Pilogalumna tenuiclava ( Berlese, 1908 )
|
publication ID |
https://doi.org/10.11646/zootaxa.5187.1.8 |
|
publication LSID |
lsid:zoobank.org:pub:852AEBF7-7B91-47FB-8F16-6D7C08047384 |
|
DOI |
https://doi.org/10.5281/zenodo.7081919 |
|
persistent identifier |
https://treatment.plazi.org/id/77704C09-FF81-B107-A2D7-FCA2AC421C3B |
|
treatment provided by |
Plazi (2022-09-14 08:38:46, last updated 2024-11-28 23:08:19) |
|
scientific name |
Pilogalumna tenuiclava ( Berlese, 1908 ) |
| status |
|
Pilogalumna tenuiclava ( Berlese, 1908) View in CoL
Oribates tenuiclavus Berlese, 1908 : Castagnoli and Pegazzano 1985.
Galumna tenuiclavus: Oudemans 1919 .
Galumna areolata Willmann, 1923 View in CoL : Hammen 1952.
Galumna radiata Sellnick, 1928 : Willmann 1931.
Allogalumna atra Mihelčič, 1957 View in CoL : Hammen 1952.
Pilogalumna tenuiclava ( Berlese, 1908) View in CoL : Grandjean 1956; Sellnick 1960; Shaldybina 1975; Karppinen and Krivolutsky 1982; Golosova et al. 1983; Schatz 1983, 2020; Karppinen et al. 1986, 1987; Marshall et al. 1987; Mahunka 1992; Pérez-Iñigo 1993; Bernini et al. 1995; Olszanowski