Chondropyga insignicosta Hutchinson & Moeseneder
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3710.3.6 |
|
publication LSID |
lsid:zoobank.org:pub:A9AF2D13-5953-476B-8D74-02B331047671 |
|
DOI |
https://doi.org/10.5281/zenodo.6165512 |
|
persistent identifier |
https://treatment.plazi.org/id/D36A61FA-5D1B-4750-A198-611C2DEC586F |
|
taxon LSID |
lsid:zoobank.org:act:D36A61FA-5D1B-4750-A198-611C2DEC586F |
|
treatment provided by |
Plazi |
|
scientific name |
Chondropyga insignicosta Hutchinson & Moeseneder |
| status |
sp. nov. |
Chondropyga insignicosta Hutchinson & Moeseneder , new species
( Figs 1–6 View FIGURES 1 – 2 View FIGURES 3 – 7. 3 )
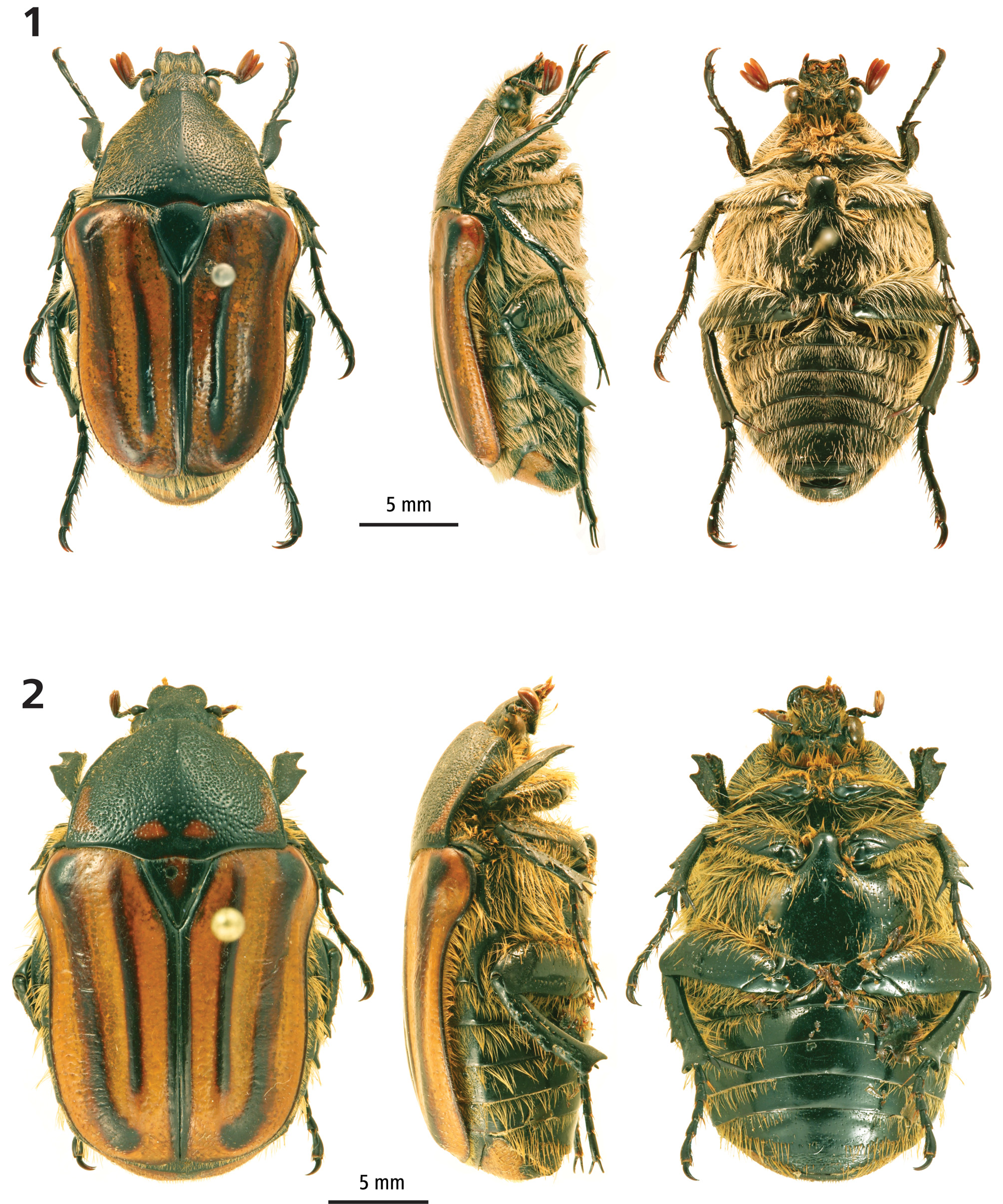
Material examined (11 specimens). Holotype male: AUSTRALIA. Upper Cameron Creek, Qld, 1.x.–18.xii.1999, D. Cook & I. Cook, intercept trap, T159367 [QM] ( Fig. 1 View FIGURES 1 – 2 ). Paratypes: AUSTRALIA. 1 female, Homebush, Qld, S.R.E. Brock collection donated to ANIC 1987, 25-055639 [ANIC] ( Fig. 2 View FIGURES 1 – 2 ); 5 males, Upper Cameron Creek, Qld, 1.x.–18.xii.1999, D. Cook & I. Cook, intercept trap, T159362, T159364, T159365, T159366 [QM], AIF46210 [AIF]; 1 male, Stoney Creek, Qld, 4.x.–17.xii.1999, D. Cook & I. Cook, intercept trap, T159363 [QM]; 1 male, Cameron Creek, Qld, 1.x.–6.xii.2002, D. Kitchin, flight intercept trap, PMH7250 [PMH], illustrated in Golding (2009); 1 male, Cameron Creek, Qld, 1.x.–27.xii.2002, D. Kitchin & T. Jack, flight intercept trap, PMH7251 [PMH]; 1 male, Cameron Creek, Qld, 1.xi.–26.xii.2003, D. Kitchin & T. Jack, flight intercept trap, PMH7252 [PMH].
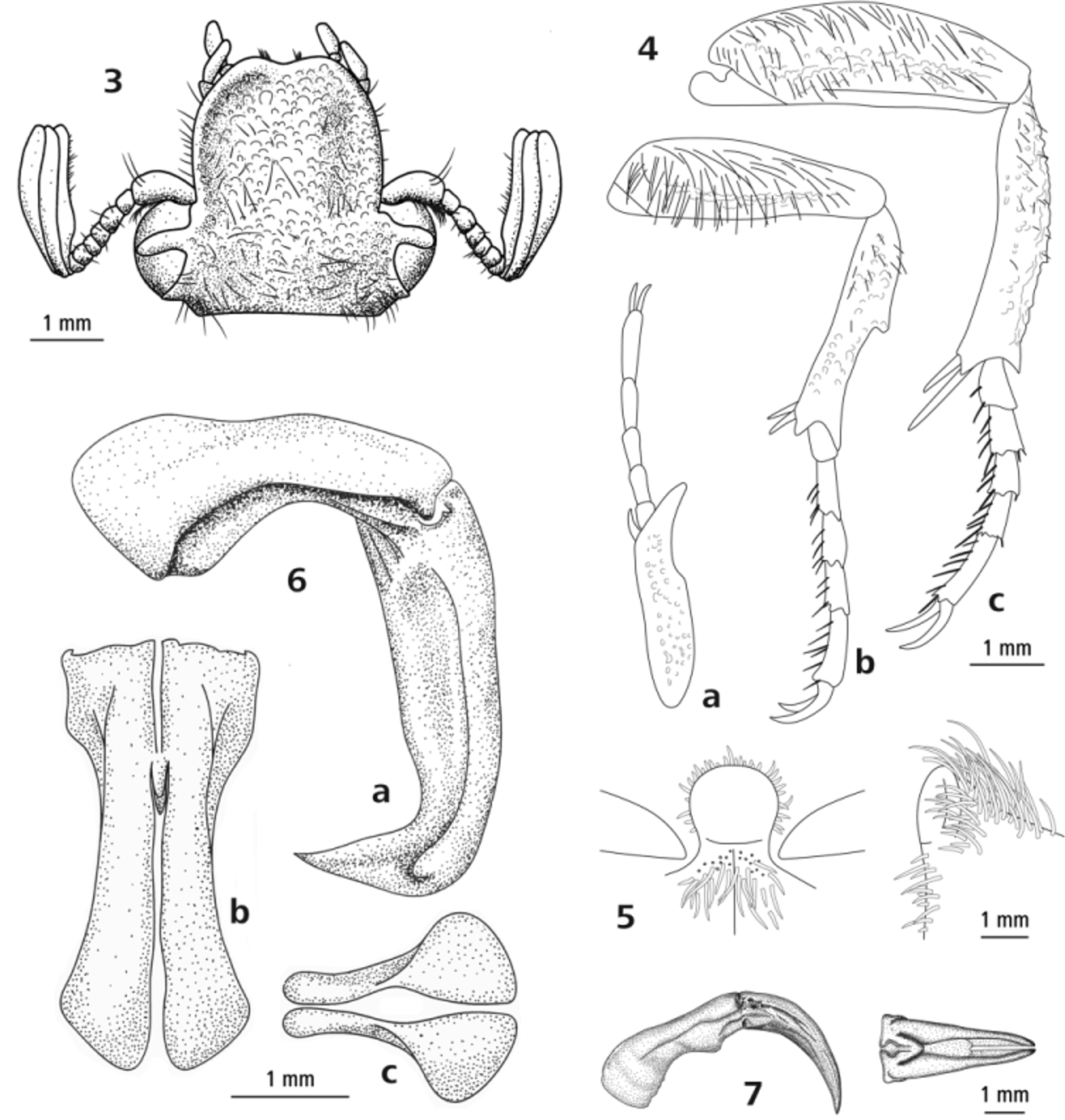
Description of holotype. Male ( Figs 1 View FIGURES 1 – 2 , 3–6 View FIGURES 3 – 7. 3 ). Length 23 mm, width 12 mm. Ovoid. Dorsally entirely glossy, ventral surface black. Head ( Fig. 3 View FIGURES 3 – 7. 3 ). Clypeus longer than wide, weakly divergent post antennal insertion; black. Lateral declivity not visible in dorsal view; lateral margins moderately raised and broadly rounded to apex, apically weakly bilobate. Antenna with 10 antennomeres; club with 3 antennomeres. Frons and clypeus coarsely punctate, often coalesced, becoming small on apical and lateral margins; bearing moderately dense, long, fine, ginger pilosity, less dense on preclypeus. Mentum broad, widely emarginate apically. Antennomeres 1–7 dark brown. Club same length as