Bythotrephes brevimanus Lilljeborg, 1901
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4379.3.2 |
|
publication LSID |
lsid:zoobank.org:pub:EEF541E1-F91C-411E-9B65-2C79ED2A2706 |
|
DOI |
https://doi.org/10.5281/zenodo.5962976 |
|
persistent identifier |
https://treatment.plazi.org/id/60573E65-D179-FF80-6EC2-6500D6610039 |
|
treatment provided by |
Plazi |
|
scientific name |
Bythotrephes brevimanus Lilljeborg, 1901 |
| status |
|
Bythotrephes brevimanus Lilljeborg, 1901 View in CoL
( Figs. 1‒7 View FIGURE 1 View FIGURE 2 View FIGURE3 View FIGURE 4 View FIGURE 5 View FIGURE 6 View FIGURE 7 )
Bythotrephes longimanus Leydig, 1860 : Lilljeborg, 1861: 268, Taf. VIII, Fig. 23‒29.
Bythotrephes cederströmii Schödler : P.E. Müller, 1868: 203 ‒204, Tab. IV, Fig. 29; Tab. V, Fig. 1 View FIGURE 1 ‒18; Tab. VI, Fig. 7 View FIGURE 7 .
Bythotrephes longimanus var. brevimanus Lilljeborg, 1901 View in CoL : Lilljeborg, 1901: 614 ‒617, Tab. 82, fig. 1‒10.
Bythotrephes longimanus balticus Ischreyt, 1930 View in CoL : Ischreyt, 1930: 253‒265, 267‒303, Fig. 2‒4 View FIGURE 2 View FIGURE3 View FIGURE 4 , 6 View FIGURE 6 c-f, 7, 13e-m, 17; Flössner, 1972: 406‒408, Abb. 189A‒E, 190B; 2000: 376, Abb. 136A‒C, E‒G.
Bythotrephes balticus Ischreyt, 1934 : Ischreyt, 1934a: 260‒266, Abb. 16a, 17; 1936: 361‒362, 367‒369 ( B. balticus livonicus , B. balticus borussicus , B. balticus danicus , B. balticus suecicus ); 1937: 60‒63; 1939: 127.
Type material. Syntypes. All speciemens certainly used by Lilljeborg (1901) for investigation and description of B. brevimanus and labeled “ B. longimanus ”, “ B. longimanus var.”, “ B. longimanus brevimanus ”, “ B. brevimanus s.str. ” can be considered as belonging to the type series of the taxon ( ICZN, 72.4.1., 72.5) and named syntypes ( ICZN, 73.2).
Lectotype. A parthenogenetic female labeled (Ecoln, Uppland, Sweden, 4.8.1872, collected by Lilljeborg, sample N 2630) and deposited in a tube with 95% ethanol in the Museum of Evolution of Uppsala University (UPSZTY 2832).
Paralectotypes are deposited in the same Museum (see their numbers below).
Material examined. Sweden: 1) sample (MNB, N 15195 View Materials ) from the unknown locality labeled “ Bythotrephes longimanus Leyd. , Schweden, Cederström”, some ad; Norrbotten: 2) sample (MEUU, old museum number 634a with specimens from Lilljeborg catalogue N 2629) labeled “ Bythotrephes longimanus brevimanus, Sw, Nb, Övertorneå, Onkijärvi , VI 1875, Lill.”, 18 ad, 2 juv. (paralectotypes, UPSZTY 2831); 3) sample (MNB, bottle N 13753 View Materials ) in a tube labeled “ No. 2, Norbothen, VII 186….”, exuviae of some specimens; Jämtland: 4) sample (MEUU, Lilljeborg catalogue N 2649) labeled “ B. longimanus var., Sw, Jmt, Storsjön, 8.08.1889, coll. Lillj.”, many ad and males (paralectotypes, UPSZTY 167720); 5) a slide (MEUU, Lilljeborg catalogue N P2327) labeled “ Bythotrephes longimanus, Sw, Jmt, Storsjön , 10.8.1889, Lillj.”, 1 ad, dissected (paralectotype, UPSZTY 167721); 6) sample (MEUU, Lilljeborg catalogue N 2660) labeled “ B. brevimanus s.str., Sw., Jmt., Bräcke Sörsjön, 19.08.1889, coll. Lilljeb.”, 5 ad, 3 males (paralectotypes, UPSZTY 167722); 7) a slide (MEUU, Lilljeborg catalogue N P2331) labeled “ Bythotrephes longimanus, Sw, Jmt, Storsjön , 10.8.1889, Lillj.”, 1 ad, 1 male, 1 juv. (paralectotypes, UPSZTY 167723); 8) sample (MEUU, Lilljeborg catalogue N 2649) labeled “ B. longimanus var., Sw, Jmt, Storsjön, 8.8.1889, Lillj.”, many ad and males (paralectotypes, UPSZTY 167724); Uppland: 9) sample (MEUU, old museum number 634b with specimens from Lilljeborg catalogue N 2630) labeled “ B. longimanus, Sw, Upl, Ekoln , 4.8.1872, Lillj.”, 39 ad, 21 juv. (paralectotypes, UPSZTY 2832, in same vial as a lectotype which was selected from this sample—see above); 10) sample (MEUU, old museum number 634c with specimens from Lilljeborg catalogue N 2637) labeled “ B. longimanus, Sw, Upl, Ekoln , 20.8.1860, Lillj.”, 6 ad, 2 juv. males (paralectotypes, UPSZTY 2833); 11) a slide (MEUU, old museum number 634d with specimens from Lilljeborg catalogue N 2322) labeled “ Bythotrephes longimanus, Sw, Upl, Ekoln , ♀, 4/8 72, Lillj.” (in Museum catalog this material is designated as “ B. longimanus brevimanus , syntypes”), 3 ad, dissected (paralectotypes, UPSZTY 2832); 12) a slide (MEUU, old museum number 634e with specimens from Lilljeborg catalogue N 2322) labeled “ Bythotrephes longimanus L, Sw, Upl, Ekoln, 4/8 72, Lillj.”, 2 ad, dissected (paralectotypes, UPSZTY 2835); 13) a slide (MEUU, old museum number 634f with specimens from Lilljeborg catalogue N P2324) labeled “ Bythotrephes longimanus, Sw, Upl, Ekoln , ♂, 20/8 60, Lillj.”, 2 males, partly dryed (paralectotypes, UPSZTY 2836); 14) a slide (MEUU, Lilljeborg catalogue N P2316) labeled “ Bythotrephes cederströmii, Sw, Upl, Ekoln , 11.8.1868, Lillj.”, 1 ad, 1 juv., dryed (parelectotypes, UPSZTY 167725); 15) sample (MNB, N 18911 View Materials ) with two tubes, one of them labeled “Upland, Ekoln, 2/9 86, Lilljeborg”, 9 ad, 1 gam, 1 male (paralectotypes, ZMB 30763); Bohuslän: 16) sample (MEUU, Lilljeborg catalogue N 2663) labeled “ B. longimanus var., Sw, Boh., Norra Bullaren, 6.8.1892, coll. Aurivillius & Svederus”, many ad and males (paralectotypes, UPSZTY 167726); Dalsland: 17) sample (MNB, bottle N 13753 View Materials ) in a tube labeled “No. 95, Dalsland, Wenern, 25. VI 1861 ”, 3 ad, strongly macerated internally; 18) sample (MNB, bottle N 13753 View Materials ) in a tube labeled “No. 136, Wenern, 2.VI 1861 ”, some ad, remains; 19) (MEUU, Lilljeborg catalogue N 2604) labeled “ B. longimanus, Sw, Dsl, Lelángen , 30.8.1882, col. Kolthoff T.”, 2 ad (paralectotypes, UPSZTY 167727); Gotland: 20) sample (MNB, bottle N 13753 View Materials ) in a tube labeled “ Gotland, No. 103, Wisbylan, IX 1863 ”, some ad; Östergötland: 21) sample (MNB, bottle N 13753 View Materials ) in a tube labeled “ II.2. Ostergothland. Osbra Langaren. Jorssnaess. U 8. VIII 1 865”, some ad; 22) sample (MNB, bottle N 13753 View Materials ) in a tube labeled “ II.1. Ostergothland, Graby, 6.VII 1865 ”, 1 juv; 23) sample (MEUU, Lilljeborg catalogue N 2602) labeled “ B. longimanus, Sw, Ög, Finspáng Bleken , 24.8.1891, coll. Lönnberg E.”, 7 ad (paralectotypes, UPSZTY 167728); Småland: 24) sample (MNB, bottle N 13753 View Materials ) in a tube labeled “No. 124, Juni 1861, Jönköpigslån”, remains of some ad; 25) sample (MNB, bottle N 13753 View Materials ) in a tube labeled “No. 131, I; 3.8.1861, Jönköpigslån”, some ad; 26) sample (MEUU, Lilljeborg catalogue N 2664) labeled “ B. longimanus var., Sw., Sm., Jönköpigslån Noen, 2.08.1893, coll. Trybom F.”, 3 ad (paralectotypes, UPSZTY 167729); 27) sample (MEUU, Lilljeborg catalogue N 2662) labeled “ B. brevimanus s.str., Sw., Sm., Gränna Bunnsjön, 12.8.1892, coll. F. Trybom”, 2 ad (paralectotypes, UPSZTY 167730); Skåne: 28) sample (MEUU, Lilljeborg catalogue N 2653) labeled “ B. longimanus var., Sw., Sk., Vombsjon, coll. G. Cederström”, 3 ad (paralectotypes, UPSZTY 167731).
Norway: 1) sample ( MEUU, Lilljeborg catalogue N 2652) labeled “ B. longimanus var. , Norway, Österdalen Tönset, 19.7.1881, coll. Esmark B. ”, 5 ad (paralectotypes, UPSZTY 167732); 2) sample ( MEUU, Lilljeborg catalogue N 2654) labeled “ B. longimanus var., Norw., Dovrefjell Elgsjön , 8.8.1886, coll. Limdman C. ”, 1 ad, 1 juv. (paralectotypes, UPSZTY 167733); 3) Lake Ringsjøen (60° 88.240 N; 10°35.465 E) GoogleMaps , 28.7.2011, 5 ad, 1 male, leg. I. Dimante-Deimantovica and B. Walseng; 4) Lake Aspern (59°1020 N; 11°4223 E) , 29.7.2011, 2 ad, leg. I. Dimante-Deimantovica and B. Walseng; 5) Lake Store Skillingen (5.6.2009, 1 ad, leg. I. Dimante-Deimantovica and B. Walseng; 6) Lake Fjellgardsvatnet , 22.6.2011, 3 ad, leg. I. Dimante-Deimantovica and B. Walseng.
Germany: 1) bottle (MNB, N 8375) with three tubes labeled “ Bythotrephes longimanus Leydig, 1860 , Wandlitzsee, leg. A. Protz, IX 1891 ”, 3 ad, 2 males, 1 juv.; 2) bottle (MNB, N 10784 View Materials with two tubes labeled “ Bythotrephes longimanus Leydig, 1860 , Gr. Stechlin See bei Neo Globsow Mark, leg. W. Hartwig, 29. VII 1896 ”, 37 ad, 12 juv.; 3) sample (MNB, N 18915 View Materials ) labeled externally “ Bythotrephes longimanus Leydig, Holstein, Apstein S.G. ” and labeled internally “ Bythotrephes, Plöner See bei Plön/Holstein, Apstein leg.”, 1 ad, 2 juv.
Poland: 1) sample (MNB, N 10785 View Materials ) labeled “ Bythotrephes longimanus Leydig, Gr. Pulssee bei Bernstein, W. Hartwig” (now Lake Jezioro Duzy Pełcz, Westpommern, Poland), 1 ad; 2) sample (MNB, N 18912 View Materials ) labeled externally “ Bythotrephes longimanus Leydig, Ostpreussen, Protz S.G. ” and labeled internally “ Bythotrephes longimanus, Lormunto utan bei Lotzen in Ostpreussen in 25 m, August 1898. A. Protz legit.”, 4 ad, 2 juv.; 3) bottle (MNB, N 18914 View Materials ) with two tubes labeled externally “ Bythotrephes longimanus Leydig, Westpreussen, Seligo G. ” and labeled internally “ Bythotrephes longimanus, Karschtafen in Massgrau Bau , 2/7 1900, Seligo”, 5 ad, 4 juv.; 4) bottle (MNB, N 18916 View Materials ‒1) with two tubes labeled externally “ Bythotrephes longimanus Leydig, Madu See, Weltner i Samter S.G.” and labeled internally “Madu, 6 X 1900, 7 X 1900 ”, 9 ad, 8 males; 5) bottle (MNB, N 18916 View Materials ‒2) labeled “ Bythotrephes longimanus Leydig, Madu See, Weltner S. ” with six tubes with specimens collected in the lake at different times, many ad, gam and males; 6) Lake Dargin, 31.7.1995, leg. A. Karabin, 6 ad, 2 juv.
Belarus: 1) Lake Obsterno , 13‒ 15.7.2010, 15 ad, coll. Zh. Buseva ; 2) Lake Sita , 25.7.2012 ‒ 29.7.2015, numerous ad, coll. V.V. Vezhnovetz; 3) Lake Drisviyaty , 18.7.2015, coll . V.V. Vezhnovetz ; 4) Lake Dolgoe, 25.7.2015, 31.7.2015, 6 ad, 2 juv., coll. V.V. Vezhnovetz ; 5) Lake Richi , 23.7.2014, 29.7.2015, 46 ad, 12 juv., coll. V.V. Vezhnovetz.
Russia: 1) sample ( ZIN, without number) labeled “ Bythotrephes longimanus F. Leydig , plankton of Lake Seliger , 14 VII 1909, coll. V.M. Rylov, N 1244”, 1 ad; 2) a slide ( ZIN) labeled “ Plankton of Lake Seliger , 7 VII 1914, N 1367, Bythotrephes , det. V. Rylov ”, 1 juv.; 3) Lake Plescheevo (Yaroslavl Province), August 2008, 11 ad, 3 juv., leg. I.K. Rivier and S . Zhdanova; 4) Sheksninskoye reservoir (Vologodskaya Province), 4.8.2011 and 22.8.2012, 26 ad, 5 juv., leg. E.V. Labunicheva ; 5) Argazinskoye reservoir (Chelyabenskaya Province), August‒ September 2011, about 40 ad and juv., leg. V.V. Rechkalov ; 6) Lake Uvildy (Chelyabenskaya Province), August‒ September 2011, 15 ad and juv., leg. V.V. Rechkalov ; 7) Lake Maloe Miassovo (Chelyabenskaya Province), July 2007, 1 ad, 1 juv., leg. V.V. Rechkalov ; 8) Hantaiskoye reservoir (north of Krasnoyarsk Region, Easter Siberia), 10.8.1980, 1 ad, 1 juv., leg. N.G. Sheveleva.
Data on body and body parts measurements of specimens of some populations are presented in Table 1.
Description. Female. General body appearance and segmentation. Body elongated and divided into four parts: head, thorax, abdomen, and postabdomen with long caudal process ( Figs. 1A View FIGURE 1 , 7A View FIGURE 7 ). Its longitudinal axis is conspicuously incurved when head is located at almost right angle to the thorax. Also highly movable abdomen can be either in a straight line with the thorax or stays at different angles to it. Head large with rounded anterior part filled by the enormously developed compound eye and bearing small antennules ventrally. Posterior part of head bears long swimming antennae and mouth parts consisting of mandibles, maxillules (mx I), and upper lip (labrum). Thorax with strongly developed muscular ventral side bearing four pairs of thoracic limbs of different sizes directed antero-ventrally. Dorsally, thorax bears a sack-like carapace transformed into a brood pouch, sometimes reaching large size. Abdomen (metasome) is elongated, cylindrical, inconspicuously three-segmented (see Korovchinsky 2015) and flexible, connected with a small postabdomen, bearing ventrally a pair of straight claws and posteriorly a very long straight caudal process with a pair of similar claws proximally. General body length of females (without caudal process) may reach 2.5 mm or slightly more (in the examined specimens it ranges from 1.18 to 2.58 mm) while the length of caudal process may exceed the body length by 1.5‒3.0 times (on average, about two times).
Head. Comparatively very large (37.8‒44.7 % of body length) and subdivided into two parts: rounded anterior part mostly filled by large compound eye and posterior part bearing dorsally a large saddle-shaped neckorgan, swimming antennae and mouth parts. Large pigment spot occupies about one-third or at most half of the eye’s volume. Ocellus (naupliar eye) is absent (see Elofsson 1966).
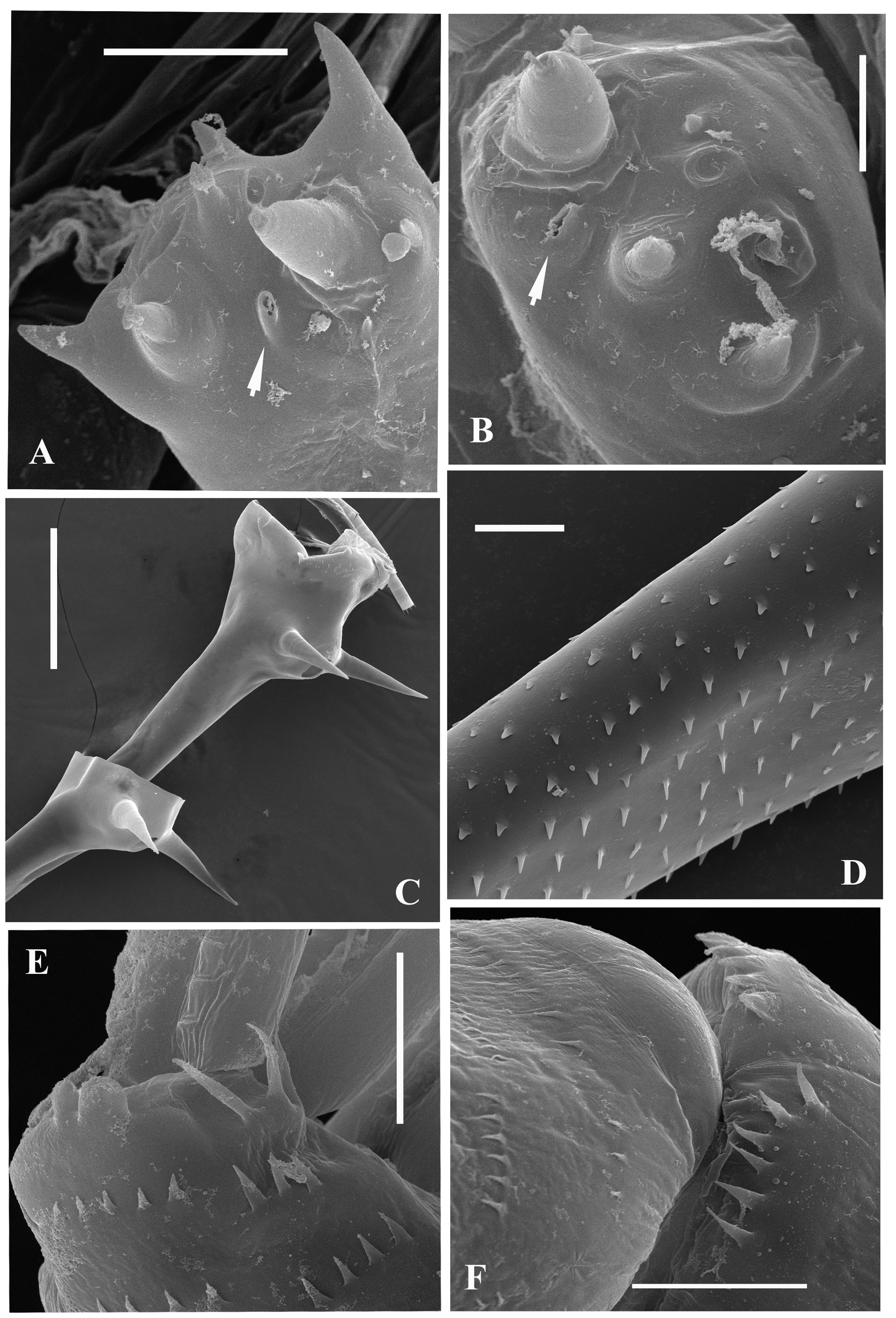
Antennules. Small and situated on the ventral side of the anterior head part beneath the eye. They are bulbous ( Fig. 1C View FIGURE 1 ) and sit on the joined basis slightly split anteriorly. Terminally they bear five regular aesthetascs in two groups of two and three, and one shorter and thinner aesthetasc-like structure, situated in a group with two regular aesthetascs, and having slightly expanded apical end (“accessory simple seta” according to Scourfield (1896)).
Swimming antennae. Comparatively long, with elongated cylindrical basipodite ( Fig. 1A, 1D View FIGURE 1 ) which has a dorsal thin feathered seta on its folded proximal part ( Fig. 1E View FIGURE 1 ). Of the two antennal branches, the lower threesegmented one (endopodite), sitting on the apical basipodital prominence, is slightly longer than the upper branch. The upper branch is four-segmented and lower branch is three-segmented ( Fig. 1D View FIGURE 1 ). Proximalmost segment of the upper branch is rudimentary and clearly visible only externally; all other segments of both branches are much more developed. Small spine with a row of neighboring minute prominences on the end of second segment of upper antennal branch ( Fig. 1H View FIGURE 1 ), similar apical prominences and spines on the distal segments of both branches ( Figs. 1F, 1G View FIGURE 1 ). Small proximalmost segment of upper branch lacks setae, while other segments possess two-segmented swimming setae of more or less similar size except distalmost of them which are shorter. All setae bilaterally armed with rows of uniform thin setulae. General formula of antennal setae: 0‒1‒2‒5/1‒1‒5 (see Korovchinsky (2015) for more details).
Mouth parts. They are represented by upper lip (labrum), mandibles, and maxillules (maxilla I). The upper lip is composed of two parts: the posterior thick and slightly flattened triangular lobe and anterior large proboscis-like outgrowth. The latter is separated from the former by a deep indention of the cuticle. The triangular lobe bears numerous papillae along its posterior-interior (oral) margin while the outgrowth is armed with tiny spinules. Mandibles are bilobed and adapted for biting ( Figs. 2A, 2B View FIGURE 2 ), with a toothed, blade-like posterior lobe and small anterior lobe (mandibular process) armed with a cluster of about 15 long outgrowths, slightly differing in size and bearing some prominences distally ( Figs. 2B, 2C View FIGURE 2 ). Posterior lobe is strongly sclerotized and divided in two toothshaped parts, the larger (posterior) of which has a small additional tooth about midway along its border ( Fig. 2A View FIGURE 2 ). Anterior side of large sclerotized lobe possesses a group of minute spinules ( Figs. 2B, 2D View FIGURE 2 ) which probably often might be broken ( Fig. 2A View FIGURE 2 ). Maxillules (mx I) resemble two cylindrical structures situated posterior to the mandibles. Distally, they bear short central seta and some spinules near it. Maxillae (mx II) are absent; the openings of maxillar glands are situated near the bases of tl I laterally (see Olesen et al., 2003).
Carapace. Resembling a bag-like structure, strongly modified into a closed brood pouch ( Figs. 1A View FIGURE 1 , 7A View FIGURE 7 ) widely connected in its base with the dorsal surface of the thorax. It may be often well developed and massive, being filled by large embryos and clearly inclined anteriorly over the head. Its length can comprise 53‒67 % of body length or more.
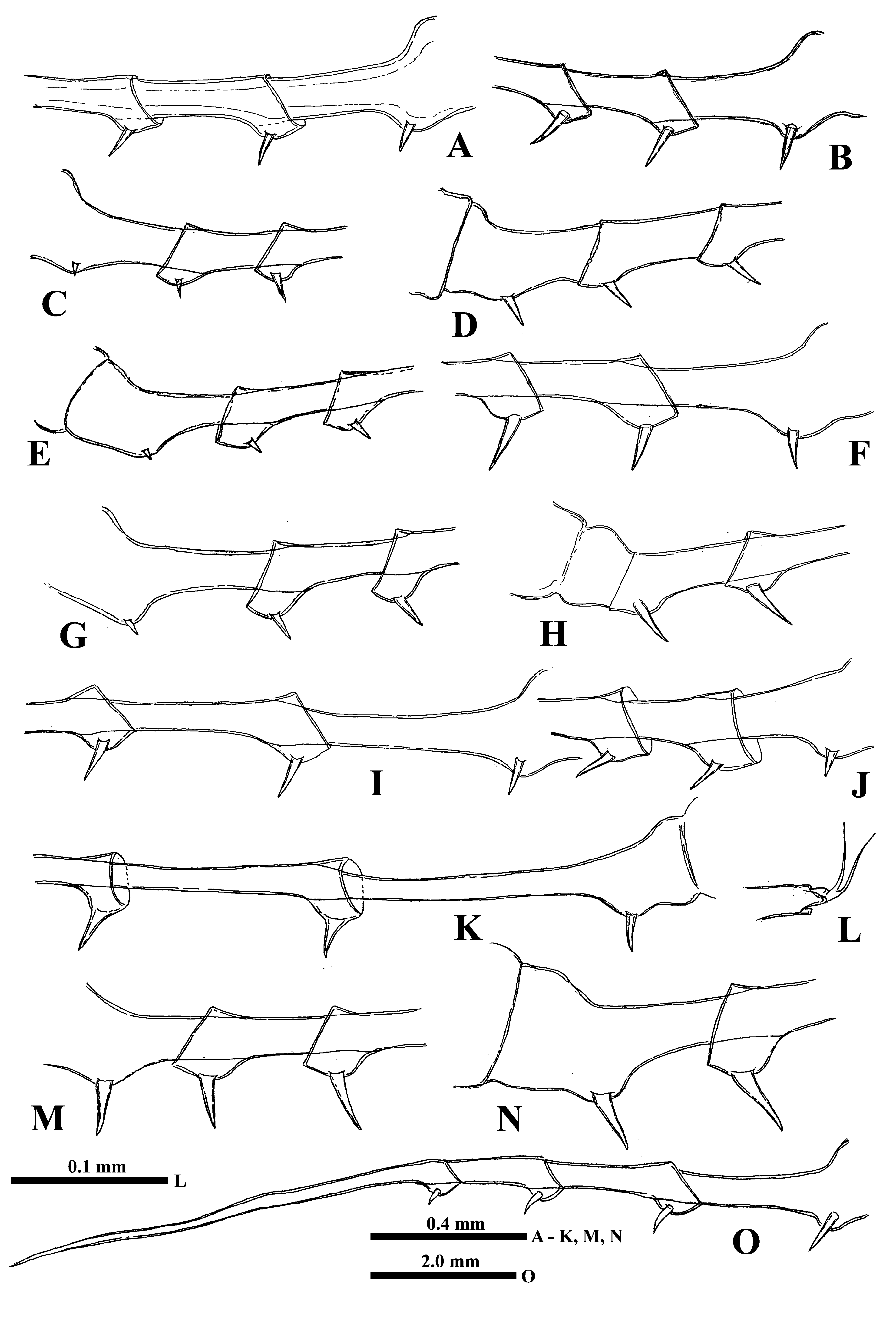
Thoracic limbs. Four pairs of strongly chitinized, stenopodous limbs are densely situated along the muscular ventral side of thorax and directed antero-ventrally ( Fig. 1A View FIGURE 1 ). All of them have complex and divers setaceous armament along their inner side. Limbs of the three anterior pairs are five-segmented, and those of the last fourth pair are three-segmented. Protopodites of all of them, covered by comparatively softer cuticle, are inconspicuously delimited into two parts (segments), coxa and basis, while the endopodites of limbs of the three anterior pairs are composed of three well developed segments being the ones of the fourth pair unisegmented ( Figs. 1I, 1L, 1M, 1N View FIGURE 1 ).
