Mormoops, Leach, 1821
|
publication ID |
https://doi.org/10.1111/j.1095-8312.2006.00605.x |
|
DOI |
https://doi.org/10.5281/zenodo.7845961 |
|
persistent identifier |
https://treatment.plazi.org/id/6C5E879A-384C-FFA9-D967-FF4AFC68FDE3 |
|
treatment provided by |
Felipe (2023-04-19 12:17:54, last updated 2024-11-29 17:09:05) |
|
scientific name |
Mormoops |
| status |
|
Mormoops View in CoL View at ENA and the Mormoopidae
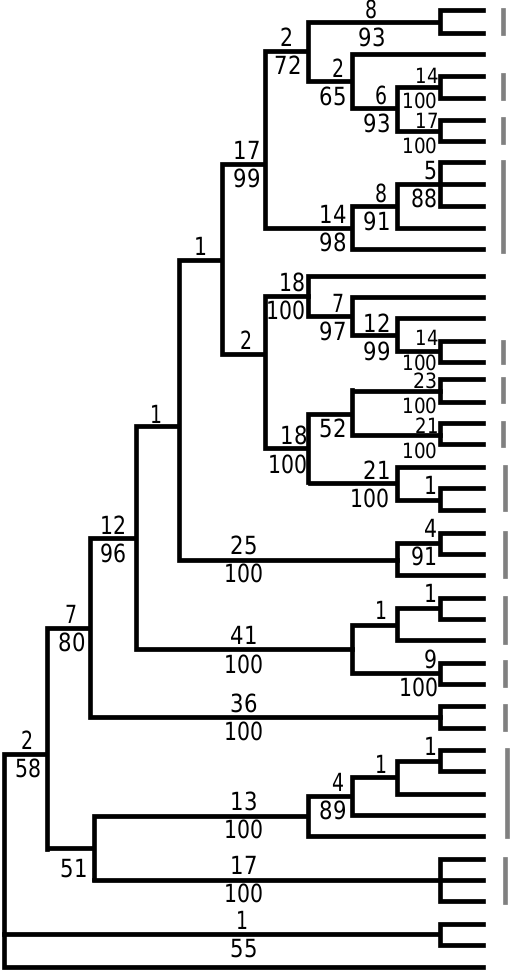
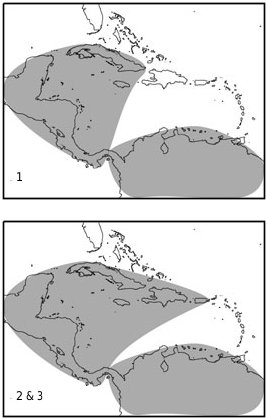
The estimated ancestral area of Mormoops and the mormoopids ( Fig. 5 View Figure 5 ) encompasses both northern South America ( Smith, 1972) and the Greater Antilles (Czaplewski & Morgan, 2003). The two biogeographical hypotheses are not mutually exclusive, and it is plausible that the most recent common ancestor of mormoopids was widespread from Mexico south to northern South America, and east to the Greater Antilles. Another interpretation of this result is that dispersal-vicariance analysis is inconclusive, and other sources of evidence are needed to clarify the geographical history of mormoopids.