antennomeres 2–7, beige to light brown. Thorax. Pronotum trapezoidal, transverse; base weakly trisinuate; basolateral angle acute, slightly produced; basolateral margin concave-linear, producing distinct angle anterior of median; lateral margin raised, rounded, continuous narrow along apical margin; midline apically forming anteromedial carina; black; surface coarsely, evenly punctate, becoming small on apical and basomedian margins, coalesced except for basal margin and midline; lateral margin glabrous; clothed in moderately long, fine, ginger pilosity, less so on midline. Scutellum slightly elongate; apex acute; black; bearing coarse punctures basally, glabrous. Elytron. Posthumeral emargination pronounced, exposing metacoxa and partially exposing dorsal surface of sternites; bicostate (not counting sutural costa), distinct; sutural costa distinct with sharply raised edge, terminating subapically of scutellum, raised in apical half, tapering apically, non-spinose, bearing micropunctures; first costa weakly raised basally, clearly so from apex of scutellum, terminating preapically; second costa broadest, reaching from humeral umbone to apical umbone, both costae bearing scattered macropunctures; elytron beige to light brown; costae, suture and margins dark brown, creating distinct striped appearance; rugulose between margin and second costa, becoming coarser apically; micropunctures forming lines in middle of costal intervals and margins of costae; few scattered setae across base, becoming more numerous near apex; epipleural pilosity reaching suture. Legs ( Fig. 4 View FIGURES 3 – 7. 3 ). Black. Profemur evenly tapering to apex, dorsoventrally flat; covered in long, fine pilosity. Protibia unidentate, incurved in anterior half, giving rise to preapical angle; apical denticle acute with rounded apex, long, reaching middle of second protarsomere; apical spur short, barely reaching apex of first protarsomere; proximal margin bearing short, dense setae along entire length; ventral surface with distinctly raised mid rib; sparsely rugulose; coarse punctures medially, less dense laterally; clothed with long, fine pilosity on either side of mid rib. Protarsi not elongate; tarsomeres 1–4 equal in length, tarsomere 5 longer with few broad setae; claws simple. Mesofemur subparallel, widest at middle, dorsoventrally flat, slightly curved; ventral surface moderately