The first pair of limbs (tl I) are especially long and strong, their length rarely surpassing the body length (up to 116.4 % of its length) but usually shorter (57.0‒80.0 % of body length, rarely even shorter) ( Figs. 1A, 1I View FIGURE 1 ). Terminally, the inner side of their protopodite bears a small triangular lobe, pseudognathobasic process (see the explanation of the term in Korovchinsky (2015)), armed laterally and distally with two outgrowths with apical setae and numerous spinules. The external part of protopodite is longer than the internal one and bears apically a small conical outgrowth. The first segment of the endopodite is long and bears 4‒7 (usually 5‒6) anterior lateral setae (their number on limbs of a single individual can vary) with a posterior row of rough incurved spines and anterior and lateral rows of fine setulae. Distally, this segment bears a shorter anterior seta of the same type and long posterior finely setulated seta ( Figs. 1I, 1J View FIGURE 1 ). The second segment of the endopodite is conspicuously shorter and bears only two apical setae similar to those on the end of previous segment but usually shorter; the segment size can vary considerably ( Fig. 1K View FIGURE 1 , Fig. 3 View FIGURE3 ). The terminal third segment of the endopodite varies in length (12.0‒ 37.0 % of body length), being mostly shorter than the proximal first endopodital segment (usually 70.0‒85.0 % of the latter one but sometimes it may be longer, being either almost equal to or even exceeding the length of the first segment (90.0‒109.5 %)) ( Figs. 1I View FIGURE 1 , 3W View FIGURE3 ), and always bearing apically four long roughly spinulated setae, two of them terminally and two subterminally. Basally, these setae are armed by a row of smaller spines, whereas distally, larger lanceolate spines are situated in two rows and directed terminally.
The limbs of second pair (tl II) are considerably shorter, their protopodite, again externally, is conspicuously longer and bears a conical outgrowth ( Figs. 1L View FIGURE 1 , 3 View FIGURE3 V-left). The first basal segment of their endopodite bears a row of 4‒7 (mostly 4‒6) rather long anterior lateral setae (their number also can vary in one individual) ( Fig. 1L View FIGURE 1 : as). There are 1‒2 posterio-lateral seta of the same type on this segment. The terminal setae of the segment differ in that the anterior one is shorter and roughly armed, whereas the posterior one is longer and finely setulated. Internally, this segment bears a stout cylindrical pseudognathobasic process, possessing some prominences of different size, one small, thin seta, and a pore apically ( Fig. 2E View FIGURE 2 ). The second segment of the endopodite is short with only two setae, the anterior one of which ( Fig. 2F View FIGURE 2 ) is similar to the anterior terminal seta of previous segment, whereas the posterior seta is longer and finely setulated. The distal, third segment of endopodite of the limb bears four setae, two terminal and two subterminal ones ( Fig. 1L View FIGURE 1 ). Of the latter, the anterior seta is comparatively short, thick and armed with a number of thin lateral denticles, while its distal end is naked and slightly hooked apically. Its neighboring posterior subterminal seta is considerably longer and similar to subterminal and terminal setae of tl I, having similar spine armament and a sharp apex. The anterior terminal seta is thick and comparatively short with longitudinal ribs, few thin lateral denticles, and slightly hooked apical end. The posterior terminal seta is similar to the neighboring anterior one but longer, with few lateral denticles and a slightly hooked apical end.
The limbs of the third pair (tl III) are generally similar to those of the previous ones, differing in some details. The external outgrowth of their protopodite is conspicuously larger ( Fig. 3V View FIGURE3 ‒ right) and lateral anterior and posterior setae (if present) of first segment of endopodite are fewer (2‒5 and 1, respectively) ( Fig. 1M View FIGURE 1 ). Distal setae of the segment are similar to other ones. The pseudognathobasic process is also similar to that of tl II ( Fig. 5A View FIGURE 5 ). Of setae of the second segment, the anterior one is similar to the respective one of tl II. Terminal and subterminal setae of the third segment are similar to those of tl II but slightly shorter and bear fewer denticles.
The limbs of the fourth pair (tl IV) are considerably reduced; their protopodite bears slightly spinulated seta sited on a short cylindrical base ( Fig. 1N View FIGURE 1 ). The only segment of the endopodite has two rows of comparatively short spine-like setae. The external row (group) ( Fig. 1N View FIGURE 1 : as) always consists of two setae, and the internal row of 5‒7 setae, which differ in their appearance and armament. Almost the whole internal part of the endopodital segment is occupied by the reduced pseudognathobasic process, also having a pore and armed by some denticles and thin seta ( Fig. 5B View FIGURE 5 ).
Abdomen (metasome) ( Fig. 1A View FIGURE 1 ) is often deformed. It is inconspicuously delimited in two segments, short proximal and long distal with a prominent fold more or less in the middle dorsal side.
“ Postabdomen” actually consists of two parts: the last small abdominal segment and the postabdomen per se (see Korovchinsky (2015)), which is comparatively small, with the anal opening situated between the postabdominal claws. These claws are comparatively small or frequently may be rudimentary (0.8‒7.8 % of body length), usually straight and directed backwards, sometimes downwards, and sometimes with the apex curved slightly forward ( Figs. 4A‒N View FIGURE 4 ).
Caudal process is directly connected with the postabdomen and proceeds as a very long, proximally rather thick and curved, then straight spine-like structure ( Figs. 1A View FIGURE 1 , 5C View FIGURE 5 , 7A View FIGURE 7 ), variable in its length (156.0‒306.0 % of body length), thus exceeding the body length by 1.5-3 times. Generally, the caudal process is strongly chitinized and its surface covered by numerous minute spinulae ( Fig. 5D View FIGURE 5 ). Basally, the caudal process bears one or two pairs of claws similar to those of the postabdomen but usually larger (e.g., proximal claws reach 2.3‒8.3 % of body length), and apically two minute setae arise from a common base ( Fig. 4L View FIGURE 4 ). Pairs of claws usually sit closely, sometimes rather distantly (e.g., distance between postabdominal claws and proximal claws of caudal process (interclaw distance) usually constitutes 12,0‒23.0 %, rarely more—up to 30.4 % of body length). Between the latter, the thickness of the structure may be different: usually 4.1‒5.2 %, sometimes reaching 7.6 % of body length ( Figs. 4A‒N View FIGURE 4 , 5C View FIGURE 5 ). Borders separating old molted integuments of caudal process with claws normally are quite conspicuous.
Size 1.18‒2.58 mm.
Gamogenetic females differ from parthenogenetic ones only in presence of large yellow-brownish resting eggs (0.40‒0.56 mm in diameter) in their brood pouches. Lilljeborg (1901) recorded the presence of only two such eggs in specimens studied by him. Body length 1.71‒ 2.06 mm.
Female of first generation hatched from resting egg. Only one such specimen was found in a sample from the lake Store Skillingen ( Norway). It had body length 3.18 mm, head length—42.2 %, comparatively short tl I— 55.3 % and short caudal process—114.1 % of body length. The distal segment of tl I is comparatively short (15.1 % of body length). There are four pairs of small claws (from proximal to distal: 4.8‒3.0 % of body length), one on postabdomen and three on comparatively thin caudal process (3.3‒2.8 % of body length), distance between which is more or less similar (22.6‒14.1 % of body length) to that in females described above, the posteriormost claws sit much closer to the end of the process ( Fig. 4O View FIGURE 4 ). The setal armament of tl I seems similar to that of females of later parthenogenetic generations; the apical setae of the second endopodital segment of tl I are long ( Fig. 3L View FIGURE3 ).
Observation of pedogenesis. In one old sample from Poland (“ Bythotrephes longimanus Leydig, Westpreussen, Karschtafen in Massgrau Bau , 2/7 1900, Seligo G.”), there was found a female of B. brevimanus with strongly swollen brood pouch filled with large embryos ( Fig. 7A View FIGURE 7 ). One of the embryos had eggs in their small brood pouches ( Fig. 7B, 7C View FIGURE 7 ), in others the eggs were ready to leave from ovaries into a brood pouch. This example unequivocally demonstrates the presence of pedogenesis in representatives of the genus Bythotrephes .
Juvenile females. The investigation of a few juvenile females (with only a pair of postabdominal claws) (body length 1.1‒1.4 mm) has revealed that they differ from adults in the presence of a somewhat longer (203.0‒282.0 %, av. 251.0 % of body length) and thicker (5.1‒7.6 %, av. 6.0 % of body length) caudal process. The setae on the first endopodital segment of tl I‒ tl III are slightly less numerous.
Males ( Fig. 6 View FIGURE 6 ) on average, are smaller than females (body length— 1.46‒1.98 mm). The thoracic limbs of first pair (tl I) are comparatively short (52.4‒70.4 % of body length) as well as each segment of them, especially the distal one (11.7‒16.5 % of body length), which is slightly swollen proximally and bears on its inner side a small strongly chitinized hook with two inner denticles, and a field of tiny prominences situated under it ( Figs. 6B, 6C, 6E View FIGURE 6 ). In juvenile males, this hook is undeveloped ( Fig. 6D View FIGURE 6 ). The copulatory appendages are small (6.0‒11.2 % of body length) and armed with numerous minute spinules terminally ( Fig. 6F View FIGURE 6 ).
Intra- and interpopulation variability of females. According to data of Table 1, the smallest adult individuals were observed in Sweden and Belarus (Lake Obsterno) whereas the largest ones were in the Russian Sheksninskoye and Argazinskoye reservoirs. At the same time, other data showed a larger body length of Scandinavian specimens, i.e. up to 2.11‒2.58 mm in Swedish Ekoln and Storsj ӧn and up to 2.14‒2.34 mm in the Norwegian lakes Aspern and Ringsj ӧn, revealing high intra- and interpopulation variability. According to Ischreyt (1939), who studied numerous populations all over the Baltic region, the body length of the adult females of the species (“ B. balticus ”) varied from 1.28 to 2.13 mm. The same is true for comparative length of tl I which is the smallest in the specimens from Swedish Ekoln (up to 47.0‒63.0 %) and much larger in other localities of the country (up to 90.5‒116.4 % in Finspang Bleken and Storsj ӧn, respectively). The specimens from the latter locality had long tl I with short apical setae of endopodital segments which is also characteristic of some other populations (e.g. Lormunto utan bei Lotzen and lake Dargin ( Poland), Lake Sita ( Belarus)). It seems that these two features are correlated. Two sets of specimens from Lake Stechlin differed in length of tl I and claw size. The length of the apical setae of endopodital segments of tl I may vary even in one individual. The comparative length of the distal segment of tl I also varies considerably, being usually short, but infrequently its length reaches or even exceeds that of the first proximal segment of the limb. The length of the caudal process vary more or less equally in different populations ( Table 1 and other data) but, in specimens from Lake Dargin ( Poland), it appeared especially prominent (up to 305.8 % of body length). According to Table 1, the length of claws of postabdomen and caudal process are especially variable, being the smallest in Lake Sita ( Belarus) and largest in Argazinskoye reservoir (up to 7.8 and 8.3 % of body length, respectively). Similarly, large claws were observed in Swedish Lake Onkijärvi (7.4 and 8.3 %, respectively). At the same time, in most populations these claws are considerably smaller, and those of the postabdomen were frequently observed to be rudimentary (this may also be a characteristic of only selected specimens of populations). The claws usually are directed backwards but sometimes downwards, either straight or with tip curved slightly forward (see Fig. 4 View FIGURE 4 and also Lilljeborg (1861: Fig. 23)). In most Swedish populations, the interclaw distance was of moderate size (up to 17.5 % of body length in Storsj ӧn) while in the Ekoln population this parameter was much larger (up to 30.4 %). Similar differences were observed in Belarussian and Russian populations (see Table 1).
Remarks. Lilljeborg’s ( 1861) original description (“ B. longimanus ”) was too generalized, however, the images (see Lilljeborg 1861: Fig. 23) unequivocally reveal the diagnostic features of the species: comparatively short tl I with comparatively long apical setae of first and second endopodital segments and short distal segment, long and straight caudal process, and small claws. The latter feature also was characteristic of P.E. Müller’s ( 1868) specimens.
Lilljeborg’s ( 1901) subsequent re-description (“ B. longimanus var. brevimanus ”) was more detailed and better illustrated. The author stressed the small size of individuals, the specificity of the brood pouch’s shape, caudal process and especially tl I which are generally short with a short distal segment.
Despite usage of the refined morphometric system, Ischreyt (1934a, 1935, 1936, 1937, 1939) did not contribute much to the morphological diagnosis of the species. But due to the investigation of numerous populations, he captured the specificity and likeness of its representatives which were proposed to be considered for species status (“ B. balticus ”). Their local morphological peculiarities were used to specify some races occurring in Eastern Baltic regions, e.g. Northern Germany, Denmark, and Southern Sweden.
Investigation of more abundant material contributed to the resolution of some difficult issues. Thus, in his monograph, Lilljeborg (1901) illustrated tl I of specimen (“ B. longimanus ”) from Mycklaflon See (Småland, Sweden) with very long and thin distal endopodital segment and small apical setae of first and second segments which seemed reminiscent of those of B. longimanus s. str. ( Korovchinsky 2015). The investigation of a number of Swedish populations of B. brevimanus has revealed the presence of a long distal segment and short apical setae of first and second endopodital segments of tl I in the representatives of some of them (see above) which might be confused with the former species. On the other hand, these might be the representatives of a new species described herein, also having long tl I with comparatively short apical setae of endopodital segments (see below).
Differential diagnosis. In comparison with B. longimanus and B. arcticus (see Korovchinsky (2015, 2016)), the species under consideration usually possesses smaller body size, shorter tl I with usually short distal segment, large setae on distal ends of their first and second segments (however, in a few populations of B. brevimanus the length of tl I, their distal segment, and the size of apical setae of the endopodital segments are comparable with those of the former species), and caudal process has a thinner basal part. Also, the adult females of B. brevimanus have more numerous claws (two‒three pairs instead of only two pairs in B. longimanus ; females of the first generation of the former species have four pairs of claws instead of three pairs in the latter one) of smaller size (av., 2.4‒6.3 % of body length in B. brevimanus vs. 8.7‒15.5 % and 6.2‒9.9 % of body length in B. arcticus and B. longimanus , respectively), which are usually straight and directed backwards, not curved backwards or straight and directed downwards as in the two latter species, respectively. Further, B. brevimanus possesses quite conspicuous borders separating the old molted integuments of the caudal process with claws.
B. brevimanus differs from B. lilljeborgi sp. nov. in the presence of smaller claws on the postabdomen (0.8‒7.8 (av. 2.4‒6.3) vs. 5.4‒12.1 (av. 6.6‒9.3) % of body length) and caudal process (2.3‒8.3 (av. 3.1‒6.4) vs. 6.1‒11.4 (av. 6.9‒9.3) % of body length) which are normally directed backwards, not downwards as in the latter species.
Another recently redescribed species, B. cederströmii ( Korovchinsky 2015) , possesses very prominent differences from B. brevimanus , having a very long caudal process with denticulated curvature (sometimes it is absent). Its postabdominal and caudal claws are much larger, curved forward apically and frequently sit quite widely separated from each other.
| ZIN |
Russian Academy of Sciences, Zoological Institute, Zoological Museum |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Bythotrephes brevimanus Lilljeborg, 1901
| Korovchinsky, Nikolai M. 2018 |
Bythotrephes cederströmii Schödler
| P.E. Müller, 1868 : 203 |
Bythotrephes longimanus var. brevimanus Lilljeborg, 1901
| Lilljeborg, 1901 : 614 |