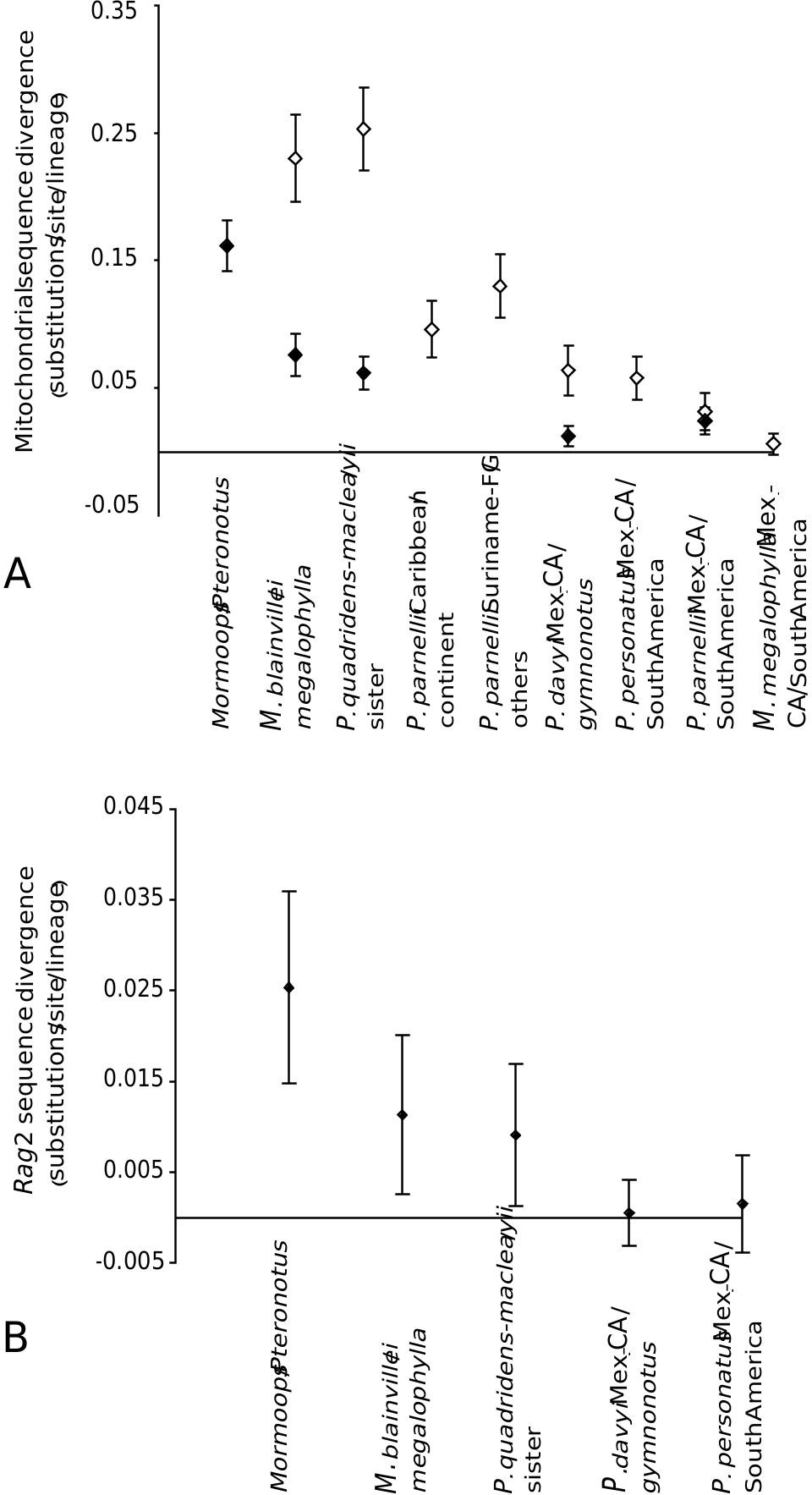
There are several reasons to doubt that the ancestor of Mormoops was as widespread as estimated in Figure 5 View Figure 5 . First, extant Mormoops species do not overlap on the continent, but both are known from the Greater Antilles (albeit, one only as fossil). Second, one additional species, Mormoops magna , is known from late Pleistocene remains on Cuba ( Silva-Taboada, 1974), adding a third Mormoops lineage to the Greater Antilles. Third, it is parsimonious to postulate that the ancestor of Mormoops reached the Greater Antilles before splitting into the extant species but, even if it did not, the divergence between the Antillean blainvillei and its sister taxon is significantly greater than that between megalophylla populations ( Fig. 6A View Figure 6 ). The combination of species diversity and depth of divergence suggests Mormoops expanded its range from north to south.