rugulose; covered in long, fine pilosity. Mesotibia dorsal margin convex basally, concave apically, with median denticle; proximal surface with shallow longitudinal groove bearing long, fine, ginger pilosity over entire length; apex with two spines of approximately equal length, deeply incurved interval; two similar length apical spurs, fine, tapering, acute, closely set, reaching tibial apex; distal surface basal half with several rows of coarse, coalesced punctures; scattered, short setae emanating from punctures; ventral margin bearing row of long, fine, ginger setae over entire length. Mesotarsi not elongate; tarsomeres 1–4 equal in length, tarsomere 5 longest, with two rows of broadened bristles on ventral surface of tarsomeres 2–5; claws simple. Metafemur anterior margin convex, posterior margin straight, widest at midlength, dorsoventrally flattened; ventral surface moderately rugulose marginally; scattered punctures medially; moderately clothed with long, fine pilosity. Metatibia curved, bent medially, medially with some small, feebly developed denticles; proximal surface with two longitudinal grooves; apically unispinose adjacent to ventral margin; two apical spurs, fine, acute, closely set, distinctly surpassing tibial spine, dorsal spur twice length of ventral spur; distal surface coarsely rugulose; few scattered, short setae along length, forming loose rows; both longitudinal grooves of proximal surface with row of long, fine ginger setae; with row of setae on ventral margin for entire length. Metatarsi not elongate, tarsomeres 1–4 equal length, tarsomere 5 longest; bristles on ventral surface of tarsomeres 1–5, forming two rows of broadened bristles on tarsomeres 2–5; claws simple. Abdomen. Mesometasternal base broad, moderately wide, hemispherical, apically and longitudinally rounded, surpassing mesocoxa, sparsely punctate, glabrous. Abdominal sternites with medial longitudinal impression on segments 3–5; punctate across all segments; bearing long, fine pilosity, becoming shorter medially. Pygidium transverse, length:width ratio 1.0:1.7, in dorsal view evenly convex, in lateral view basally flat, apically rounded; light brown, with broad macula medially from both sides through apex, basomedial macula; entire surface concentrically rugose; bearing sparse, ginger setae, basally shorter. Genitalia ( Fig. 6 View FIGURES 3 – 7. 3 ). Parameres as long as phallobase, gradually broadening from base to pre-apex where bent anteriorly through 90 degrees, then abruptly narrowed, twisted to apex; dorsal cleft narrow, widening to pre-apex.