et al. 1996; Niemi et al. 1997; Mahunka and Mahunka-Papp 2000; Ryabinin and Pankov 2002; Weigmann 2006; Siepel et al. 2009; Bayartogtokh 2010; Norton and Ermilov 2014; Miko 2016; Murvanidze and Mumladze 2016; Ermilov and Klimov 2017; Behan-Pelletier and Lindo 2019.
Diagnosis
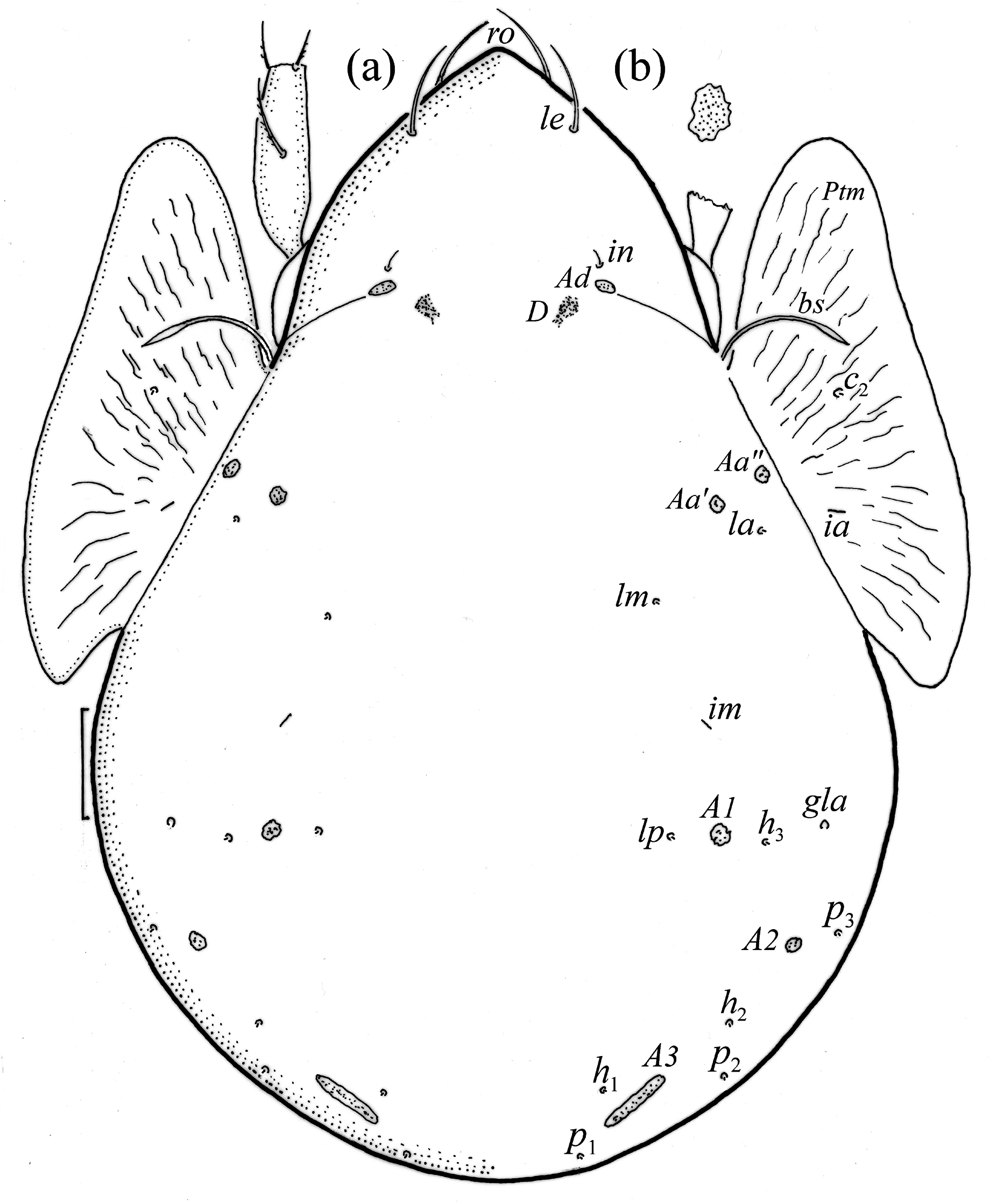
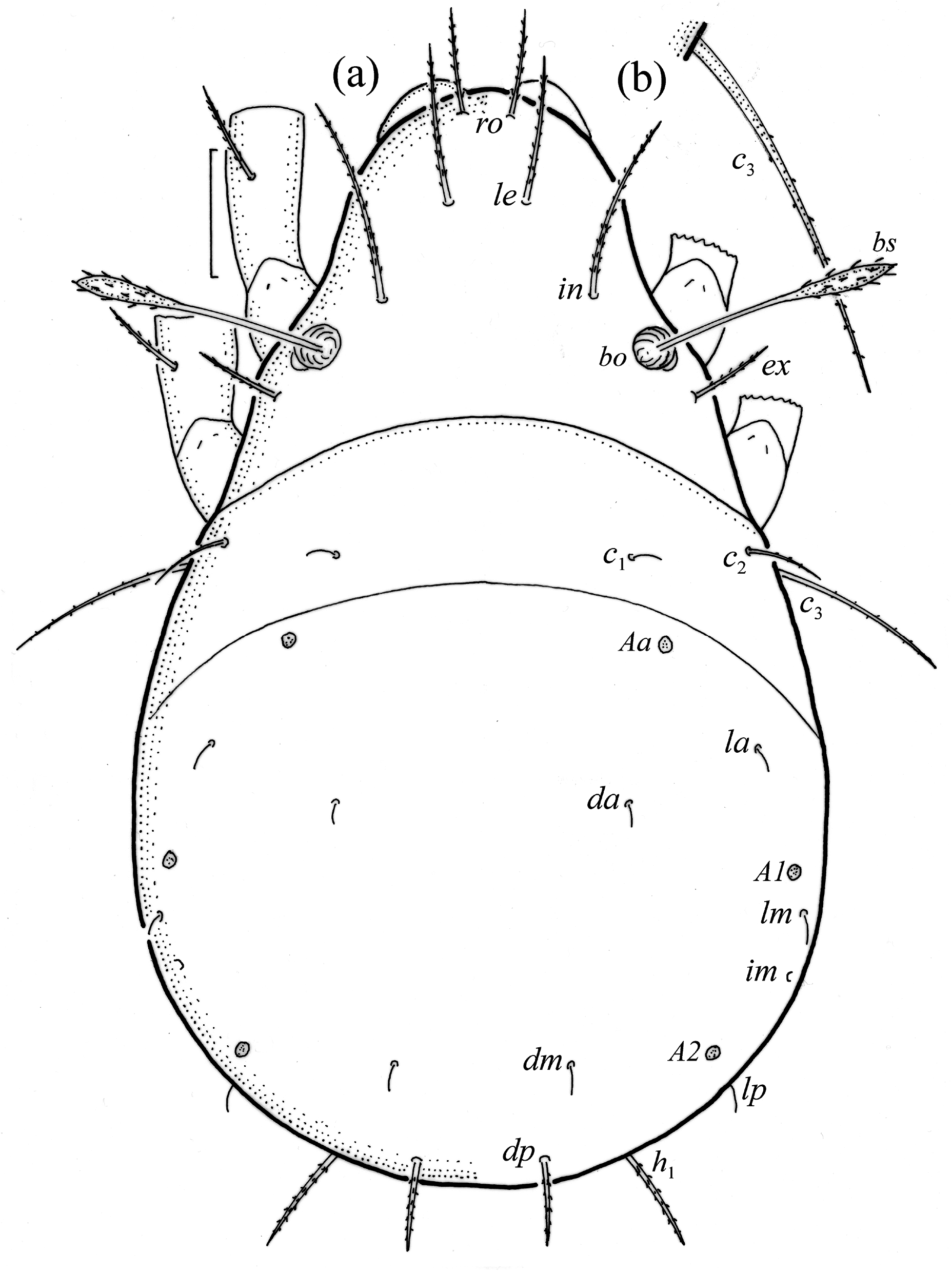
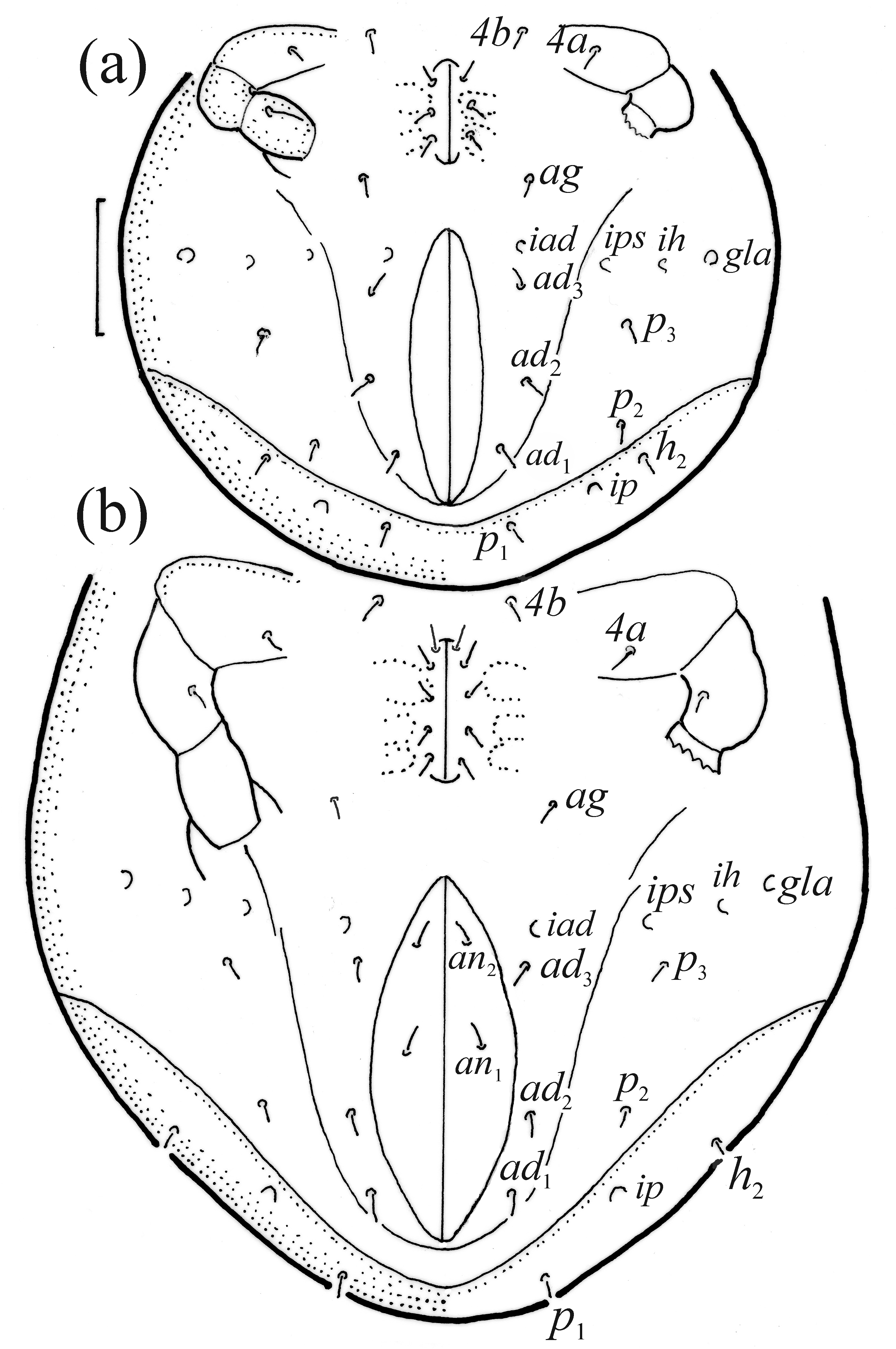
Adults brown to dark brown, of medium size (462–540), and with characters of Pilogalumna ( Ermilov & Klimov 2017) . Interlamellar setae short, bothridial seta fusiform, with long, narrow, finely barbed head. Notogaster with ten pairs of microsetae and five pairs of porose areas, including rounded Aa’ and Aa” of similar size, and elongated A3. Postanal porose area narrow and elongated.
Juveniles light brown, prodorsal setae of medium size or long and barbed, bothridial seta fusiform, with thick, barbed head. Larva with 11 pairs of gastronotal setae, including h 2; most setae short and smooth, except for mediumsized c 3, dp and h 1, and slightly shorter c 2 and h 2, most of these setae barbed, h 2 finely barbed. Nymphs with 15 pairs of these setae, most short and smooth, except for medium-sized and barbed c 3. Gastronotal shield poorly developed, with setae d -, l -series and h 1 in larva, and d -, l -, h -series and p 1 in nymphs; setae of c -series, p 2 and p 3 inserted on unsclerotized integument. In all juveniles, humeral organ present.
Morphology of adult
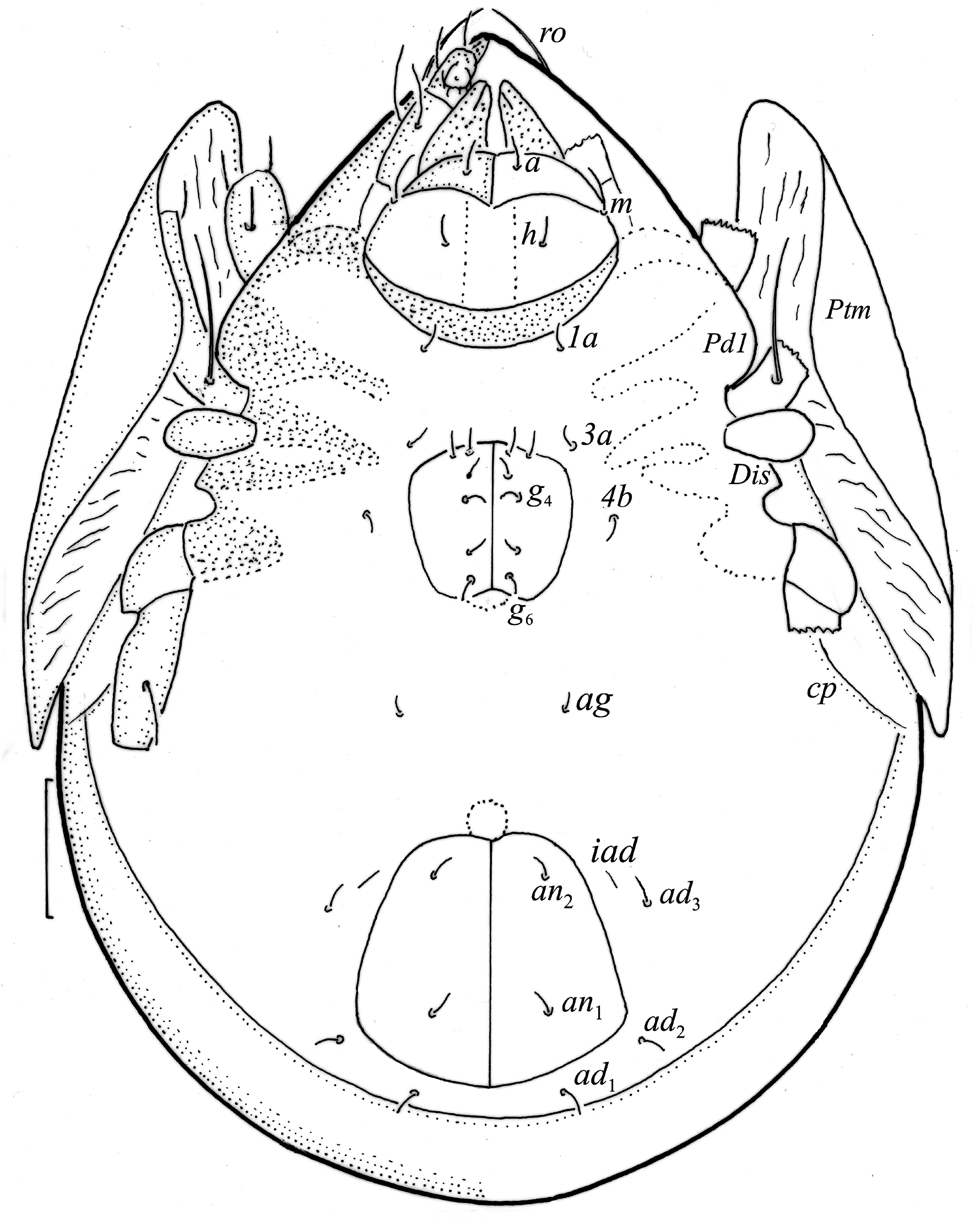
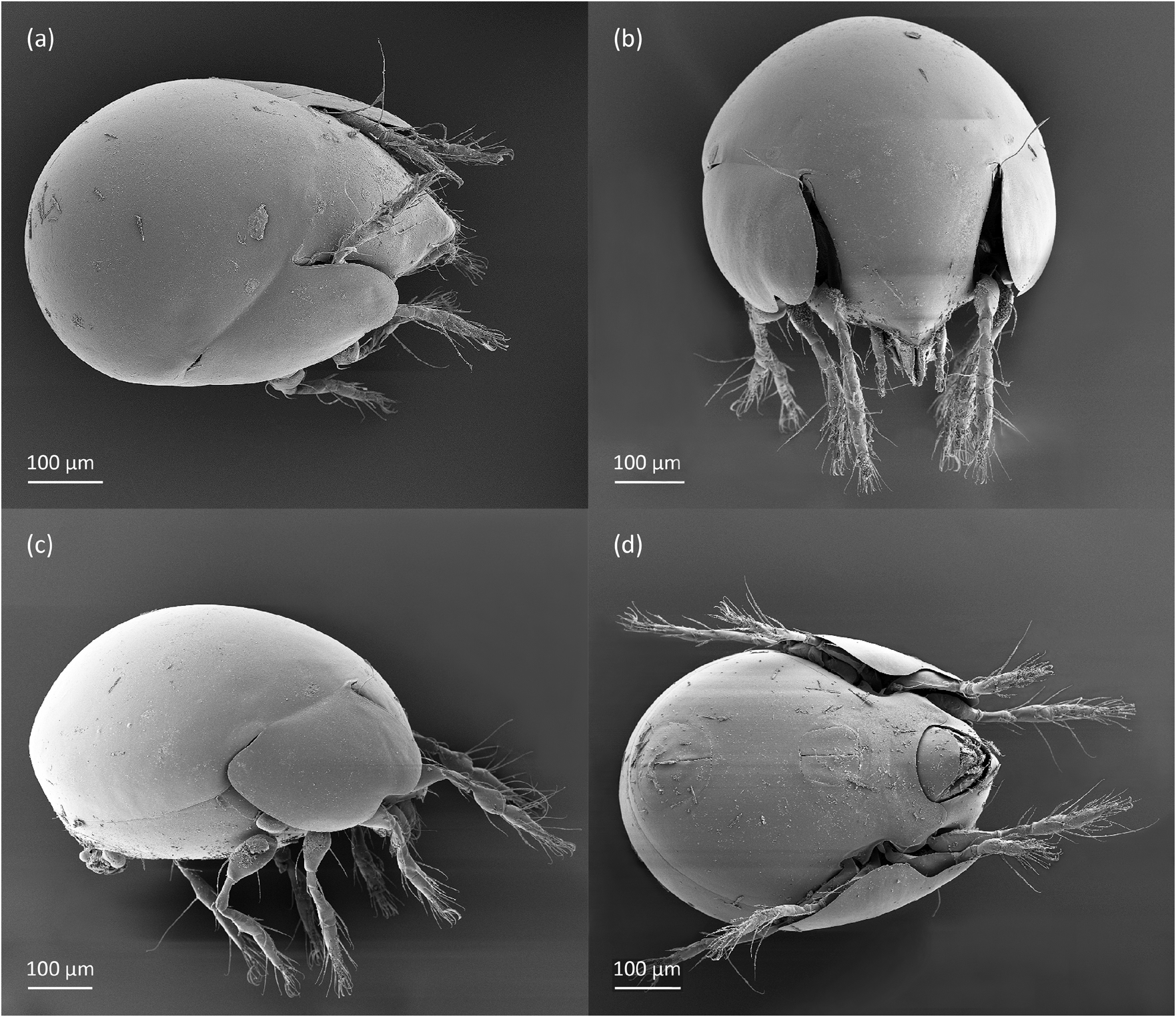
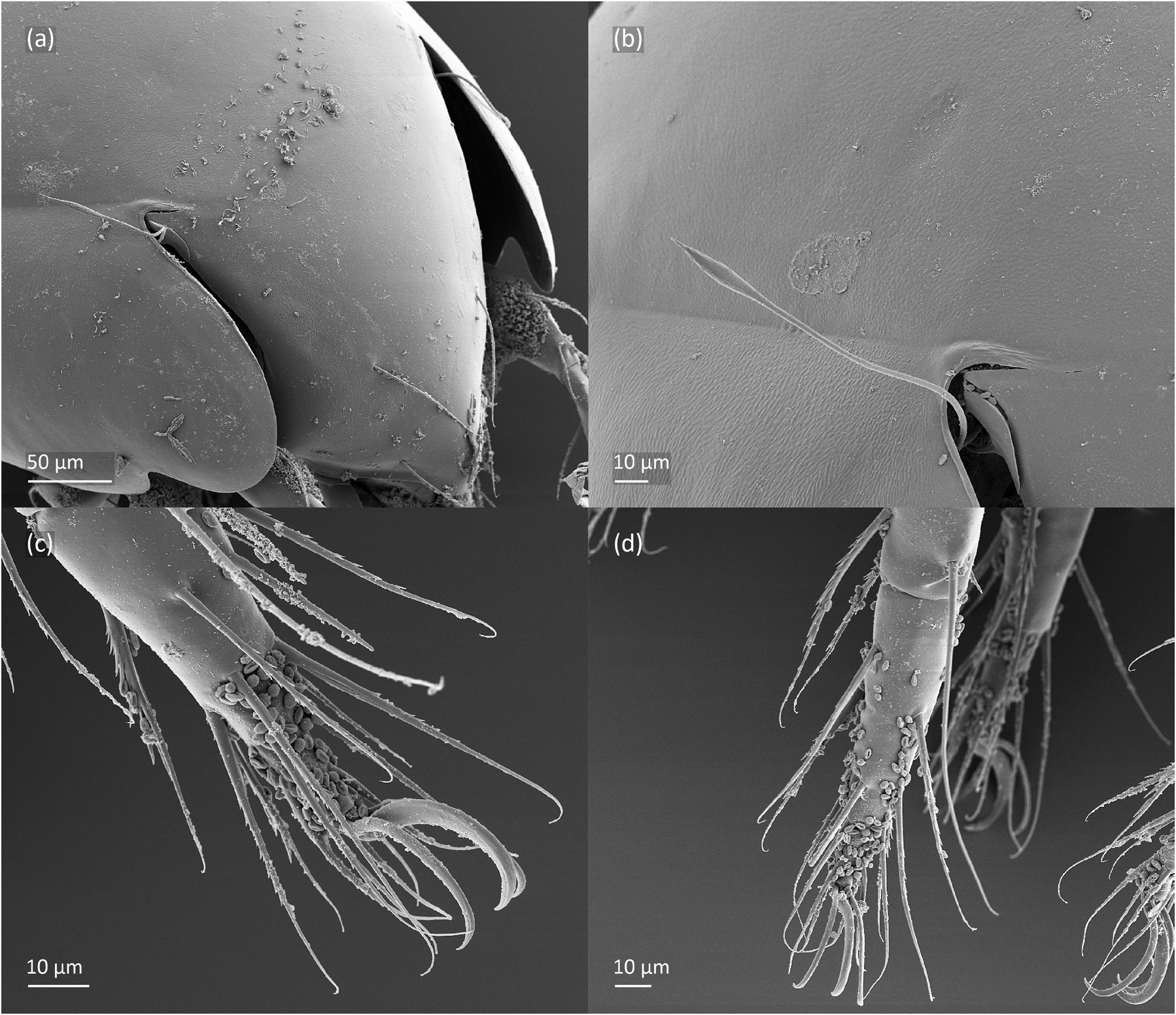
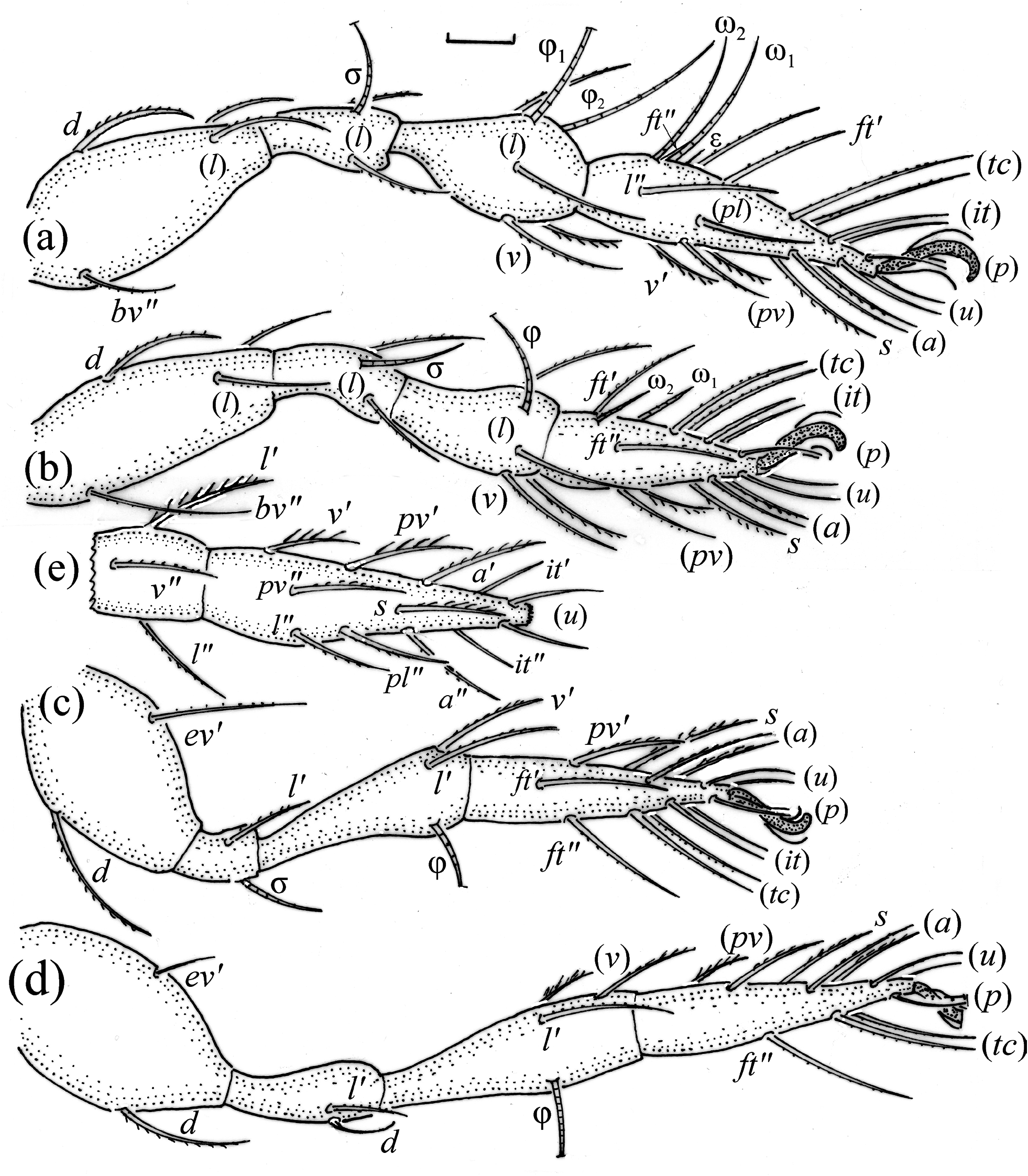
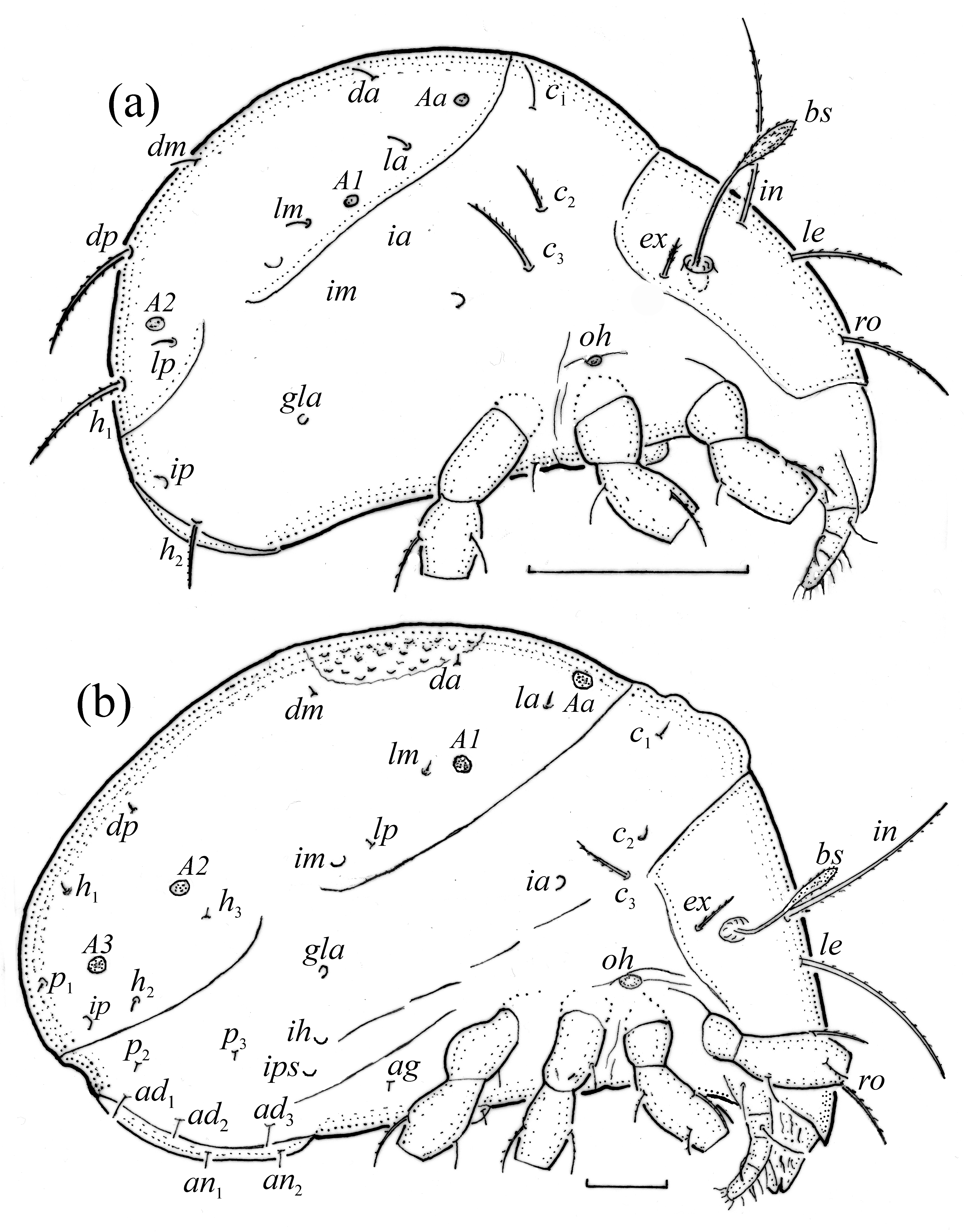
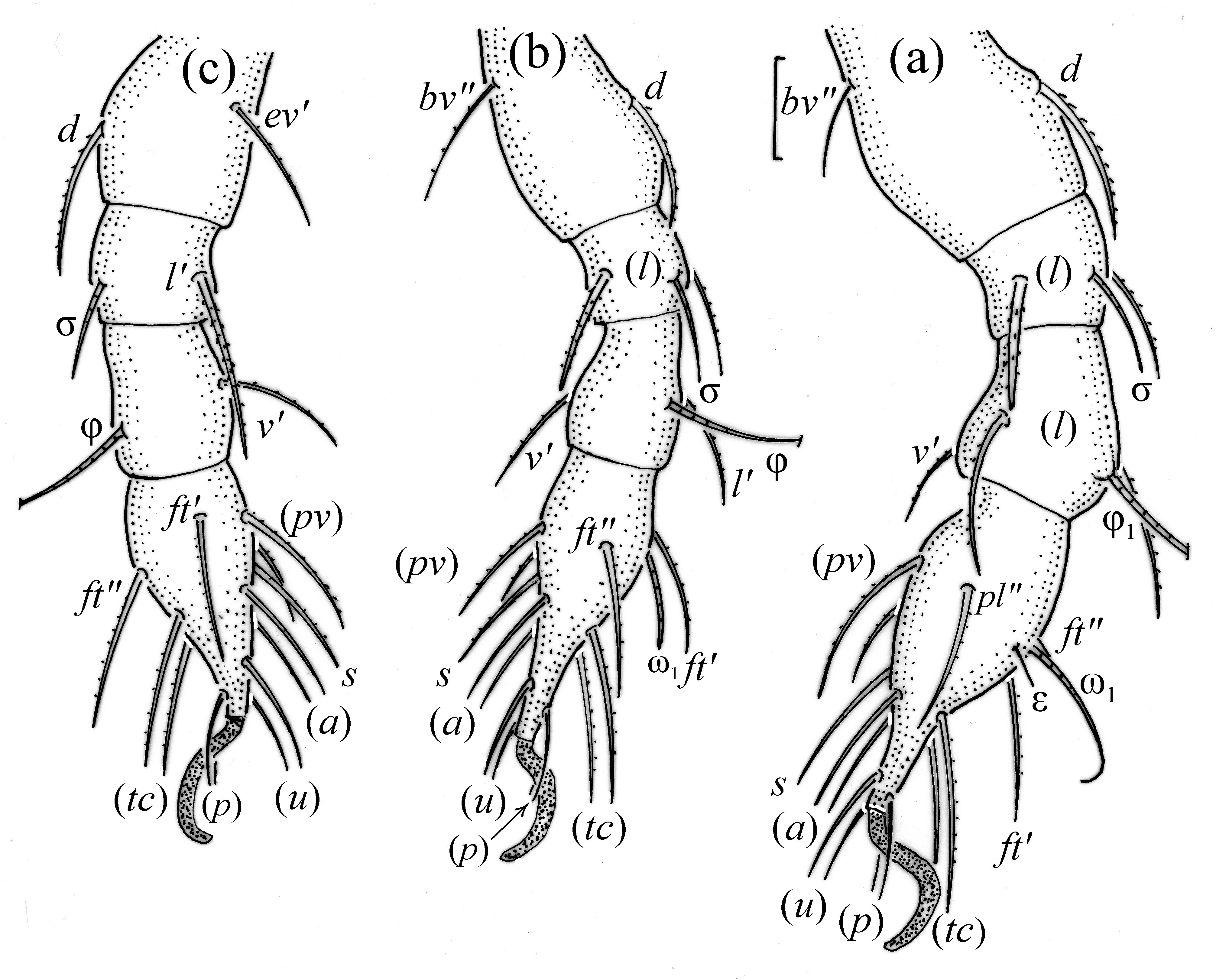
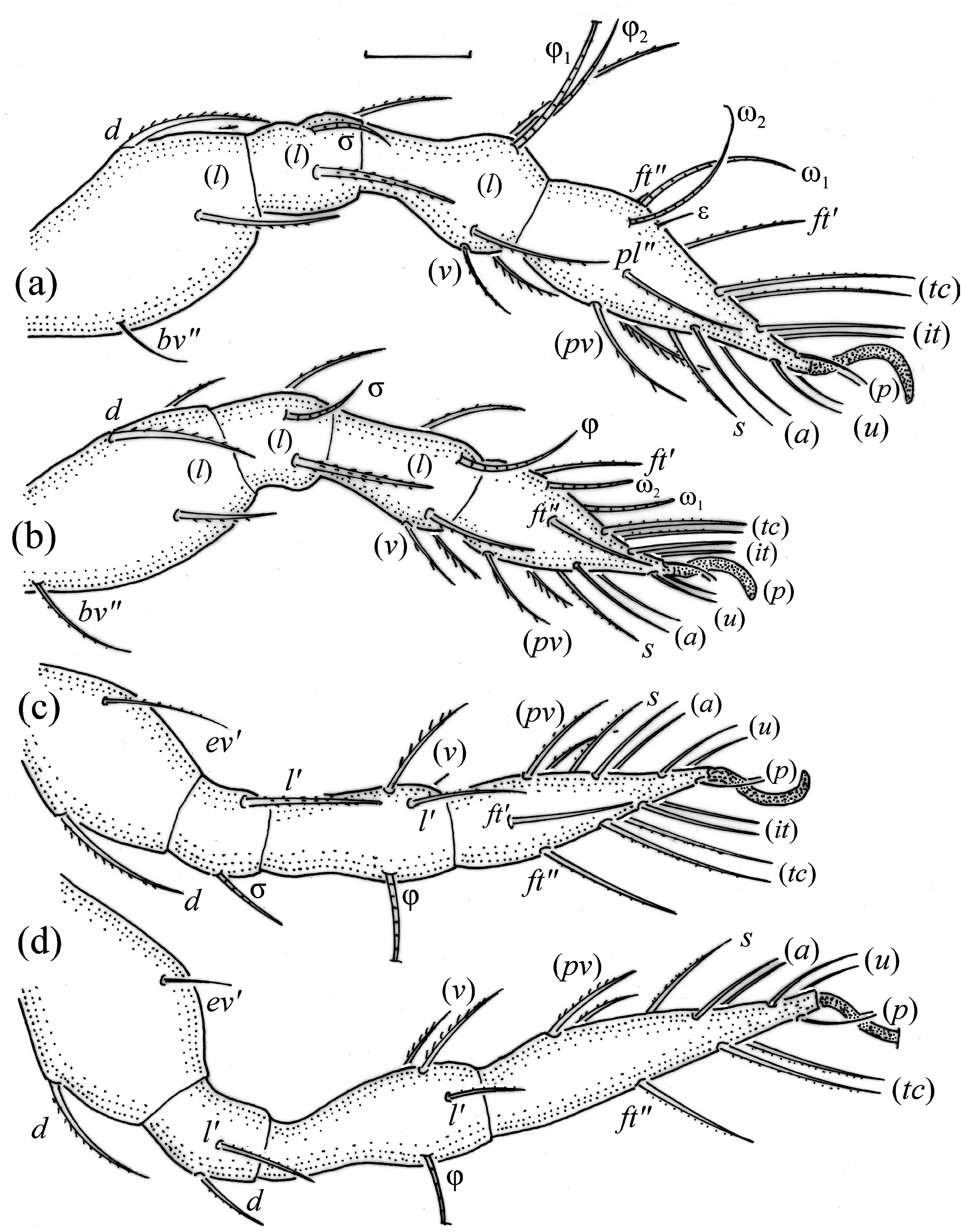
Adult ( Figs. 1–7 View FIGURE 1 View FIGURE 2 View FIGURE 3 View FIGURE 4 View FIGURE 5 View FIGURE 6 View FIGURE 7 ) similar to that described by Berlese (1908) and investigated by Mahunka (1992), but see Remarks. Mean length (and range) of females 620.2±24.6 (553–650, n= 17) and males 552.5±46.6 (455–585, n= 13), mean width (and range) of females 461.8±25.7 (422–488) and males 411.5±22.3 (390–455). Notogastral microsetae 10 pairs, including c 2 on pteromorph, porose area Aa divided in Aa’ and Aa” of similar size, all porose areas small and oval, except for elongated A3 ( Figs. 1a View FIGURE 1 , 3 View FIGURE 3 , 4a View FIGURE 4 , 5a–c View FIGURE 5 , 6a, 6b View FIGURE 6 ). Postanal porose area Ap narrow and elongated. All hypostomal setae short and smooth ( Figs. 2 View FIGURE 2 , 5d View FIGURE 5 ). Chelicera chelate, seta cha longer and thicker than chb, both barbed ( Fig. 4b View FIGURE 4 ). Most palp setae smooth, except for finely barbed sup and inf on femur, and l” on genu and tibia ( Fig. 4c View FIGURE 4 ), formula of palp seta 0-2-1-3-9(1). Leg femora oval in cross section, most leg setae barbed, seta v on paraxial side of tibiae, v’ on tarsus I and pv on all tarsi with longer barbs than those on antiaxial side ( Figs. 5 View FIGURE 5 , 6c, 6d View FIGURE 6 , 7 View FIGURE 7 ). Solenidia ω 1 and ω 2 on tarsus I of similar length, seta ft” short. Formulae of leg setae [trochanter to tarsus (+ solenidia)]: I – 1-4- 2(1)-4(2)-20(2); II – 1-4-2(1)-4(1)-15(2); III – 2-2-1(1)-3(1)-15; IV – 1-2-2-3(1)-12. Leg tarsi heterotridactylous.
Remarks. The mean length and width of females of P. tenuiclava investigated herein are slightly larger than those described by Berlese (1908) – length 590, but smaller than those investigated by Sellnick (1960) – length 660, Pérez-Iñigo (1993) – length 620–690 and Weigmann (2006) – length 560–690 (in all papers sex not investigated). In our specimens, the porose area A3 is shorter than that drawn by Weigmann (2006), and longer than drawn by other authors. The distribution of porose areas and prodorsal setae are similar as in figures drawn by Berlese (1908) and Mahunka (1992). Only Mahunka (1992) showed the distribution of notogastral setae, which is similar to our adults, except for alveolus of seta la that is placed laterally to porose area Aa’ (vs. in our adults posterolateral to porose area Aa’), and dark point anterior to alveolus lm, which in our adults is absent.
Description of juveniles
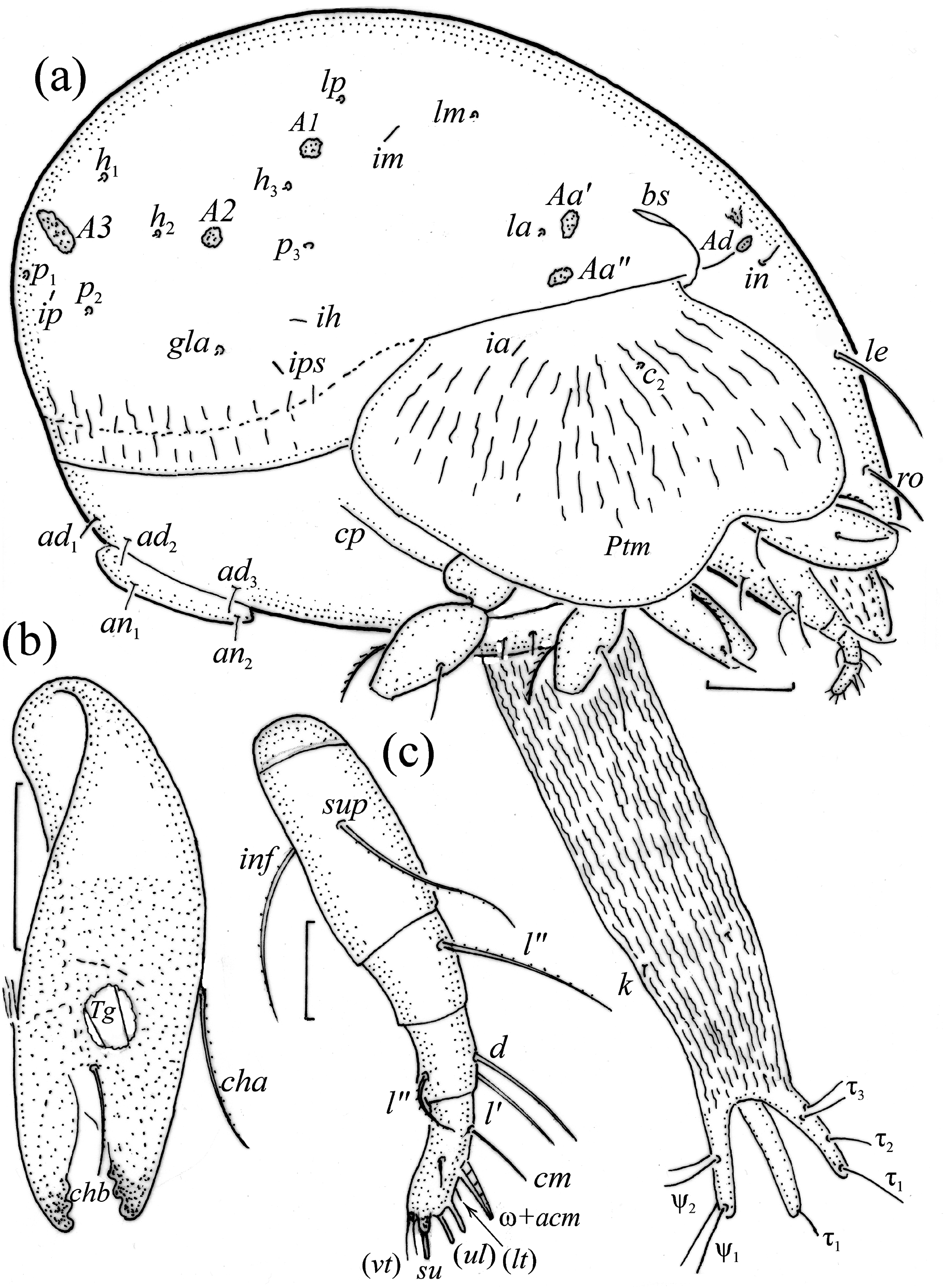
Larva egg-shaped in dorsal view ( Figs. 8 View FIGURE 8 , 9a View FIGURE 9 , 10a View FIGURE 10 , 11a–c View FIGURE 11 ), and unpigmented, except for light brown prodorsal and gastronotal shields and legs. Prodorsum subtriangular, prodorsal setae barbed, in long, ro and le of medium size, and ex shorter ( Table 1 View TABLE 1 ). Mutual distance between setal pair le about 1.5 times longer than between pair ro, and distance between setal pair in about three times longer than between pair ro, pair le inserted closer to pairs in than ro. Opening of bothridium rounded, with anteromedial addition; bothridial seta fusiform, with thick, barbed head ( Figs. 8 View FIGURE 8 , 10a View FIGURE 10 , 11a, 11b, 11d View FIGURE 11 ). Most palp setae smooth ( Figs. 10a View FIGURE 10 , 12a View FIGURE 12 ).
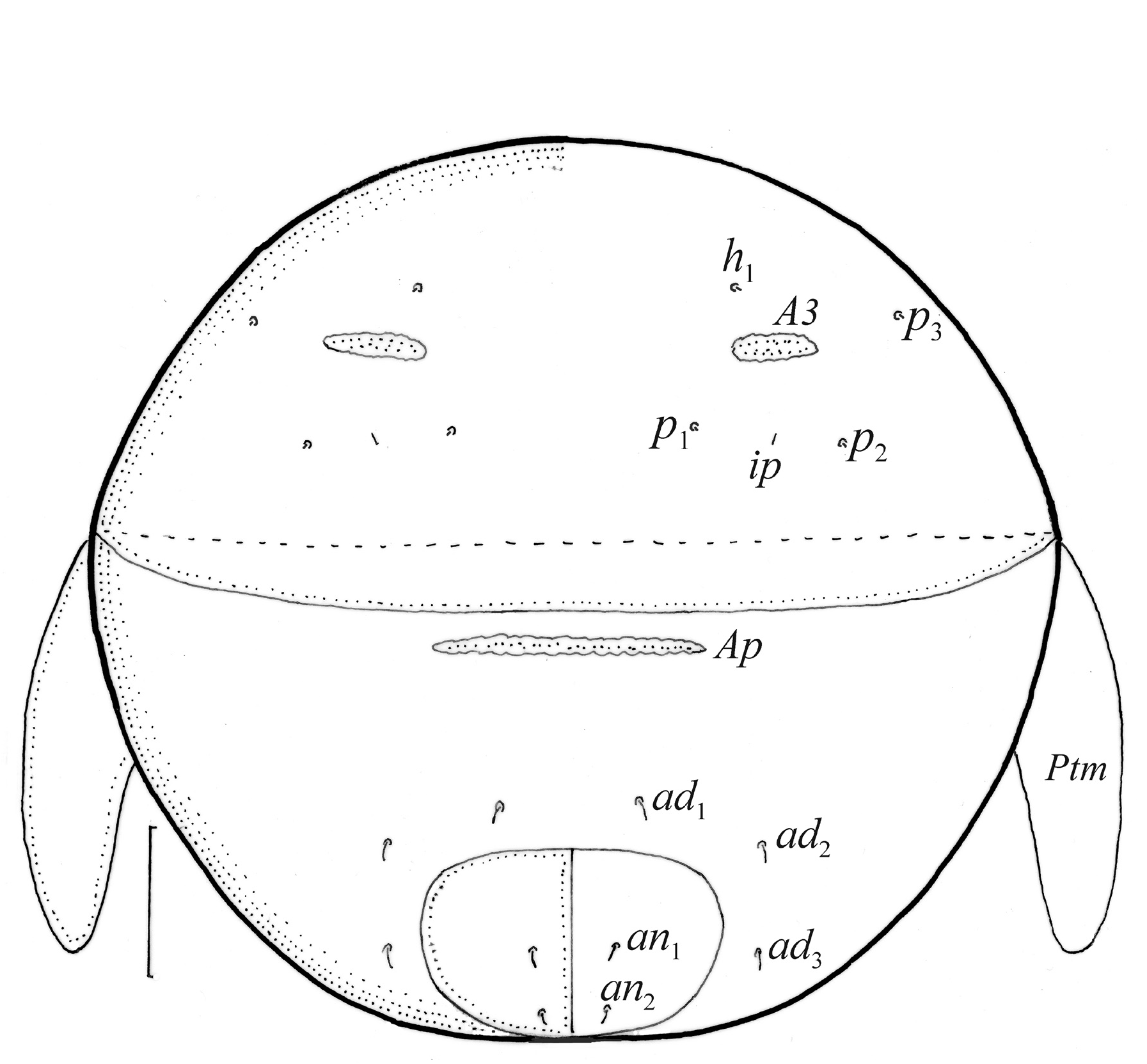
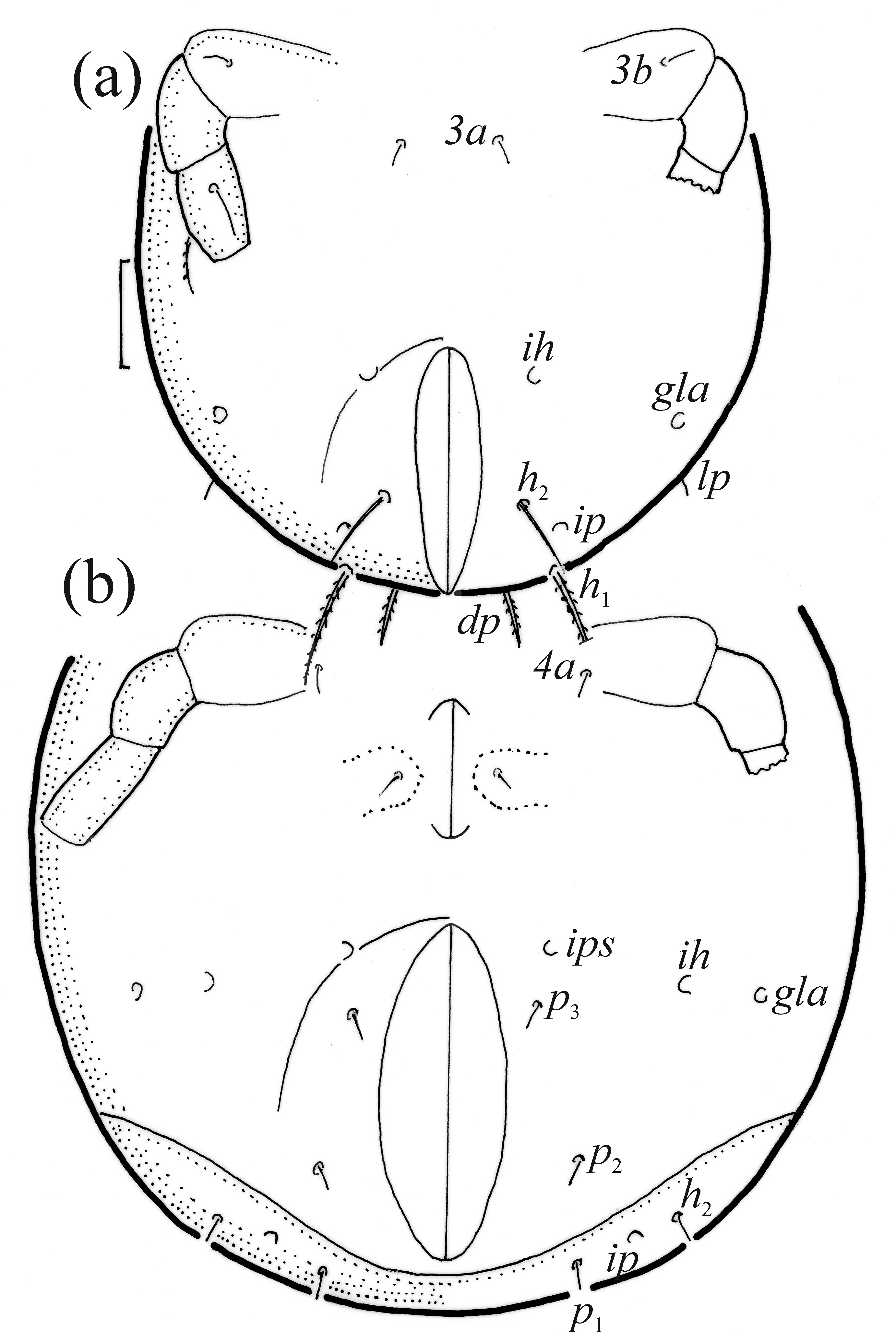
Gastronotum of larva ( Figs. 8 View FIGURE 8 , 9a View FIGURE 9 , 10a View FIGURE 10 , 11a–c View FIGURE 11 ) with 11 pairs of setae, including h 2 inserted lateral to medial part of anal valves; most short and smooth, except for medium-sized c 3, dp and h 1, and slightly shorter c 2 and h 2, most of these setae barbed, but h 2 finely barbed. Setae of c -series inserted on unsclerotized integument, length increasing from c 1 to c 3 ( Table 1 View TABLE 1 ). Gastronotal shield poorly developed, with seven pairs of setae (d -, l -series, h 1), seta h 2 on unsclerotized integument. Small porose areas present, Aa anteromedial to seta la, A1 anterior to seta lm, and A2 anteromedial to seta lp ( Fig. 8 View FIGURE 8 ). Cupule ia posterior to seta c 3, cupule im posterior to seta lm, cupule ip between setae h 1 and h 2, cupule ih lateral to anterior part of anal valves. Opisthonotal gland opening gla lateral to seta lp, without dark sclerotized surrounding ( Figs. 10a View FIGURE 10 , 12b View FIGURE 12 ). Humeral organ located above insertions of leg II ( Figs. 10a View FIGURE 10 , 12c View FIGURE 12 ). Paraproctal valves (segment PS) glabrous. Legs of larva stocky, all femora oval in cross section, most leg setae barbed ( Fig. 11a–c View FIGURE 11 , 13 View FIGURE 13 ).
Shape of prodorsum of protonymph, prodorsal setae, bothridium and bothridial seta as in larva, but bothridial seta slimmer, and prodorsal setae relatively longer than in larva; in deutonymph and tritonymph le nearly as long as in. Gastronotum oval, with 15 pairs of setae because h 3 and p -series added, and retained in subsequent nymphs ( Figs. 9b View FIGURE 9 , 10b View FIGURE 10 , 14a, 14b View FIGURE 14 ); all short and smooth, except for longer and barbed c 3. Setae of c -series inserted on unsclerotized integument. Gastronotal shield poorly developed, with ornamentation, well observed in lateral aspect ( Fig. 10b View FIGURE 10 ), and 10 pairs of setae (d -, l -, h -series, p 1), setae p 2 and p 3 inserted on unsclerotized integument ( Figs. 9b View FIGURE 9 , 14 View FIGURE 14 ). Four pairs of porose areas present, Aa anteromedial to seta la, A1 posteromedial to seta lm, A2 posteromedial to seta lp and A3 anterior to seta h 1. In protonymph, one pair of genital setae inserted lateral to genital valves, two pairs added in deutonymph and in tritonymph each ( Figs. 9b View FIGURE 9 , 14 View FIGURE 14 ), all short and smooth. In deutonymph, one pair of aggenital setae appearing and three pairs of adanal setae, and remained in subsequent instars; all short and smooth. In protonymph and deutonymph, anal valves glabrous, in tritonymph two pairs of short and smooth anal setae present. In tritonymph, cupule ia as in larva, cupule im posterior to seta lp, and ip between setae p 1 and p 2, cupule iad lateral to anterior part of anal valves, cupules ips and ih displaced lateral to iad ( Figs. 10b View FIGURE 10 , 11b View FIGURE 11 , 14 View FIGURE 14 ). Opisthonotal gland opening gla anterolateral to seta h 3 ( Figs. 10b View FIGURE 10 , 12d View FIGURE 12 ), without dark sclerotized surrounding, humeral organ located as in larva. Legs of tritonymph stocky, all femora oval in cross section ( Figs. 12d View FIGURE 12 , 16 View FIGURE 16 ), most leg setae barbed, but some setae on paraxial side of tibia (v’) and tarsi (v’, pv’) with longer barbs than those on antiaxial side (v’’ and v’’, respectively).
Summary of ontogenetic transformations
In all juveniles, the prodorsal setae le and in are longer than ro and ex, whereas in the adult seta ro and le are of medium size, and in and ex are short. The bothridium is rounded in all instars, but in the adult it is covered by anterior tectum of notogaster. In all instars, the bothridial seta is fusiform, with barbed head, which in juveniles has thicker head than in the adult, especially in the larva. The larva has 11 pairs of gastronotal setae, including h 2, the nymphs have 15 pairs (h 3 and p -series appearing in protonymph, and present in other instars), whereas the notogaster of adult loses setae c 1, c 3 and of d -series, such that 10 pairs of microsetae remain. The formula of gastronotal setae in P. tenuiclava is 11-15-15-15-10 (from larva to adult), the formula of epimeral setae are 3-1-2 (larva, including scaliform 1c), 3-1-2-1 (protonymph), 3-1-2-2 (deutonymph and tritonymph), and 1-0-1-1 (adult). The formula of genital setae is 1-3-5-6 (protonymph to adult), and formula of aggenital setae is 1-1-1 (deutonymph to adult), and setal formula of segments PS-AN is 03333-0333-022. The ontogeny of leg setae and solenidia of P. tenuiclava is shown in Table 2 View TABLE 2 .
Distribution, ecology and biology
Pilogalumna tenuiclava has a Holarctic distribution ( Subías 2004, updated 2022), and is hygrophilous, mucicol and tyrphobiotic ( Rajski 1968; Höpperger & Schatz 2013). This species is usually not abundant. For example, in mires of western Norway, P. tenuiclava occurred only in one sample, and only six specimens were found (out of almost 60,000 Oribatida studied). It was present only in Sphagnum riparium Ångstr. collected from poor carpet ( Seniczak et al. 2020a). It was found in feathers of herring gull ( Larus argentatus Pontoppidan ) ( Krivolutsky & Lebedeva 2004), and among fossil mites of the Quaternary Period ( Krivolutsky et al. 1990). It belongs to intermediate hosts of cestode tapeworms of the family Anoplocephalidae ( Denegri 1993) .
Note: structures are indicated where they are first added and are present through the rest of ontogeny; pairs of setae in parentheses, dash indicates no additions.
Based on ten samples of peat mosses collected on 18 June 2010 at the pond Zakręt 1 ( Poland), P. tenuiclava had a mean density of 34 individuals per 500 cm 3 (±SD = 45, range 0‒137 individuals per 500 cm 3), it occurred in 90% of samples and made 11% of oribatid mites. The juveniles were more abundant in this season than adults and made 83% of population ( Nowicka 2014) .
In the studies on the seasonal dynamics in the same pond, P. tenuiclava had similar abundance in four studied seasons (spring, summer, autumn and winter), and the juveniles were present all over the year. However, when we consider the mean value from two successive years, the participation of juvenile stages was the following: spring— 55%, summer—60%, autumn—34%, and winter—22% ( Seniczak et al. 2019a). In 30 randomly selected adults, the sex ratio (females to males) was 1:0.6, and 42% of females were gravid and carried 3–7 large eggs (211 x 132 each), comprising 34% of the length of females.
Bayartogtokh, B. (2010) Oribatid mites of Mongolia (Acari: Oribatida). Russian Academy of Sciences. KMK Scientific Press Ltd., Moscow, 400 pp.
Behan-Pelletier, V. M. & Lindo, Z. (2019) Checklist of oribatid mites (Acari: Oribatida) of Canada and Alaska. Zootaxa Monograph, 4666 (1), 1 - 180. https: // doi. org / 10.11646 / zootaxa. 4666.1.1
Berlese, A. (1908) Elenco di generi e specie nuovi di Acari. Redia, 5, 1 - 15.
Bernini, F., Castagnoli, M. & Nannelli, R. (1995) Arachnida, Acari. In: Minelli A., Rufo S. & La Posta S. (Eds.), Checklist delle specie della fauna italiana, 24. Bologna: Calderini, 131 pp.
Castagnoli, M. & Pegazzano, F. (1985) Catalogue of the Berlese Acaroteca. Istituto Sperimentale per la Zoologia Agraria, Firenze: 490 pp.
Denegri, G. M. (1993) Review of oribatid mites as intermediate hosts of the Anoplocephalidae. Experimental & Applied Acarology, 17 (8), 567 - 580. https: // doi. org / 10.1007 / BF 00053486
Ermilov, S. G. & Klimov, P. B. (2017) Generic revision of the large-winged mite superfamily Galumnoidea (Acari, Oribatida) of the world. Zootaxa Monograph, 4357 (1), 1 - 72. https: // doi. org / 10.11646 / zootaxa. 4357.1.1
Golosova, L. D., Karppinen, E. & Krivolutsky, D. A. (1983) List of oribatid mites (Acarina, Oribatei) of northern Palaearctic region. II. Siberia and the Far East. Acta Entomologica Fennica, 43 (1), 1 - 14.
Grandjean, F. (1956) Observations sur les Galumnidae. 1 re serie. (Acariens, Oribates). Revue francaise d'entomologie, 23 (1), 137 - 146.
Hammen, L. van der (1952) The Oribatei (Acari) of the Netherlands. Zoologische Verhandelingen, Leiden, 17 (1), 1 - 139.
Hopperger, M. & Schatz, H. (2013) Hornmilben (Acari, Oribatida) von Castelfeder (Sudtirol, Italien). Gredleriana, 13, 71 - 98.
Karppinen, E. & Krivolutsky, D. A. (1982) List of oribatid mites (Acarina, Oribatei) of northern Palaearctic region. I. Europe. Acta Entomologica Fennica, 41 (1), 1 - 18.
Karppinen, E., Krivolutsky, D. A. & Poltavskaja, M. P. (1986) List of oribatid mites (Acarina, Oribatei) of northern Palaearctic region. III. Arid lands. Annales Entomologici Fennici, 52 (3), 81 - 94.
Karppinen, E., Krivolutsky, D. A., Tarba, Z. M., Shtanchaeva, U. Ya. & Gordeeva, E. W. (1987) List of oribatid mites (Acarina, Oribatei) of northern Palaearctic region. IV. Caucasus and Crimea. Annales Entomologici Fennici, 53 (1), 119 - 137.
Krivolutsky, D. A., Druk, A. Ja., Eitminaviciute, I. S., Laskova, L. M. & Karppinen, E. (1990) Fossil oribatid mites. Mokslas Publ., Vilnius: 109 pp. [In Russian]
Krivolutsky, D. A. & Lebedeva, N. V. (2004) Oribatid mites (Oribatei, Acariformes) in bird feathers: non-passerines. Acta Zoologica Lithuanica, 14 (1), 26 - 47. https: // doi. org / 10.1080 / 13921657.2004.10512570
Mahunka, S. (1992) Pelops and Oribates species in the Berlese collection (Acari). Acta Zoologica Hungarica, 38 (3 - 4), 213 - 260.
Mahunka, S. & Mahunka-Papp, L. (2000) Checklist of the oribatid mites of Hungary (Acari: Oribatida). Folia Entomologica Hungarica, 61 (1), 27 - 53.
Marshall, V. G., Reeves, R. M. & Norton, R. A. (1987) Catalogue of the Oribatida (Acari) of Continental United States and Canada. Memoirs of the Entomological Society of Canada, 139 (1), 1 - 418. https: // doi. org / 10.4039 / entm 119139 fv
Miko, L. (2016) Oribatid mites (Acarina, Oribatida) of the Czech Republic. Revised check-list with a proposal for Czech oribatid nomenclature. Klapalekian a, 52 (Suppl.), 1 - 302.
Murvanidze, M. & Mumladze, L. (2016) Annotated checklist of Georgian oribatid mites. Zootaxa, 4089 (1), 1 - 81. https: // doi. org / 10.11646 / zootaxa. 4089.1.1
Niemi, R., Karppinen, E. & Uusitalo, M. (1997) Catalogue of the Oribatida (Acari) of Finland. Acta Zoologica Fennica, 207 (1), 1 - 39.
Norton, R. A. & Ermilov, S. G. (2014) Catalogue and historical overview of juvenile instars of oribatid mites (Acari: Oribatida). Zootaxa, 3833 (1), 1 - 132. https: // doi. org / 10.11646 / zootaxa. 3833.1.1
Nowicka, A. (2014) Roztocze (Acari) strefy brzegowej wybranych jezior srodlesnych Warmii i Mazur ze szczegolnym uwzglednieniem mechowcow (Oribatida). Dissertation, Forest Research Institute in Sekocin Stary.
Olszanowski, Z., Rajski, A. & Niedbala, W. (1996) Roztocze Acari - Mechowce Oribatida. Katalog Fauny Polski - Catalogus faunae poloniae, Poznan, Poland, 34 (9), 1 - 243.
Oudemans, A. C. (1919) Notizen uber Acari, 26. Reihe (Oribatoidea) Gruppe der Galumnae. Archiv fur Naturgeschichte (A), 83 (4) (1917), 1 - 84.
Perez-Inigo, C. (1993) Acari, Oribatei, Poronota. In: Ramos Sanchez, M. A. et al. (Eds.), Fauna Iberica. Museo de Ciencias Naturales, Madrid, vol. 3, 1 - 320.
Rajski, A. (1968) Autecological-zoogeographical analysis of moss mites (Acari, Oribatei) on the basis of fauna in the Poznan Environs. Part II. Fragmenta Faunistica, 14 (12), 277 - 405. https: // doi. org / 10.3161 / 00159301 FF 1968.14.12.277
Ryabinin, N. A. & Pankov, A. N. (2002) Catalogue of oribatid mites of the Far East of Russia. Part II. Continental part of the Far East. DVO, Vladivostok, Khabarovsk: 92 pp. [In Russian]
Schatz. H. (2020) Catalogue of oribatid mites (Acari: Oribatida) from Vorarlberg (Austria). Zootaxa, 4783 (1), 1 - 106. https: // doi. org / 10.11646 / zootaxa. 4783.1.1
Schatz, H. (1983) U. - Ordn.: Oribatei, Hornmilben. Catalogus Faunae Austriae, Wien, Teil IXi, 1 - 118.
Sellnick, M. (1960) Formenkreis: Hornmilben, Oribatei. Nachtrag. In: Brohmer, P. Ehrmann, P. & Ulmer, G. (Eds.), Die Tierwelt Mitteleuropa. Leipzig, 3 (4), Erg., 45 - 134.
Seniczak, A., Seniczak, S., Graczyk, R., Waldon-Rudzionek, B., Nowicka, A. & Pacek, S. (2019 a) Seasonal dynamics of oribatid mites (Acari, Oribatida) in a bog in Poland. Wetlands, 39 (6), 853 - 864. https: // doi. org / 10.1007 / s 13157 - 019 - 01125 - 2
Seniczak, A., Seniczak, S., Iturrondobeitia, J. C., Solhoy, T. & Flatberg, K. I. (2020 a) Diverse Sphagnum mosses support rich moss mite communities (Acari, Oribatida) in mires of western Norway, Wetlands, 40, 1339 - 1351. https: // doi. org / 10.1007 / s 13157 - 019 - 01236 - w
Shaldybina, E. S. (1975) Family Galumnidae Jacot, 1925. In: Ghilarov, M. S. (Ed.), Key to soil-inhabiting mites - Sarcoptiformes. Moscow, Nauka Publisher, pp. 347 - 363. [in Russian]
Siepel, H., Zaitsev, A. & Berg, M. (2009) Checklist of the oribatid mites of the Netherlands (Acari, Oribatida). Nederlandse Faun. Mededelingen, 30, 83 - 111.
Subias, L. S. (2004, electronic updated in 2022) Listado sistematico, sinonimico y biogeografico de los Acaros Oribatidos (Acariformes, Oribatida) del mundo (1758 - 2002). Graellsia, 60 (numero extraordinario), 3 - 305. 15 ª actualizacion, 527 pp. (accessed on 01 April 2022) https: // doi. org / 10.3989 / graellsia. 2004. v 60. iextra. 218
Weigmann G. (2006) Hornmilben (Oribatida). In: Dahl, F., series founder, Die Tierwelt Deutschlands part 76. Keltern, Goecke & Evers, pp. 1 - 520.
Willmann, C. (1931) Moosmilben oder Oribatiden (Cryptostigmata). In: Dahl, F. (Ed.), Die Tierwelt Deutschlands. Gustav Fischer, Jena, Bd. 22 (5), 79 - 200.
FIGURE 1. Pilogalumna tenuiclava, female. (a) Dorsal aspect, I pair of legs partially drawn, scale bar 50 μm; (b) shape of porose are A1 (enlarged).
FIGURE 4. Pilogalumna tenuiclava. (a) Female with ejected ovipositor, lateral aspect, legs partially drawn; scale bar 50 μm; mouthparts, right side, scale bars 20 μm; (b) chelicera, Trägårdh organ in ‘transparent’ area), (c) palp.
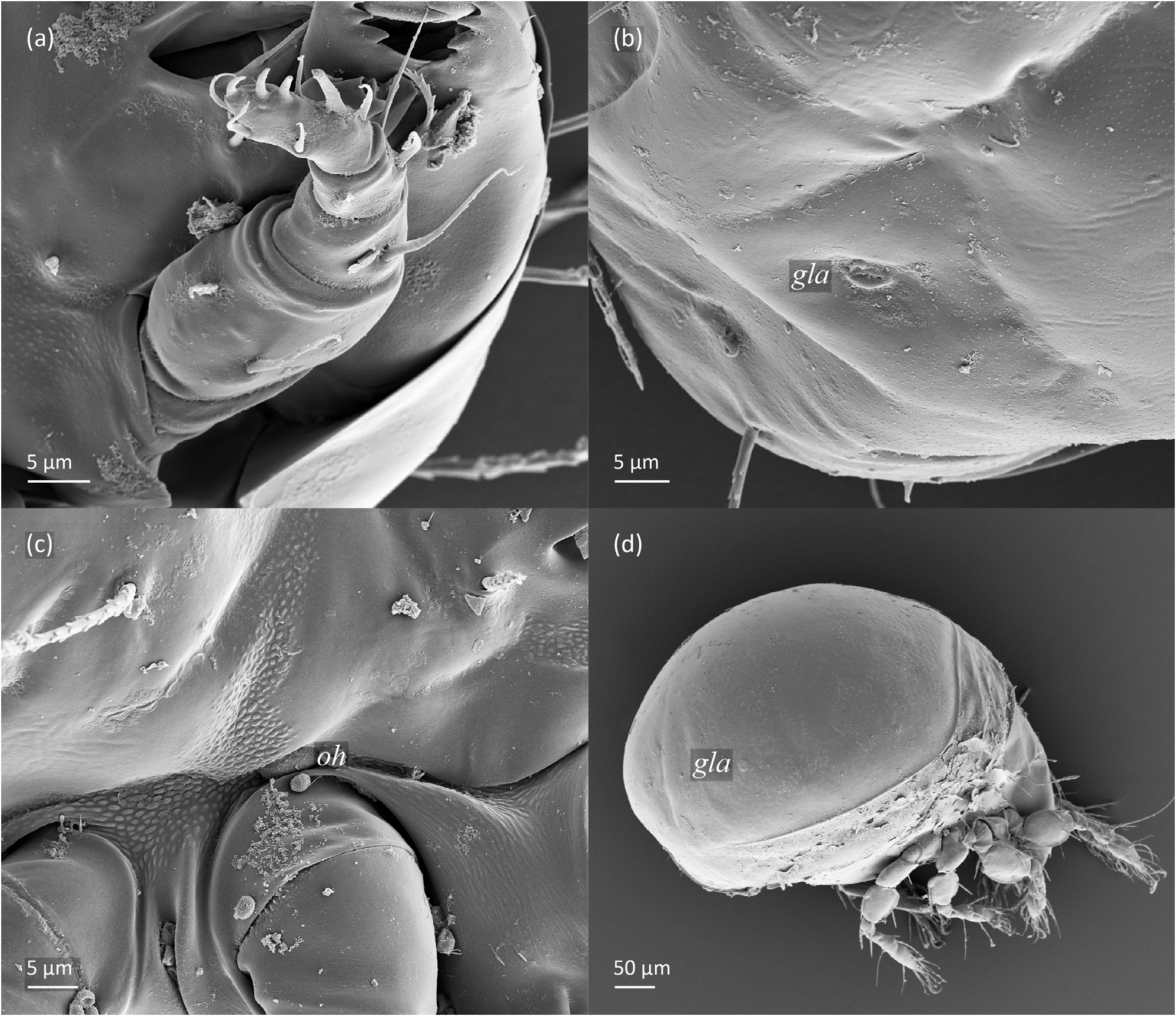
FIGURE 5. Pilogalumna tenuiclava, adult, SEM micrographs. (a) Dorsolateral view, (b) frontal view, (c) lateral view, (d) ventral view.
FIGURE 6. Pilogalumna tenuiclava, adult, SEM micrographs. Dorsolateral view, (a) anterior part, (b) bothridial seta, (c) leg II, (d) leg III.
FIGURE 7. Pilogalumna tenuiclava, leg segments of adult (part of femur to tarsus), right side, antiaxial aspect, setae on the opposite side not illustrated are indicated in the legend, scale bar 20 μm. (a) Leg I; (b) leg II; (c) leg III, tibia (v’’), tarsus (pv’’); (d) leg IV; (e) part of tibia and tarsus I, ventral aspect.
FIGURE 8. Pilogalumna tenuiclava, larva. (a) Dorsal aspect, legs I and II partially drawn, scale bar 20 μm; (b) shape of seta c3 (enlarged).
FIGURE 9. Pilogalumna tenuiclava, ventral part of hysterosoma, legs III and IV partially drawn, scale bar 20 μm. (a) Larva, (b) protonymph.
FIGURE 10. Pilogalumna tenuiclava, lateral aspect, legs partially drawn, scale bars 50 μm. (a) Larva, (b) tritonymph.
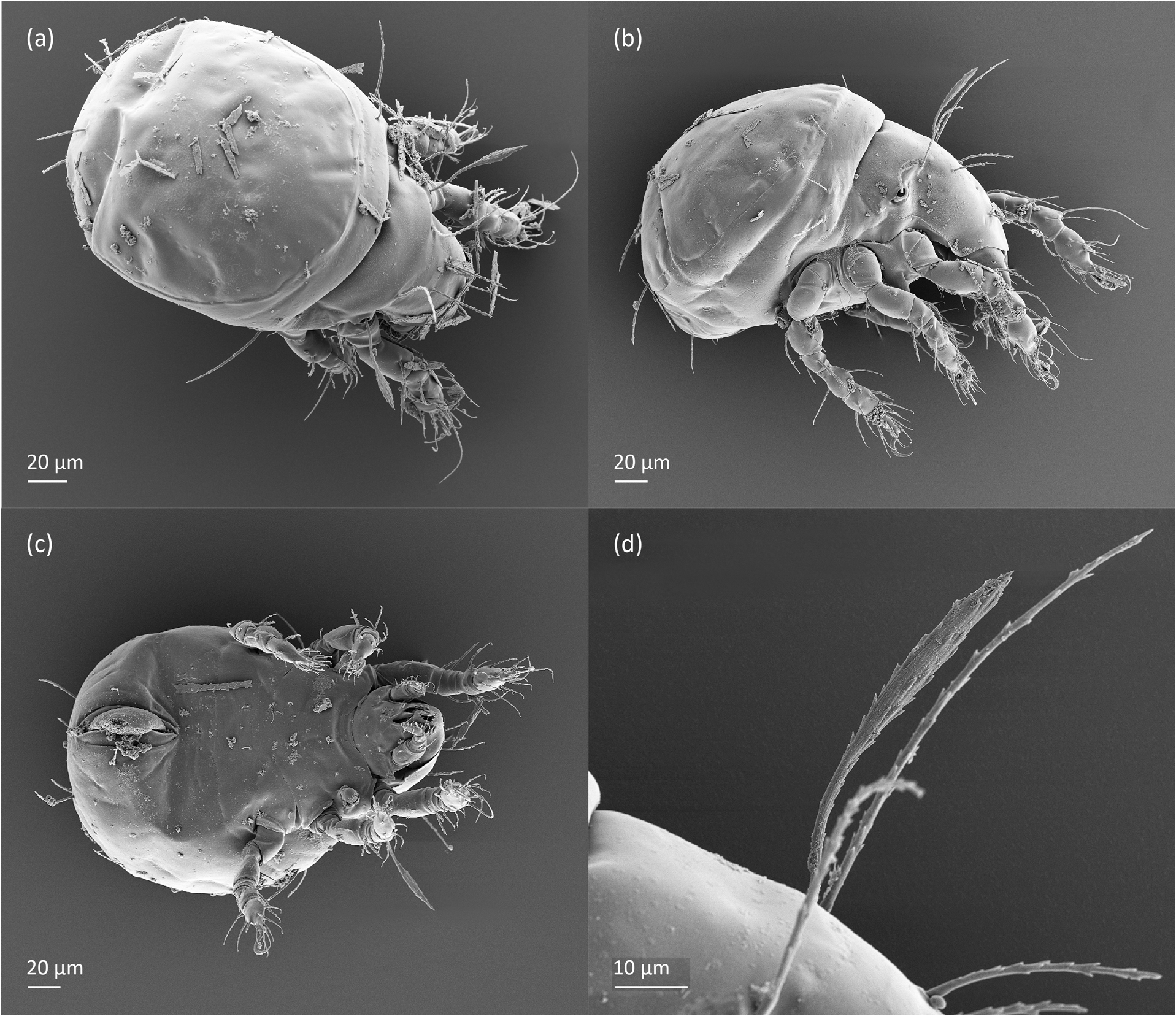
FIGURE 11. Pilogalumna tenuiclava, larva, SEM micrographs. (a) Dorsal view, (b) lateral view, (c) ventral view, (d) bothridial seta.
FIGURE 12. Pilogalumna tenuiclava, SEM micrographs. Larva, (a) palp, ventral view; lateral view, (b) gla opening, (c) humeral organ, (d) tritonymph.
FIGURE 13. Pilogalumna tenuiclava, leg segments of larva (part of femur to tarsus), right side, antiaxial aspect, seta on the opposite side not illustrated is indicated in the legend, scale bar 10 μm. (a) Leg I, tarsus (pl’); (b) leg II, (c) leg III.
FIGURE 14. Pilogalumna tenuiclava, ventral part of hysterosoma, IV pair of legs partially drawn, scale bar 50 μm. (a) Deu- tonymph, (b) tritonymph.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
SubOrder |
Oribatida |
|
Family |
|
|
Genus |
Pilogalumna tenuiclava ( Berlese, 1908 )
| Seniczak, Anna & Seniczak, Stanisław 2022 |
Allogalumna atra Mihelčič, 1957
| Mihelcic 1957 |
Galumna radiata
| Sellnick 1928 |
Galumna areolata
| Willmann 1923 |
Galumna tenuiclavus:
| Oudemans 1919 |
Oribates tenuiclavus
| Berlese 1908 |