If Mormoops ranged into the Greater Antilles even before blainvillei and megalophylla differentiated, Caribbean colonization in this family can be traced back to the divergence between the mormoopid genera, and might be as ancient as the Oligocene or Miocene (Czaplewski & Morgan, 2003). A northern neotropical (and perhaps insular) origin for the genus can be overturned by the discovery of a basal Mormoops species in South America. An extensive fossil record shows that M. megalophylla ranged from Florida through the Greater Antilles to Bahia in Brazil during the Late Pleistocene ( Ray, Olsen & Gut, 1963; Silva-Taboada, 1974; Czaplewski & Cartelle, 1998). Studies of morphological variation are necessary to determine the relationships among extant and fossil megalophylla populations and test the hypothesis presented here because more than one species might be involved ( Morgan, 2001).
One prediction following Czaplewski & Morgan’s (2003) biogeographical model is borne by the molecular data: divergences between Antillean and continental mormoopids are greater than those between Central American and northern South American populations ( Fig. 6 View Figure 6 ). There is only one exception in the P. parnellii lineage (subgenus Phyllodia ), where two northern South American populations might not share a most recent common ancestor ( Figs 3 View Figure 3 , 5 View Figure 5 ). For every other mormoopid lineage, and even in one instance within Phyllodia , the divergence between Mexico /Central America and South America appears to be recent ( Fig. 6 View Figure 6 ), and might correspond to the completion of the Isthmus of Panama in the late Pliocene. Either Mexico /Central America or north-western South America was recently colonized by all mormoopid lineages. As discussed above, the direction of this expansion appears to be from north to south in Mormoops and P. personatus s.l., but the evidence is ambiguous for Phyllodia , as well as for P. davyi and gymnonotus .
Because Mormoops is at the base of the mormoopid radiation, restricting its ancestral distribution to the northern Neotropics constrains the geographical origin of the family to that region. Other than differences in branch length (longer for northern neotropical splits, shorter for divergences between Mexico /Central America and South America), the fossil record also supports a north-to-south expansion. The oldest mormoopid diverged before the two extant genera (G. Morgan, pers. comm.), and ranged into Florida in the Oligocene ( Czaplewski, Morgan & Naeher, 2003). In general, mormoopids appear to have reached South America late in their history, after diversifying in Mexico, Central America, and/or the Greater Antilles ( Fig. 6 View Figure 6 ).
This finding is critical to the biogeographical history of noctilionoids. Both morphology ( Simmons & Conway, 2001) and large concatenated molecular datasets ( Teeling et al., 2005) indicate that mormoopids and phyllostomids are each other’s closest relative (this topology was not always recovered in this study, probably because taxon sampling among bat families was poor relative to the higher-level analyses cited above). Two phylogenetic hypotheses have been proposed to explain relationships among phyllostomids. One, based on analyses of mostly morphological data ( Wetterer, Rockman & Simmons, 2000) identified the vampires ( Desmodus , Diaemus , and Diphylla ) as the oldest phyllostomid lineage. A second hypothesis based on mtrDNA and Rag 2 (Baker, Porter, Hoofer & Van Den Bussche, 2003) suggests that Macrotus diverged before any other phyllostomid.
The geographical distribution of the basal lineage of the phyllostomids would have a disproportionate effect on ancestral area reconstructions for that family. Vampires range from Mexico to Chile and Uruguay, and fossils have been found on Cuba ( Koopman, 1994). This lineage would not constrain the ancestral area of the phyllostomids because of its widespread distribution. Since the greatest diversity of phyllostomids is concentrated in northern South America and the vampires include it in their range, this would likely be the most parsimonious ancestral area for the family. By contrast, Macrotus is only known from the southwestern United States south to Guatemala, through the Greater Antilles and Bahamas ( Koopman, 1994). If Macrotus is at the base of the phyllostomid radiation, then the ancestral distributions of mormoopids and phyllostomids were adjacent in the northernmost Neotropics. Phyllostomid fossils are known from the middle Miocene of La Venta ( Czaplewski, 1997), indicating phyllostomids reached South America early in their history. The geographical distribution of these closely related families during their early history might help explain the remarkable differences in taxonomic and adaptive diversity between the two groups.
Czaplewski NJ. 1997. Chiroptera. In: Kay RF, Madden RH, Cifelli RL, Flynn JJ, eds. Vertebrate paleontology in the neotropics: the Miocene fauna of la Venta, Colombia. Washington, DC: Smithsonian Institution Press, 410 - 431.