Variation in males. Length 20–23 mm, width 11–13 mm. Pronotal anterior margin sometimes produced medially. Some specimens with beige basomedial macula and macula in pronotal basolateral angle to premedian lateral area and a macula prebasally near lateral extent of scutellum. Scutellum sometimes basomedially light brown; with paramedial punctures extending in v-shape parallel to lateral margins. Posthumeral emargination sometimes narrowly exposing abdominal sternites. Protibia distal margin in some specimens with preapical angle developed to slight remnant of second denticle.
Female ( Fig. 2 View FIGURES 1 – 2 ). Length 25 mm, width 14 mm. Differing from male in the following characters. Broader. Head, pronotal and elytral features less angulate, giving more rounded appearance. Clypeus shorter, as long as wide, strongly bilobate, glabrous; frons glabrous. Pronotum less trapezoidal, anterior margin wider, lateral margins less angulate, basolateral angle 90 degrees; pilosity shorter. Scutellum shorter, nearly equilateral. Elytron with few, scattered, short setae. Mesometasternal process shorter, reaching anterior edge of mesocoxa. Profemur dorsoventrally thicker. Protibia with two broad denticles; base of distal margin more convex; apical spur surpassing first tarsomere. Protarsomeres 2–5 missing on both prolegs of female paratype. Mesotibia median denticle larger, apex wider; apical spines larger. Mesotarsomere bristles not in rows, not broadened. Metafemur not rugulose; with scattered punctures. Metatibia more parallel; groove on proximal surface barely discernible; bearing pronounced truncated tooth apical of median of dorsal margin; apex wider, unispinose with indication of second spine; dorsal spurs curved, parallel-sided, almost equal length. Metatarsi shorter; ventral bristles not in rows, not broadened. Abdominal sternites convex, without medial impression; glabrous across middle. Pygidium strongly transverse, height:width ratio 1.0:2.5; with black median macula, a black macula extending along apical edge from centre to two-thirds to distal corners, small black macula immediately above apical macula. Variation in females unknown since only one female in collections.
Discussion. Females can be reliably distinguished from males by their convex abdominal sternites, bidentate protibiae, dentate metatibiae, smaller antennal clubs and glabrous head. The single known female shows heavy abrasion and consequently its head, thorax and legs are duller in appearance. This has been observed in other cetoniines and is most likely due to burying behaviour associated with oviposition in soil.
Within the scope of this study and our ongoing work on Australian cetoniines we inspected all Australian genera and the known species of Diaphonia Newman, 1840 , Chondropyga Kraatz, 1880 , Pseudoclithria van der Poll, 1886, Aphanesthes Kraatz, 1880 , Hemichnoodes Kraatz, 1880 , Tapinoschema Thomson, 1880 , Phyllopodium Schoch, 1895 and Grandaustralis Hutchinson & Moeseneder, 2013 . With the exception of the genera Phyllopodium and Grandaustralis , these genera are in need of review. This is especially the case for Diaphonia and Pseudoclithria , where several undescribed species should be placed and misplaced ones moved elsewhere. We describe C. insignicosta in the genus Chondropyga because it shares several easily visible characters with the other species of the genus Chondropyga : a short bilobate clypeus, transverse and trapezoidal pronotum and the general form of the mesometasternal process (Table 1) and because there are insufficient similarities for it to be placed in any other genus. A diagnosis of easily visible generic characters between the genera Grandaustralis , Diaphonia and Tapinoschema was provided previously (Hutchinson & Moeseneder 2013) and can be used to separate these taxa from Chondropyga . While C. insignicosta shares many characters with the five other species that were examined, it is the only species with prominent costae in both sexes. Parameres in the other species of Chondropyga are simple, evenly arcuate in lateral view and taper apically ( Fig. 7 View FIGURES 3 – 7. 3 ) whereas in C. insignicosta they are bent and twisted apically ( Fig. 6 View FIGURES 3 – 7. 3 ).
Regarding the species that are currently listed in Chondropyga we note that Allard (1995) assigned Schizorrhina (Diaphonia) suturata Nonfried, 1891 to the genus Chondropyga without having seen any specimens and suggested it may be synonymous with Aphanesthes trapezifera (Thomson, 1878) . No other author seems to have inspected specimens of this species first-hand since its description in 1891. Lea (1914) suggested that S. suturata may have been founded on a common form of Aphanesthes trapezifera though he was unable to reach a conclusion in the absence of A. trapezifera specimens. The institution that was expected to hold the type of this taxon, the MNHUB, is unable to locate any type material. Consequently, Schizorrhina (Diaphonia) suturata is not represented in Table 1.
TABLE 1. Differences and similarities between species in the genus Chondropyga . Notes: 1 type species of the genus Chondropyga , 2 Chondropyga frenchi (Schoch, 1898) male based on image of syntype from the MVMA website, 3females of C hondropyga allardi Rigout & Allard, 1997 are unknown.
Etymology. The name insignicosta refers to the prominent elytral costae of this species. It is a concatenation of the Latin words insignis, meaning remarkable or prominent and costa for ribs.
Ecology. Very few specimens of this large and attractive species are known from a small number of insect collections. All male specimens were caught in flight intercept traps in rainforest. A collection method was not noted for the single female specimen. The small area of occurrence, the few captured specimens and the specialized collection method by which they were captured suggests that Chondropyga insignicosta is rarely found and localized. This is consistent with Allsopp’s (1997) prediction that the likelihood of discovering an undescribed Australian scarab is more related to the size of its range and to accessibility of its region of occurrence rather than the size of the insect. Since flight intercept traps were only installed from early November until mid-December the overall period of adult activity is unknown. Specimens were collected in notophyll to microphyll rainforest. One adult was collected in dry weather with local bushfires (D. Kitchin, personal communication). Females of this species presumably fly less than males as has been observed by the authors for other Australian cetoniines in which female specimens are generally under-represented in catch from flight intercept traps (Hutchinson & Moeseneder 2013).
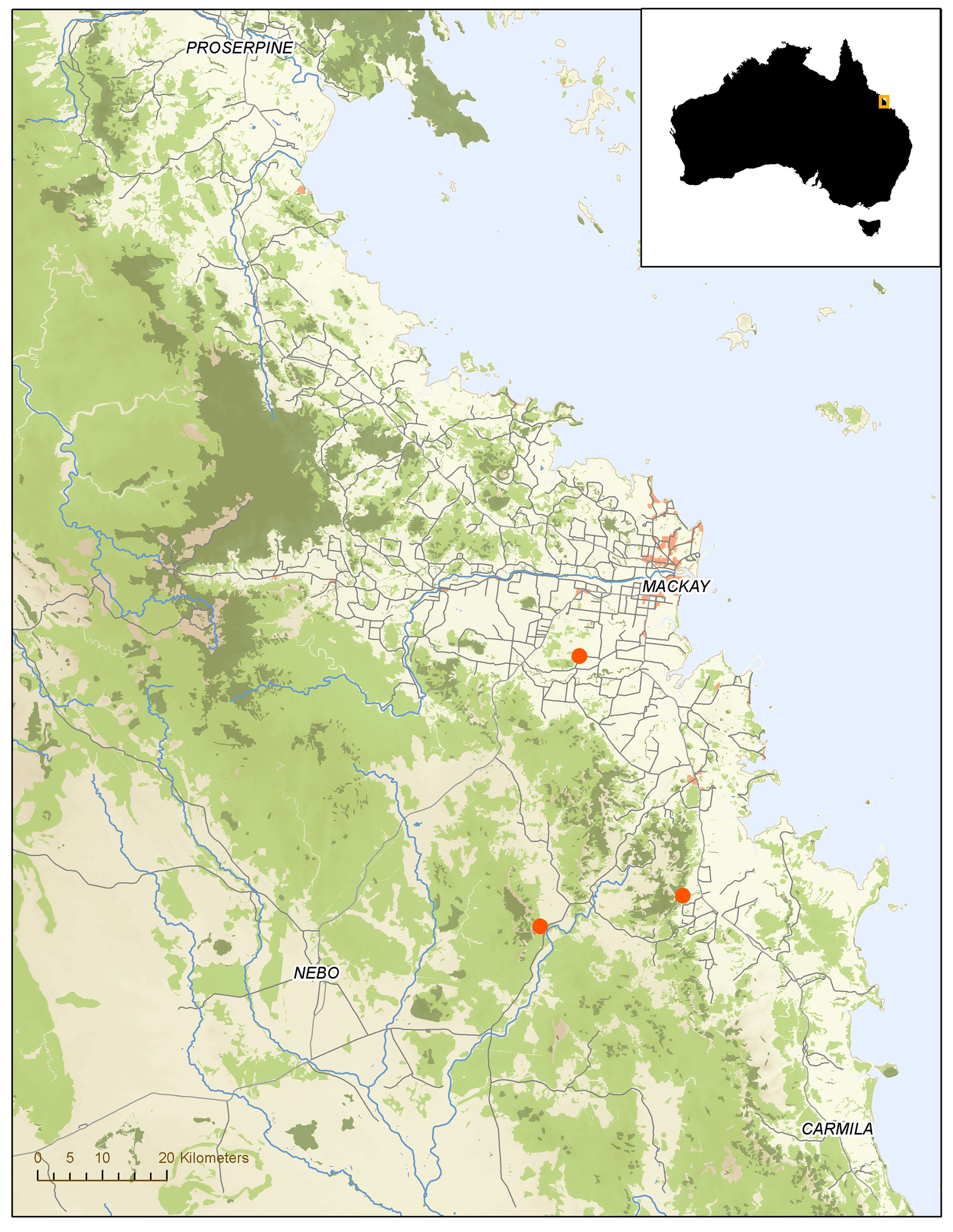
Geographical distribution. This species is known only from three localities along the coast of Central Queensland, Australia ( Fig. 8 View FIGURE 8 ). Eight specimens are from rainforest habitat in the Upper Cameron Creek valley at the foot of Black Mountain near Koumala. One specimen comes from rainforest at the banks of the Stoney Creek at Blue Mountain near Sarina. The solitary female is from Homebush.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.