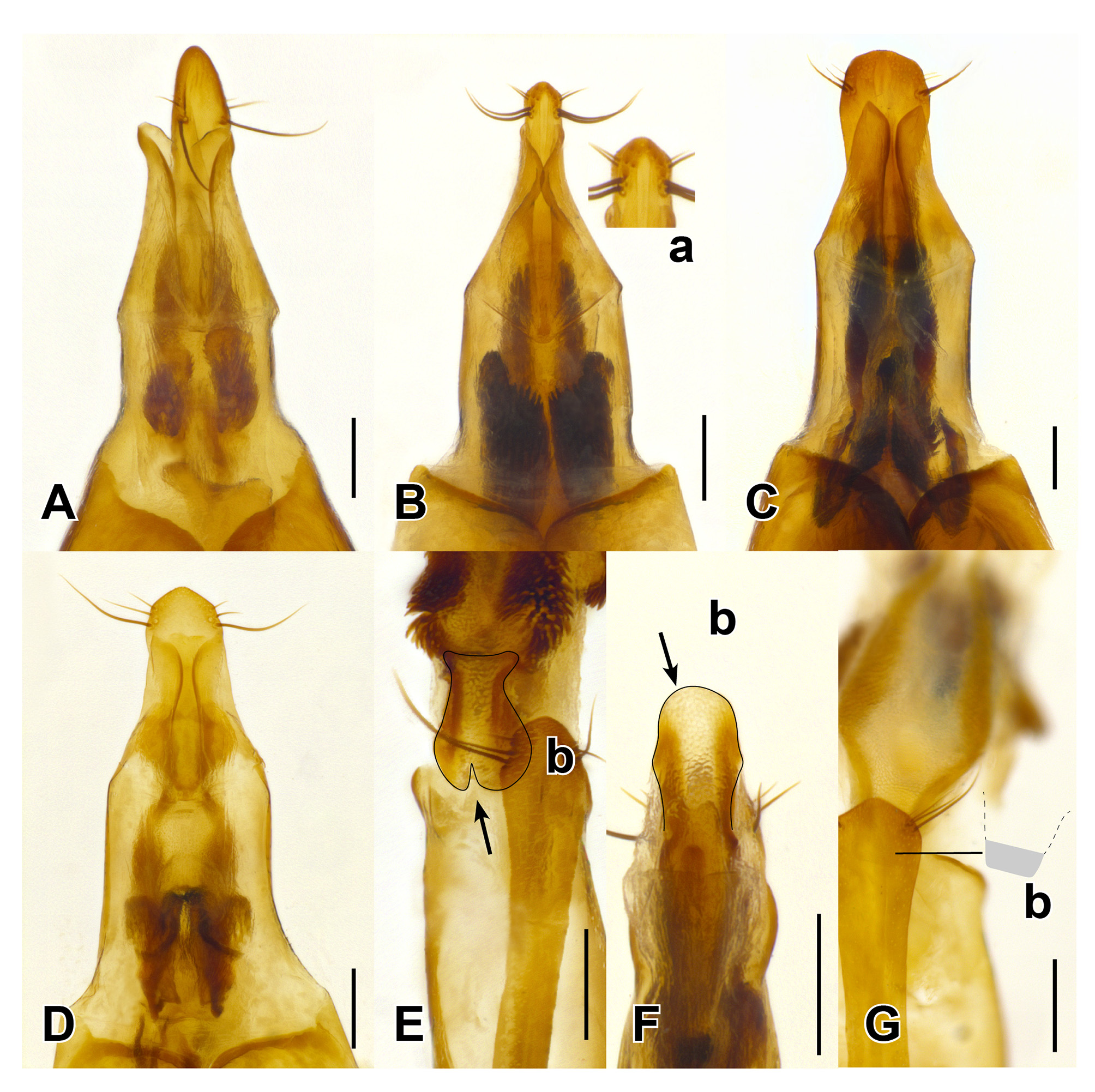
Head capsule with fully developed neck constriction but without, or with only weakly developed nuchal ridge laterally; eyes large, longer than temples, their outline not protruding beyond outline of head in dorsal view; infraorbital ridge short, extending anteriad at most to the level of posterior margin of eye; dorsal basal ridge absent; postgenal ridge present; posterior area of ventral head capsule with depression anteriad to postgenal ridge, depression anteriorly margined by more or less pronounced ridge medially confluent with wide gula; postmandibular ridge distinct, short, extending basad to the level of basal margin of maxillae; “taxonomic chaetotaxy” visible in dorsal view with two pairs of frontoclypeal punctures, anterior and posterior frontal punctures present (“a” and “c”, respectively, in Fig. 3 View FIGURE 3 A), one oculomarginal puncture between frontal punctures, one vertical puncture near neck constriction (“b” and “d”, respectively, in Fig. 3 View FIGURE 3 A); labrum large, transverse, bilobed, with distinct membranous extension of lobes; ligula apically broadly rounded, entire, but very slightly notched; mandible moderately long, with distinct short dorsolateral groove, visible in dorsal view; last segment of maxillary and labial palpi glabrous, narrowly fusiform, distinctly narrower than apex of their respective penultimate segment, apical maxillary palpomere as long as penultimate segment, apical labial palpomere slightly shorter than penultimate segment; penultimate segment of labial palpi with row of stout setae along anterior margin; antennae rather long, their first antennomere about as long as antennomeres 2 and 3 combined; antennomeres 1–3 with only long, sparse setae, pubescence present on antennomere 4 onwards.
Pronotum with two punctures in dorsal row: one very close to anterior margin of pronotum, and one on disc of pronotum before middle; superior marginal line of hypomeron not deflexed under anterior angle; inferior marginal line of hypomeron not intersecting with superior line and not continued anteriad of the level of base of anterior coxa; anterior angle strongly produced anteriad of anterior margin of prosternum; hypomeron strongly inflexed, not visible in lateral view, very wide posteriad of anterior coxae, without translucent postcoxal process; pronoto-sternal suture distinct; prosternum with median longitudinal projection but without ridge; basisternum with sparse brown setae but without pairs of long stout black macrosetae.
Elytra evenly densely punctate, without subbasal ridge or spines on humeri; scutellum only with one (anterior) scutellar ridge, with punctation as on elytra; wing with veins CuA and MP4 fused, but distinct as two veins basally, vein MP3 clearly present though reduced to a dark marking ( Fig. 2 View FIGURE 2 G).
Legs moderately long, all tarsi five segmented; protarsomeres with dorsal surface setose, dilated in both sexes, with white adhesive setation underneath; anterior tibiae with very dense pale setation medially; middle and posterior tibiae with long dark spines; mesocoxae contiguous; metacoxae without transverse carina; meso- and metatarsomeres with longer and denser setae ventrally, but without any clusters of white adhesive setae or dark combs; each tarsus with one short empodial seta much shorter than tarsal claws.
Abdomen evenly punctate, with punctation much sparser than on elytra, punctures becoming sparser towards abdominal apex; protergal gland manifested as well-developed invaginated capsulae with smaller opening; base of abdominal tergites III–IV with anterior and posterior transverse carinae, tergites V–VIII with only anterior transverse carina; apical margin of tergite VIII with whitish seam of microsetae. Lateral tergal sclerites IX elongate, apically obtuse.
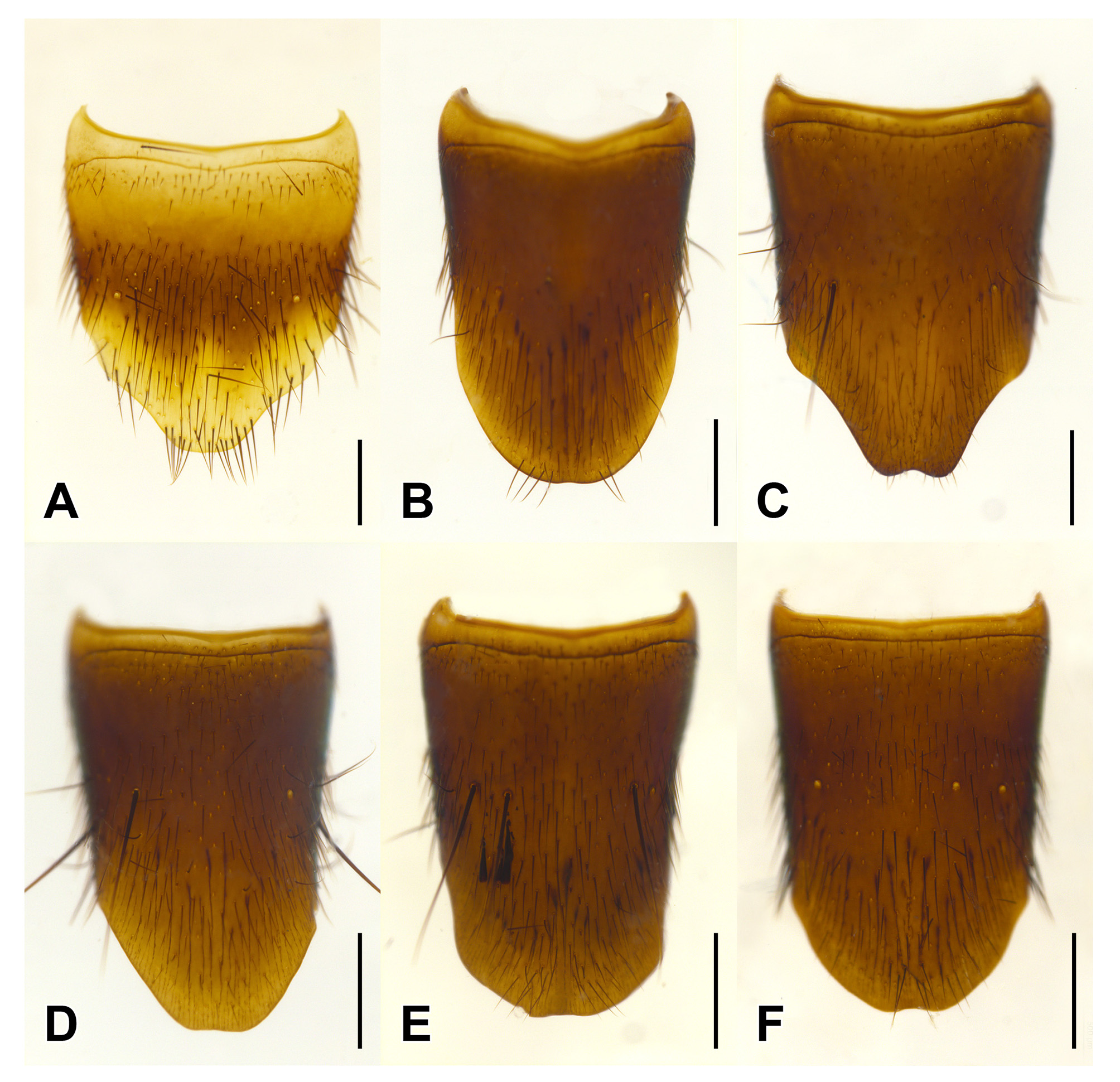
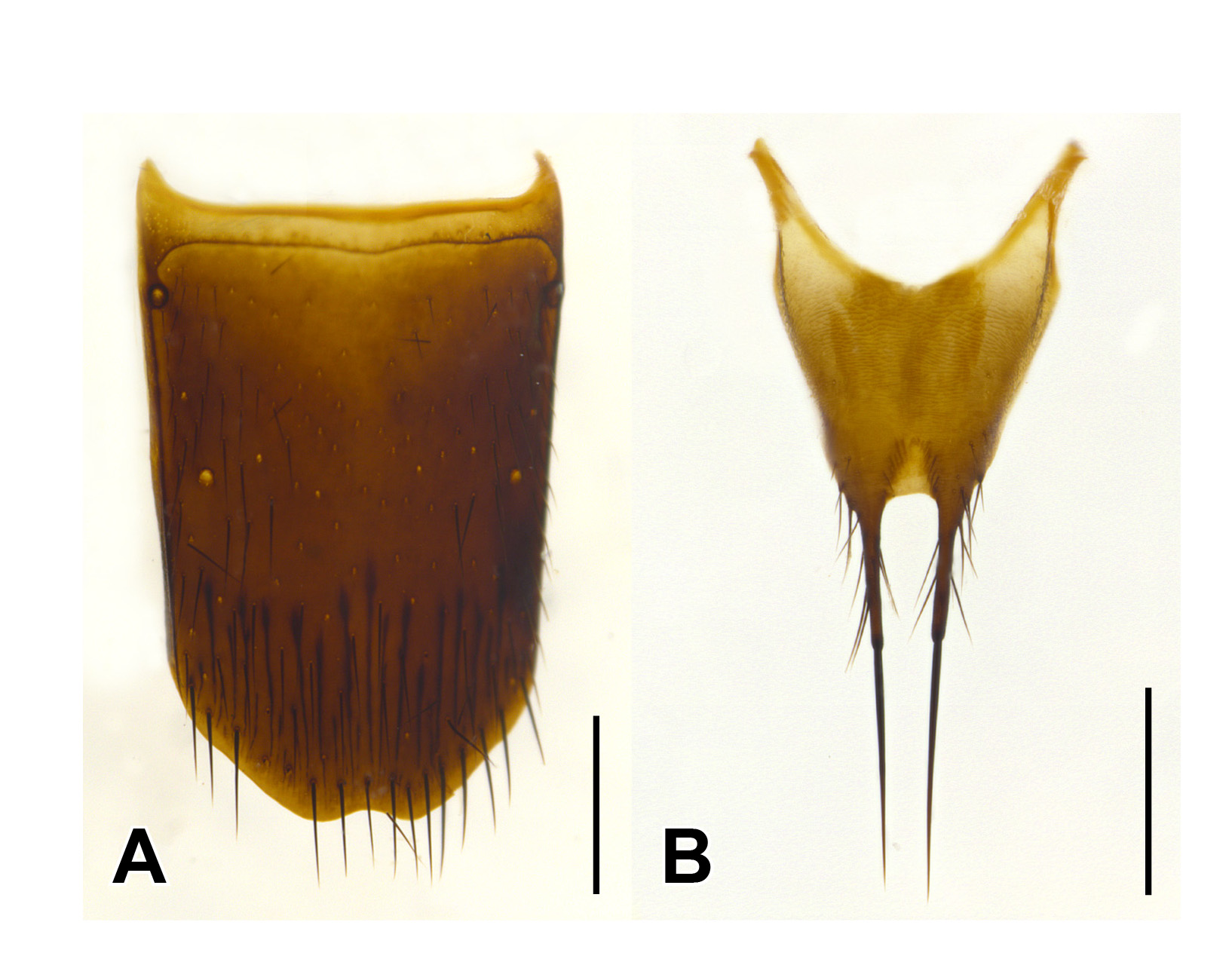
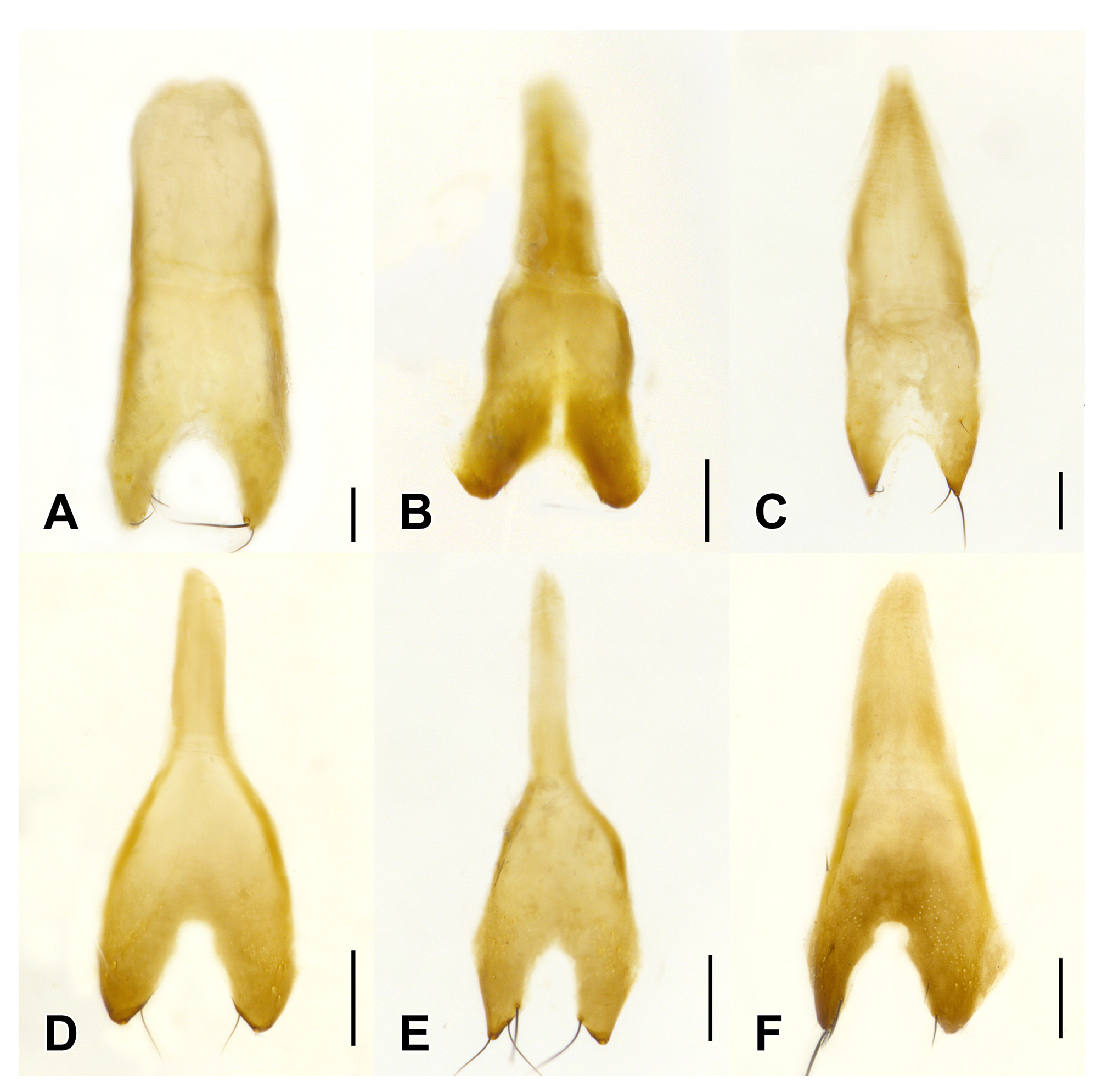
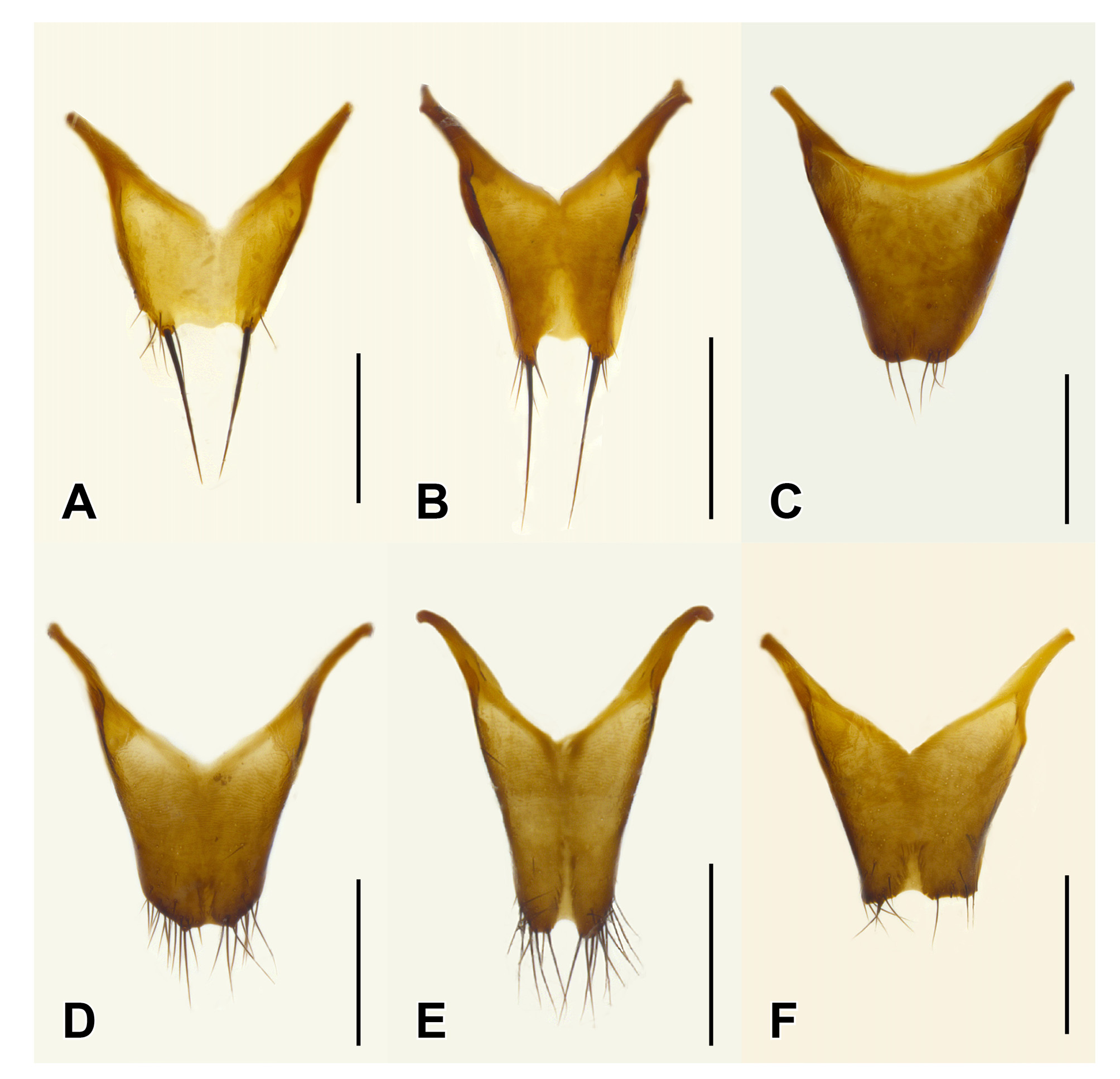
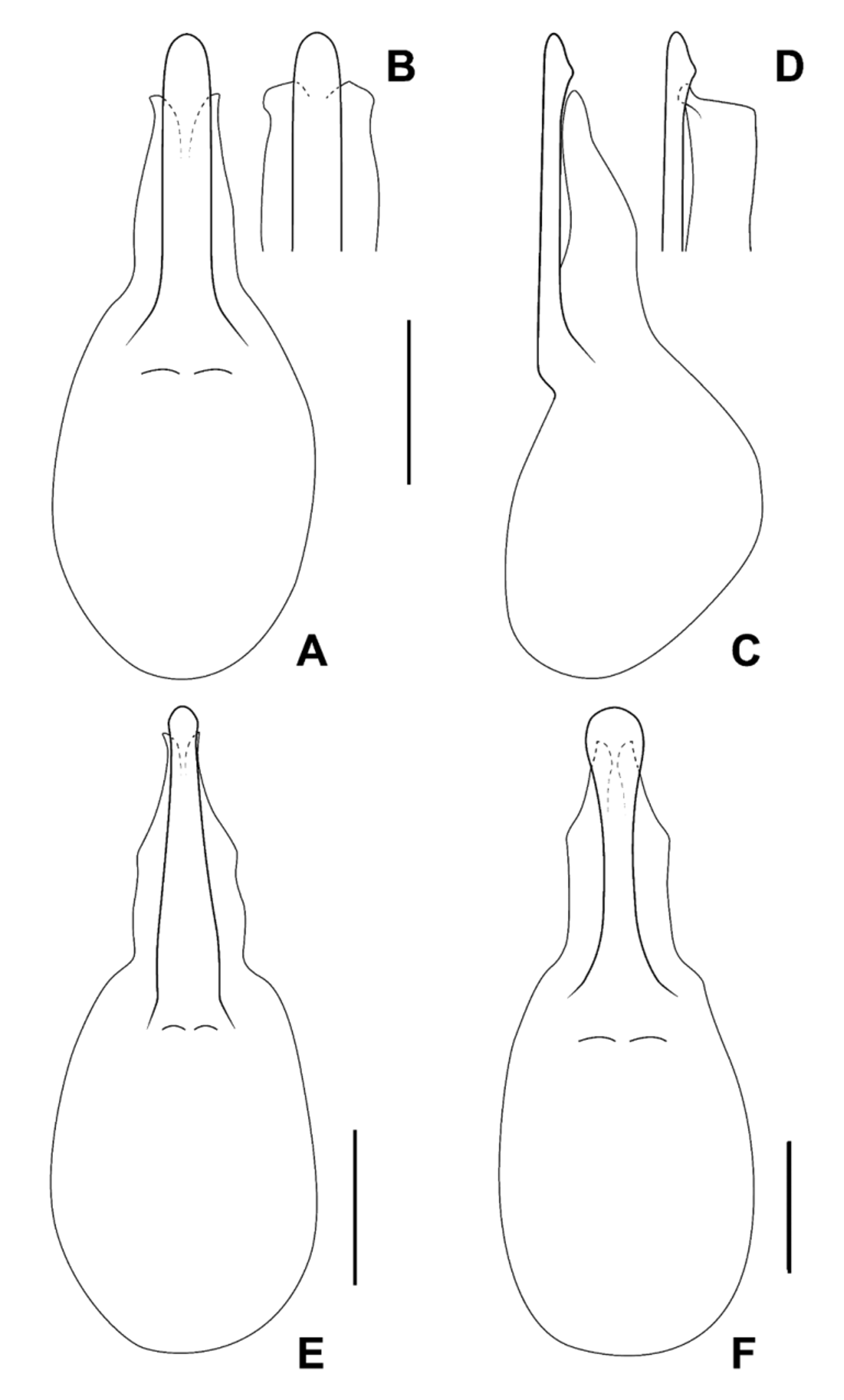
Male. Middle legs with black iridescent combs on posterior margin of trochanters and base of femora ( Fig. 2 View FIGURE 2 F). Abdominal sternite VIII with apical margin somewhat truncate but at most weakly emarginate at middle ( Fig. 4 View FIGURE 4 ); sternite IX nearly to entirely symmetrical with long narrow basal part and short, broader, emarginate apical portion ( Fig. 6 View FIGURE 6 ); tergite X short, with slight to distinct median emargination ( Fig. 7 View FIGURE 7 ). Aedeagus symmetrical, with very large bulbous basal part of median lobe and narrow apical portion ( Fig. 8 View FIGURE 8 ), with internal sac more or less armoured with complex sclerites ( Fig. 9 View FIGURE 9 ); parameres fused into single lobe closely adjoining median lobe, paramere apically with few long regular setae, without or with small, sharp and pale, tooth-like structures superficially similar to peg setae on its underside.
Female. Abdominal sternite VIII with apical margin somewhat truncate but not emarginate at middle; ovipositor consisting of a pair of broad proximal gonocoxites and a pair of rather long, narrow distal gonocoxites, without styli. Female tergite X with attenuate apico-lateral angles and with paired apical projections ( Fig. 5 View FIGURE 5 B).
Sexual dimorphism. Males are easily separated from females by the black combs on the mesotrochanters and base of mesofemora, and by the more strongly dilated anterior tarsi.
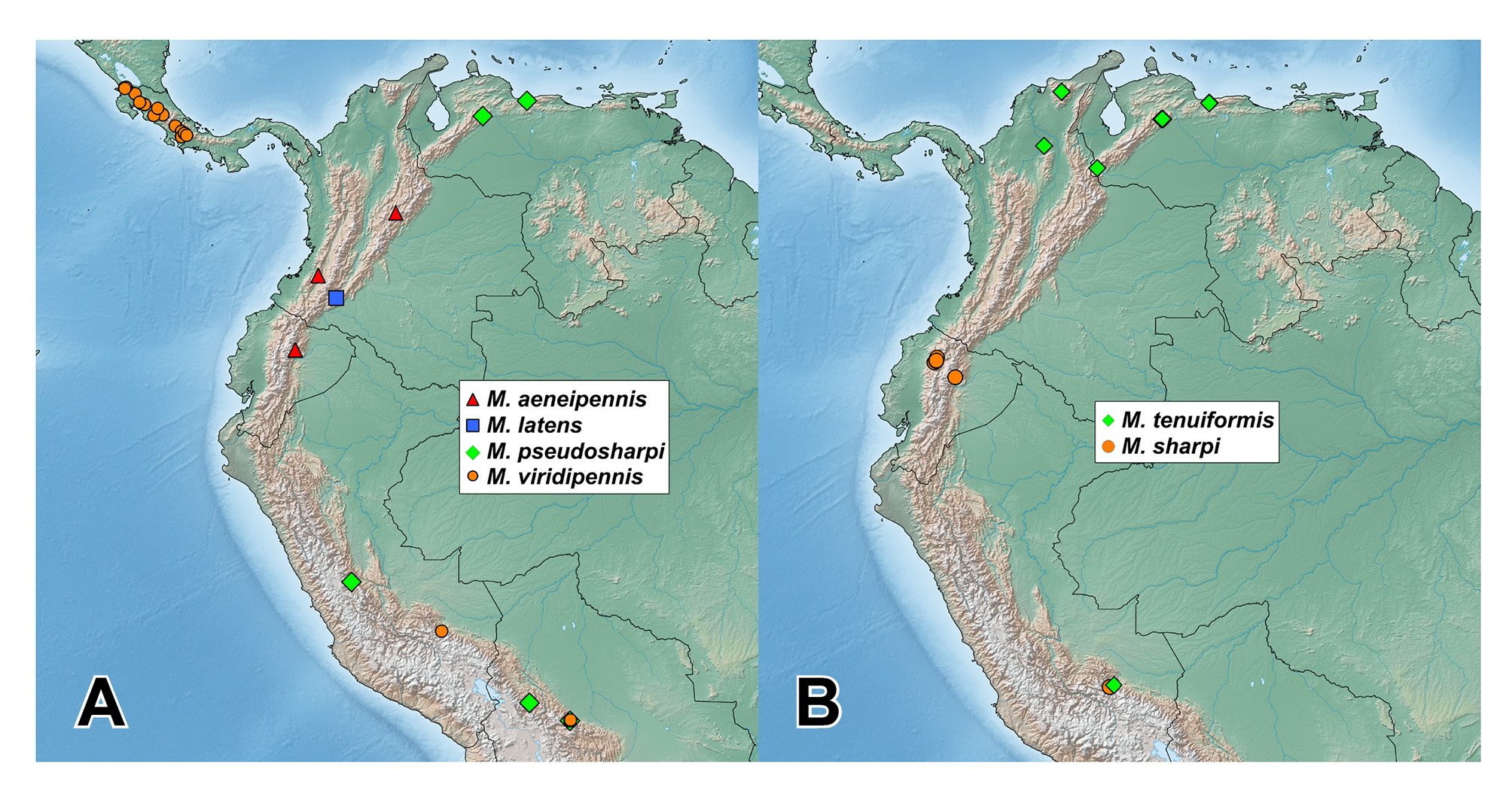
Distribution and bionomics. The genus Mimosticus is broadly distributed along the southern Neotropical Cordilleras, from northern Costa Rica to western Panama, and through Venezuela, Colombia and Ecuador south to Peru and Bolivia ( Fig. 10 View FIGURE 10 ). It is mostly confined to montane forests at elevations between 1000 and 2000 m, however the lowest elevation record is from 670 m ( M. viridipennis , Costa Rica), and the highest from 2850 m ( M. aeneipennis sp.n., Colombia). Based on collection records it is apparent that individuals of Mimosticus occur in various ground-based forest microhabitats including leaf litter, fungusy logs and other plant-based decaying substrates. As can be judged from their frequent occurrence in flight intercept and Malaise traps, they often disperse by flight. One species, M. viridipennis , seems to have a more distinct association with freshly rotten debris such as fruits or fungi compared to the other species.
Systematic position. The genus Mimosticus belongs to the subtribe Amblyopinina (in the sense of Chatzimanolis et al. 2010) of the tribe Staphylinini based on the analysis in Solodovnikov (2006) and the unique combination of characters diagnostic of the subtribe mentioned in Brunke & Solodovnikov (2013): mesoscutellum with only one basal ridge (symplesiomorphic); elytra with subbasal ridge absent or reduced (synapomorphic) and aedeagus with paramere closely attached to or fused with median lobe (in this case, fused at base) (synapomorphic). The earlier placement of Mimosticus in Quediina was based on a non-phylogenetic, classic concept of that subtribe, which does not accurately reflect evolutionary history (e.g., Solodovnikov 2006). Thus, it is noteworthy that Sharp (1884) had long ago suggested an affinity of Mimosticus with the Australian genus Quediopsis Fauvel, 1878 , a genus also now included in the Amblyopinina ( Chatzimanolis et al. 2010).
The black iridescent combs on the posterior margin of the mesotrochanters and the base of the mesofemora in males are phylogenetically noteworthy. One New Zealand species of Amblyopinina , “ Quedius ” fuscatus Broun, 1893, possesses a double row (single in Mimosticus ) of black combs but only on the mesofemur. Otherwise, similar combs are located either on the posterior coxae or on the mesotarsi in males of many, mostly Australian species of Amblyopinina but are not known to occur on the legs of any other Staphylinini . As the taxa now included in Amblyopinina , some very species-rich, have never been phylogenetically explored, the sister relationships of Mimosticus within this subtribe are currently unknown.
Comparison with other Staphylinini . In the Neotropical region, species of Mimosticus ( Fig. 1 View FIGURE 1 ) superficially resemble some similarly coloured members of the subtribes Xanthopygina and Philonthina that can even be found in the same microhabitats. However, species of Mimosticus can be easily distinguished from most Neotropical Xanthopygina and Philonthina by the strongly inflexed hypomera, which are not visible in lateral view. Exceptional members of the latter subtribes with inflexed hypomera (mostly myrmecophilic taxa) can be separated from Mimosticus by the presence of a dorsal basal ridge and either no empodial setae ( Philonthina ) or a welldeveloped pair of empodial setae (Xanthopygina). Mimosticus has one short empodial seta on each tarsus; within Staphylinini this occurs only within the Amblyopinina .
Among the Neotropical amblyopinine genera Loncovilius Germain, 1903 , Cheilocolpus Solier, 1849 , Rolla Blackwelder, 1952 and Heterothops Stephens, 1829 , Mimosticus is distinguished by a combination of its relatively large body size (~ 11–12 mm), strong metallic lustre (dull in most other Amblyopinina ), wide pronotal hypomera lacking the translucent postcoxal process (present in Loncovilius ), non-aciculate apical segment of maxillary palpi (aciculate in Heterothops , and in most Cheilocolpus and Rolla), mesotarsi of males without clusters of pale setae (developed in males of some Loncovilius ), and mesotrochanters and mesofemora of males with black combs (unknown in any other Amblyopinina ).
Species of Mimosticus most closely resemble some relatively large Neotropical species of ‘ Quedius ’ such as Q. viridulus Erichson, 1840 from Colombia and, especially, Q. germaini Bernhauer, 1917 from Bolivia. Both of these ‘ Quedius ’ only superficially resemble true Quedius and, in fact, belong to the Loncovilius-Quediomimus Cameron, 1948 lineage of the subtribe Amblyopinina , of which Mimosticus may be a member. However, the postcoxal process present in these taxa will readily distinguish them from Mimosticus . Additionally, Q. viridulus has distinct posterior angles of the head (indistinct in Mimosticus ), while Q. germaini has spines on the humeri and distinctly transverse subapical antennomeres (no humeral spines and antennomeres much more elongate in Mimosticus ). A proper systematic treatment of Q. viridulus , Q. germaini , and other, similarly misplaced Neotropical ‘ Quedius ’ or ‘Quediina’ will be provided in a separate paper.
Species recognition. Three of the six currently recognized species of Mimosticus ( M. viridipennis , M. tenuiformis and M. aeneipennis ) may be distinguished by external characters alone. The remaining three species ( M. sharpi , M. pseudosharpi , and M. latens ) belong to the ‘ sharpi species complex’ where species are very similar externally and males are needed for confident identification. It is interesting to note that, unlike many groups of rove beetles, the structure of the aedeagus in this species complex does not provide the most useful characters for species delimitation. Instead, tergite X and sternite IX in males of the ' sharpi species complex' are characteristically shaped in each species ( Fig. 6–7 View FIGURE 6 View FIGURE 7 ) and can often be seen without dissection.
Differences in the aedeagus between some species involve the shape of the paramere and patterns of sclerotization of the internal sac, which bears multiple small, spine-like sclerites and several larger ones. Since the internal sac is very complex in Mimosticus , and, at the same time, displays only subtle interspecific variation among some species, the ideal observation of this morphology would require the complete eversion of the sac in a long series of specimens. However, as the eversion of internal sac is a very laborious (sometimes impossible) procedure for routine species determinations, we focused only on diagnostic, interspecific differences in the internal sac that can be seen in situ after a conventional dissection of the aedeagus (see Methods). With this approach, additional, even more subtle variation in the internal sac may have been overlooked due to its densely packed conformation in situ. Future, more extensive studies of the everted internal sac may reveal additional variation. Finally, striking variation in the shape of the apex of the median lobe was observed when aedeagi are macerated in KOH. This variation is artefactual: as the internal sac everts, the soft dorso-lateral portions of the median lobe apex move laterally and change the outline in lateral and parameral view ( Fig. 8 View FIGURE 8 A vs. 8B, 8C vs. 8D).
Species distributions. Another noteworthy feature of some Mimosticus species recognized herein is a wide and, at the same time, highly patchy distribution. For example, most of the 65 examined specimens of M. viridipennis were collected from a nearly continuous band from north-western Costa Rica to western Panama, while some were from one locality each in southern Peru and Bolivia. This pattern of records ( Fig. 10 View FIGURE 10 A) leaves a huge area in the Andes where M. viridipennis either does not occur, or was not previously sampled. Mimosticus sharpi is known from 69 specimens, some of which originate from several localities in northern Ecuador and the remainder from one locality in southern Peru, again leaving a large gap along the Andes without any records. Another, similar pattern is displayed by our 79 records of M. tenuiformis . Most records of M. tenuiformis are from several localities in the northern Andes (north-western Venezuela and northern Colombia), but one female was found in southern Peru. Given our sample of the Mimosticus material available, which is fragmentary considering the size of South and southern Central America, it is difficult to judge whether the observed disjunct distribution patterns are real or, rather, artefacts of poor sampling. The single female of M. tenuiformis from Peru may even be a misidentified new species, as males are very important for reliable species determination in this genus. Finally, the overlapping distributions within the ' sharpi species complex' are worth mentioning because these three morphologically similar species have never been collected together at the same locality, raising the question of whether they are truly syntopic or not. More extensive sampling in South America is needed to reassess species distributions and sympatry in this genus.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
SubFamily |
Staphylininae |
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
SubFamily |
Staphylininae |
|
Genus |