Czaplewski NJ, Cartelle C. 1998. Pleistocene bats from cave deposits in Bahia, Brazil. Journal of Mammalogy 79: 784 - 803.
Czaplewski NJ, Morgan GS, Naeher T. 2003. Molossid bats from the late Tertiary of Florida with a review of the Tertiary Molossidae of North America. Acta Chiropterologica 5: 61 - 74.
Koopman K. 1994. Chiroptera: systematics. Handbuch der Zoologie 8: 1 - 217.
Morgan G. 2001. Patterns of extinction in West Indian bats. In: Woods CA, Sergile FE, eds. Biogeography of the West Indies. Boca Raton, FL: CRC Press, 369 - 407.
Ray CE, Olsen SJ, Gut HJ. 1963. Three mammals new to the Pleistocene fauna of Florida, and a reconsideration of five earlier records. Journal of Mammalogy 44: 373 - 395.
Silva-Taboada G. 1974. Fossil Chiroptera from cave deposits in central Cuba, with description of two new species (Genera Pteronotus and Mormoops) and the first West Indian record of Mormoops megalophylla. Acta Zoologica Cracoviensia 19: 33 - 73.
Simmons NB, Conway TM. 2001. Phylogenetic relationships of mormoopid bats (Chiroptera: Mormoopidae) based on morphological data. Bulletin of the American Museum of Natural History 258: 1 - 97.
Smith JD. 1972. Systematics of the Chiropteran family Mormoopidae. University of Kansas Museum of Natural History Miscellaneous Publication 56: 1 - 132.
Teeling EC, Springer MS, Madsen O, Bates P, O'Brien SJ, Murphy WJ. 2005. A molecular phylogeny for bats illuminates biogeography and the fossil record. Science 307: 580 - 584.
Wetterer AL, Rockman MV, Simmons NB. 2000. Phylogeny of phyllostomid bats (Mammalia: Chiroptera): data from diverse morphological systems, sex chromosomes, and restriction sites. Bulletin of the American Museum of Natural History 248: 1 - 200.
Figure 3. A, strict consensus of eight most parsimonious cladograms resulting from analysis of cytochrome b (L = 1792 steps, consistency index = 0.439, retention index = 0.775). Numbers below branches are Bremer support values, above branches are percent of 1000 jackknife replicates. Names of outgroups are in bold; for sequence data, see Appendix. B, phylogram resulting from maximum likelihood analysis using a rate-constant GTR+I+Γ model of DNA evolution (–lnL = 9181.23). Numbers above or below branches are percent of 300 50% jackknife replicates, thicker lines indicate 100% jackknife support.
Figure 5. Majority rule (50%) consensus of 19 000 cladograms resulting from Bayesian analysis of concatenated molecular data for all diagnosable mormoopid taxa (– lnL = 24 910; 95% confidence interval = 24,890–24 920). Dashed branches had posterior probabilities between 0.50 and 0.95. All other branches had posterior probabilities between 0.95 and 1. Names of outgroups are in bold; for sequence data, see Appendix. The top panel shows the ancestral area inferred for branch 1, the bottom panel shows the ancestral area of branches 2 and 3. DIVA Optimizations were constrained to a maximum of two areas, and all solutions are shown. Three alternatives to the polytomy of Pteronotus davyi and Pteronotus gymnonotus, two alternatives to the sister of Pteronotus quadridens and macleayii (davyi and gymnonotus, or personatus), and two taxonomies (the traditional species taxonomy of Smith (1972), or that shown in Figure 3 were analysed, and all result in the same composite estimates. Geographic distributions are as shown in Table 1. Pteronotus pristinus and Mormoops magna were not analysed. FG, French Guiana; Hon., Honduras; Mex., Mexico; PR, Puerto Rico; Ven., Venezuela.
Figure 6. Confidence intervals around observed sequence divergence resulting from parametric bootstrapping of rate-constant mormoopid phylogenies. A, estimates of divergence for mitochondrial ribosomal DNA (black diamonds) and the cytochrome b gene (white diamonds). B, estimates of divergence for nuclear Rag2. CA, Central America; FG, French Guiana; Mex., Mexico.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |