Stenaspis Audinet-Serville, 1834
|
publication ID |
https://doi.org/10.5281/zenodo.5041512 |
|
publication LSID |
lsid:zoobank.org:pub:37900822-FF60-4386-BF30-9434678DD39B |
|
persistent identifier |
https://treatment.plazi.org/id/445E87AE-2B53-FF8F-FF06-8C37E1E5FCD7 |
|
treatment provided by |
Carolina (2021-06-29 14:39:03, last updated by Plazi 2023-11-03 02:24:46) |
|
scientific name |
Stenaspis Audinet-Serville, 1834 |
| status |
|
Stenaspis Audinet-Serville, 1834 View in CoL
Type species: Stenaspis verticalis Audinet-Serville, 1834 (monobasic).
Stenaspis Audinet-Serville 1834: 51 View in CoL ; Dupont 1838: 50; Castelnau 1840: 419; Blanchard 1845: 145; LeConte 1854: 441; Strauch 1861: 127; Thomson 1861: 211, 1864: 208, 434; Lacordaire 1869: 171; Chenu 1870: 311; Gemminger and von Harold 1872: 2967; LeConte 1873a: 314; Bates 1880: 76; LeConte and Horn 1883: 299; Leng 1886: 60; Casey 1912: 318; Aurivillius 1912: 458; Bradley 1930: 241; Zajciw 1960: 144, 1961: 401; Arnett 1962: 863, 881; Linsley 1962: 98; Monné 1994: 34; Monné 2005: 642; Monné 2012: 62; Eya 2015: 361.
Smileceras LeConte 1850: 8 ; Thomson 1861: 380, 1864: 208, 434.
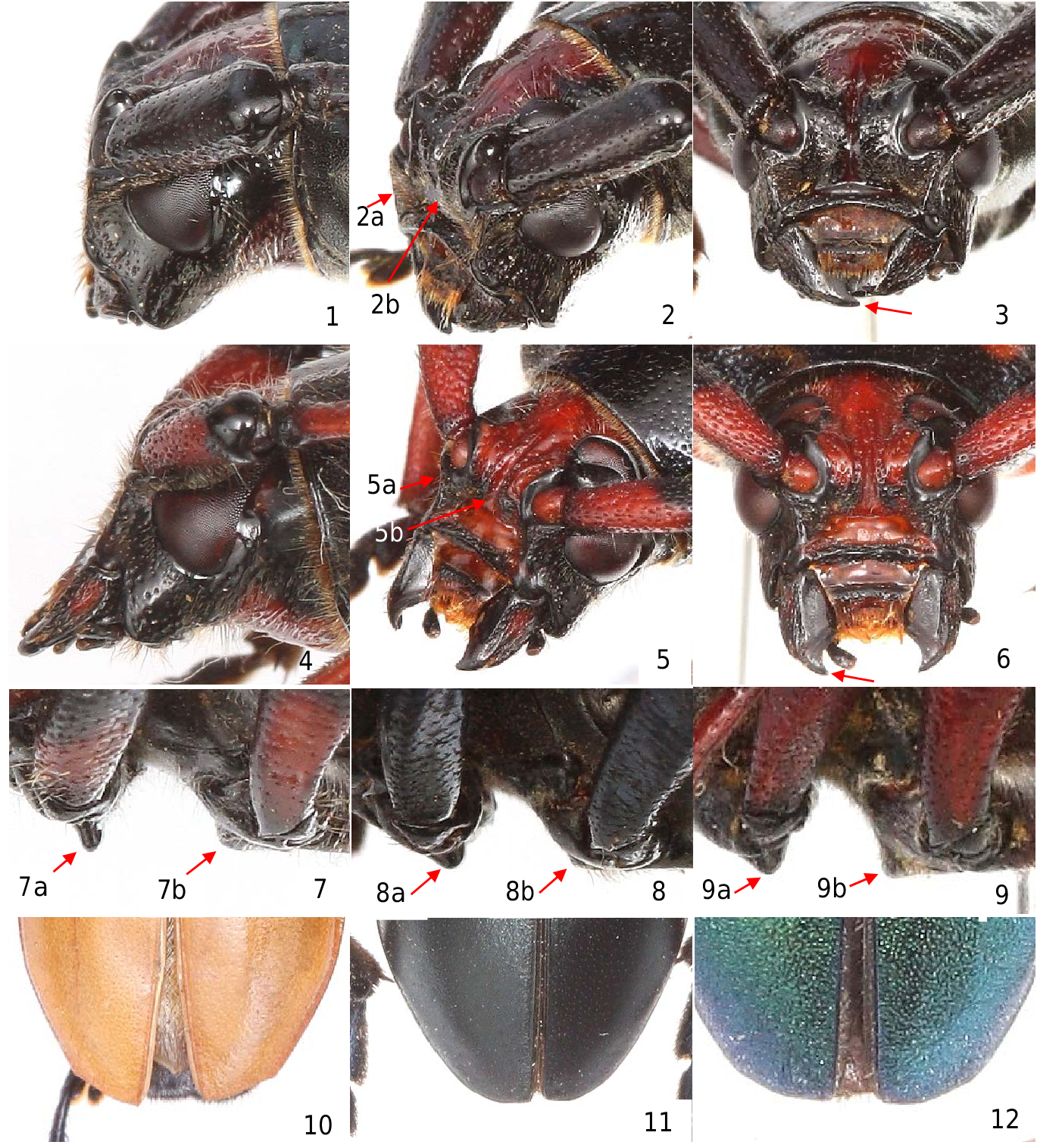
Redescription. Form, robust, parallel-sided to slightly tapered posteriorly, dorsum sparsely pubescent. Head with frons square, perpendicular, abruptly separated from anteocular space, impressed transversely above clypeus with deep pit on each side of transverse impression, median line canaliculate, extending to vertex between eyes, vertex and frons rugulose, irregularly punctate ( Fig. 1–6 View Figures 1–12 ); mandible arcuate, striate-punctate, outer edge excavate, apices simple; palpi short, subequal, last segment not expanded, outer edge impressed, apex truncate; gena quadrate, lower lobe of eye well separated from base of mandible ( Fig. 1, 4 View Figures 1–12 ); antennal tubercles prominent; eyes moderately large, finely faceted, upper lobes small, well separated; antennae elongate, 11-segmented, scape conical (without excavated or impressed area in basal half), apices of antennomere 3–7 darker, slightly enlarged and expanded, 11 th antennomere appendiculate. Pronotum broader than long, narrower than base of elytra at humeri, sides rounded, angulate or tuberculate; anterior angles inflated, broadly rounded or with obtuse callus; prosternum with intercoxal process narrower than coxal cavity, apex protuberant, ridged between coxae, subvertical, abruptly declivous behind ( Fig. 7a–9a View Figures 1–12 ), coxal cavities wide open behind; mesosternal intercoxal process not protuberant, about level with top of coxae ( Fig. 7b–9b View Figures 1–12 ) and abruptly declivous and concave in front. Scutellum triangular, elongate toward apex, acutely pointed, glabrous. Elytra with apices unarmed, rounded to suture ( Fig. 10–12 View Figures 1–12 ). Legs slender, hind femora linear, compressed, not attaining apex of elytra; hind tarsi with tarsomeres triangular, explanate, first tarsomere shorter than following two together, third tarsomere cleft to base.
Discussion. According to Audinet-Serville (1834: 52), Stenaspis is a genus with a thorax that is laterally dilated, almost transverse, square (especially in males), tuberculate on each side at the middle, and obliquely tapered from the tubercle to the posterior angle. The pronotal disc is glabrate with sides irregularly punctate. The scutellum is large, elongate triangular, and narrowly acute at the apex. The antennae are glabrous, longer than the body in males and is described as having 12 articles with the second segment short, globular, and segments 3–8 cylindrical, and the following segments flattened, elongate and the terminal segment longest.
The antennae of four species of Stenaspis (i.e., S. castaneipennis , S. solitaria , S. superba and S. verticalis ) examined are 11-segmented with the last antennomere appendiculate as described later by Linsley (1962: 98). The intercoxal process of the prosternum is cuneiform, compressed and protruding ( Fig. 7a, 8a, 9a View Figures 1–12 ). The mandibles are short and thick. The elytra are almost parallel-sided, slightly narrowed apically, the humeral angles are rounded, and the apices are rounded and unarmed. The legs are medium length, the femora are clubbed and elongate. The body of Stenaspsis is glabrate and more or less shining.
The prothorax of Stenaspis is sexually dimorphic as in Crioprosopus and Callistochroma . Linsley (1962: 98–99) described the two males of North American Stenaspis (i.e., S. verticalis and S. solitaria ) as having the dorsal surface of the pronotum coarsely, irregularly punctate, the lateral surface finely, densely punctate, and the prosternum with transverse subrectangular impressed areas on each side of the middle that are finely and densely punctate. Furthermore, he stated that females of both species have the pronotum coarsely, irregularly punctate on the lateral surface, and the prosternum coarsely punctate and transversely rugose. The sides of the male pronotum (or the prosternal episternum or proepisternum) are finely, densely punctate and inflated ( Fig. 15, 16a View Figures 15–23 ), and this finely punctate area is clearly demarcated from the coarsely punctate dorsum of the disc ( Fig. 16b View Figures 15–23 ), and is vaguely divided from the subrectangular impressed area of the prosternum ( Fig. 17a–b View Figures 15–23 ). There is considerable variation in the shape of the male pronotum in S. verticalis and S. castaneipennis ( Fig. 15, 18 View Figures 15–23 , 24, 27 View Figures 24–32 ). Some individuals have rounded sides due to well developed and inflated anterior angles (or proepisternum, Fig. 15–16 View Figures 15–23 , 24–25 View Figures 24–32 ), which merges over the lateral tubercles behind middle, whereas other males with less developed anterior angles have more prominent lateral tubercles ( Fig. 18 View Figures 15–23 , 27 View Figures 24–32 ). Based on the specimens examined, the male S. solitaria all have well developed lateral tubercles ( Fig. 33a View Figures 33–41 ) with rounded anterior angles ( Fig. 33b View Figures 33–41 ), which do not extend and merge over the lateral tubercles. In the females, the coarse punctures on the side of the pronotum merge with punctures on the dorsum and are not clearly demarcated as in the males ( Fig. 22 View Figures 15–23 , 31 View Figures 24–32 ). The anterior angles of the pronotum in females are obtusely callused and obliquely tapered toward the apex ( Fig. 21a View Figures 15–23 , 30a View Figures 24–32 ). Females of all four species (i.e., S. castaneipennis , S. solitaria , S. superba and S. verticalis ) have prominent lateral tubercles behind the middle and anterior angles broadly callused, and proepisternum and prosternum coarsely punctate. As in other trachyderine genera there are five discal calli on the pronotum, two antemedial, one on each side of the middle, and three postmedial near the base, one in the middle and one each on either side ( Fig. 15 View Figures 15–23 , 33 View Figures 33–41 ). These five discal calli in Stenaspis are vaguely protuberant, and the spaces in the middle between these calli are usually flattened. In some specimens; however, the three calli near the base, especially the one in the middle, are more visible than the two in the anterior half. Stenaspis superba is the only species with the two anterior dorsal calli more prominent ( Fig. 39 View Figures 33–41 ) and the pronotal disc convex instead of flattened ( Fig. 40 View Figures 33–41 ).
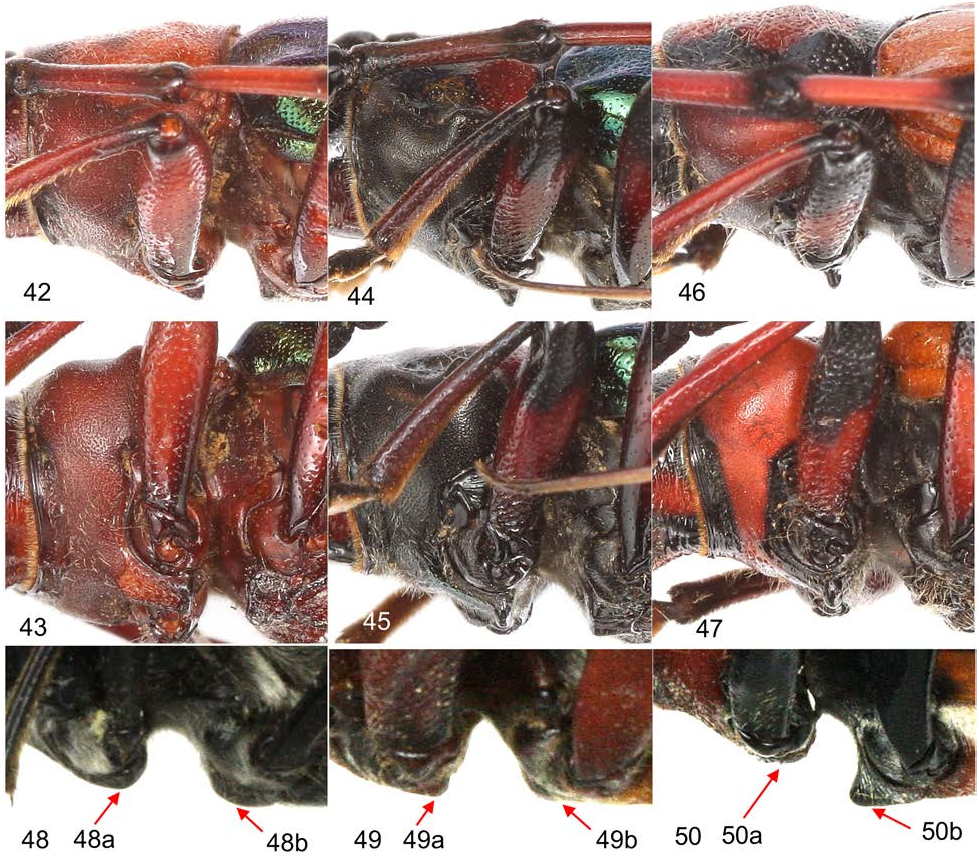
The prosternal intercoxal process of S. castaneipennis , S. solitaria and S. verticalis is also variable in shape ranging from uniformly sloping to the apex and abruptly declivous or concave behind ( Fig. 42–43 View Figures 42–50 ) to concave and abruptly acuminate at the apex ( Fig. 44–47 View Figures 42–50 ). Variation in the shape of prosternal process is more evident in S. verticalis , as compared to S. castaneipennis where the prosternal process is more commonly concave and abruptly acuminate at the apex. In S. solitaria the prosternal process is usually more uniformly sloped to the apex.
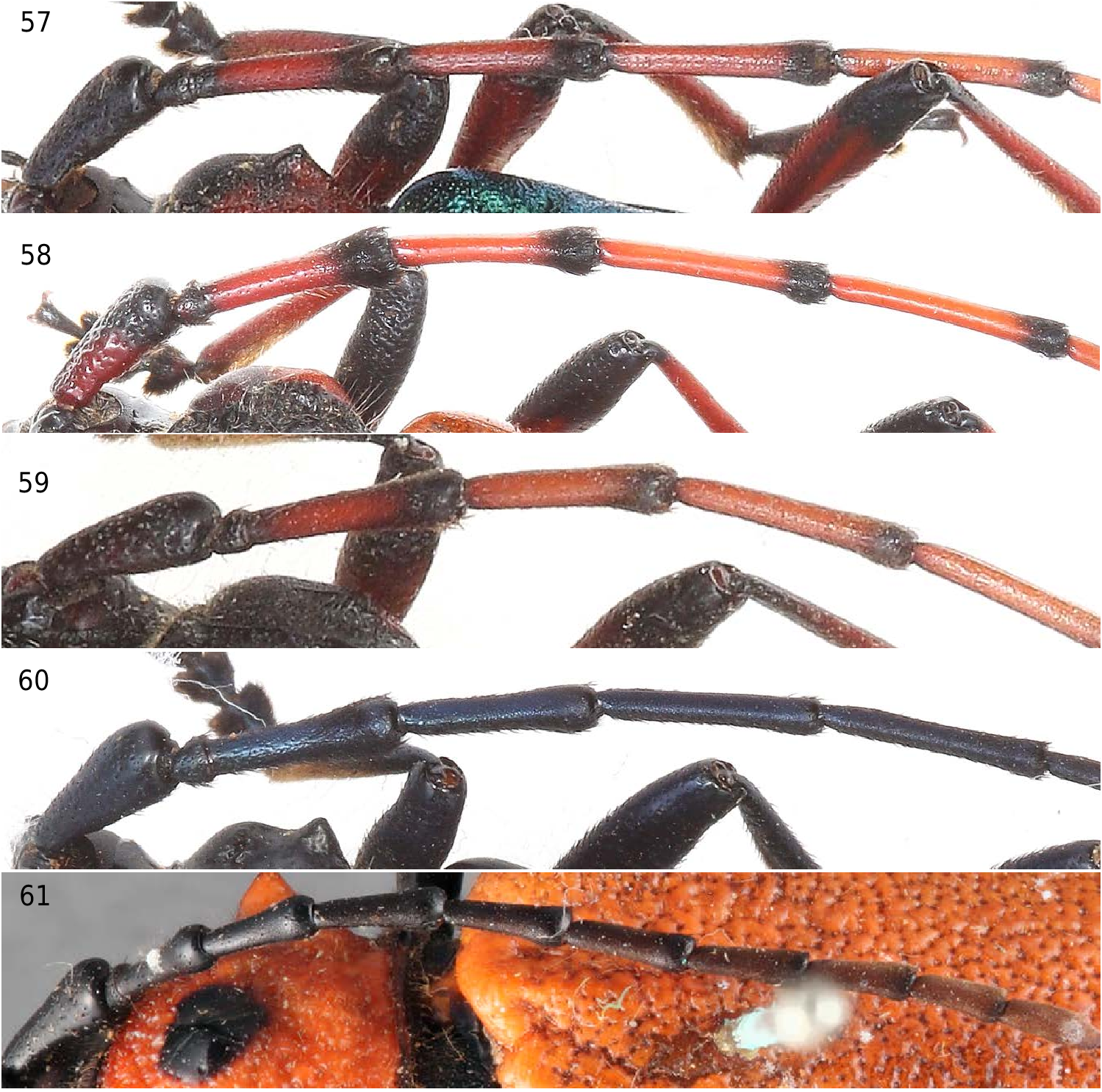
The scape in S. castaneipennis , S. solitaria , S. superba and S. verticalis is conical (without excavated area in basal half), usually glabrate and sparsely punctate or coarsely, separately punctate ( Fig. 51–53 View Figures 51–56 ; 57–61 View Figures 57–61 ). The antennae of S. verticalis and S. castaneipennis are bicolored and antennomeres III–V are glabrous and sparsely punctate except apically darker and more densely punctate and pubescent ( Fig. 57–58 View Figures 57–61 ). In S. solitaria , which is concolorous (usually all black or reddish-brown) the antennomeres III–V are more densely, uniformly punctate throughout except for the basal half of the third antennomere, which is less densely punctate ( Fig. 60 View Figures 57–61 ). In the holotype female of S. superba the antennomeres are black to dark brown with scape to the fourth antennomere sparsely punctate, and densely, finely punctate from antennomere V to XI ( Fig. 61 View Figures 57–61 ).
Other commonalities between these four species of Stenaspis include the elongate triangular scutellum which is narrowly acute at the apex (i.e., length ≥ width) and the elytra which are distinctly margined laterally and the apices unarmed as in Crioprosopus . The lateral margination of each elytron usually ends just prior to reaching the apex, and the elytra are obliquely truncate or rounded apically.
Herein, the genus Stenaspis is characterized as follows: (1) head with frons large, square, perpendicular, abruptly separated from anteocular space ( Fig. 1–6 View Figures 1–12 ); (2) dorsum of scape without excavated or impressed area in basal half ( Fig. 51–53 View Figures 51–56 ); (3) prosternal intercoxal process protuberant and ridged between coxae and subvertical to vertical or concave behind ( Fig. 7–9 View Figures 1–12 ); (4) mesosternal intercoxal process not protuberant, about level with top of coxae and abruptly declivous in front ( Fig. 7–9 View Figures 1–12 ); (5) pronotum narrower than base of elytra at humeri with sides rounded, angulate or frequently tuberculate slightly behind middle, and anterior angles rounded or broadly callused ( Fig. 15–21 View Figures 15–23 , 24–30 View Figures 24–32 , 33, 36, 41 View Figures 33–41 ); (6) the area between the five discal calli on the pronotum almost flat, usually glabrate, and dorsal calli usually absent or vague (e.g., Fig. 15–16, 21–22 View Figures 15–23 , 24, 30–31 View Figures 24–32 , 33–34, 36–37 View Figures 33–41 ); (7) hind tarsi with tarsomeres triangular, explanate, first tarsomere slightly shorter than following two together ( Fig. 62–65 View Figures 62–68 ); (8) males with proepisternum and prosternum finely, densely punctate, and proepisternum inflated and clearly demarcated from the coarsely punctate pronotal disc ( Fig. 16, 19 View Figures 15–23 , 25, 28 View Figures 24–32 , 34 View Figures 33–41 ), and prosternum with densely punctate transverse subrectangular area on each side of middle ( Fig. 17a, 20 View Figures 15–23 , 26, 29a View Figures 24–32 , 35a View Figures 33–41 ); (9) females with sides of pronotum laterally tuberculate behind middle ( Fig. 21 View Figures 15–23 , 30 View Figures 24–32 , 36, 39 View Figures 33–41 ) and coarsely punctate with punctures merging with coarse dorsal punctures ( Fig. 22 View Figures 15–23 , 31 View Figures 24–32 , 37, 40 View Figures 33–41 ), proepisternum not clearly demarcated from the pronotal disc as found in males, and anterior angles frequently broadly or obtusely callused ( Fig. 21a View Figures 15–23 , 30a View Figures 24–32 , 36a, 41 View Figures 33–41 ); and (10) elytra distinctly margined laterally, and apices of elytra unarmed and truncate or rounded at sutural angle ( Fig. 10–12 View Figures 1–12 ).
Crioprosopus and Callistochroma can be differentiated from Stenaspis by the prosternal intercoxal process that is arcuate at the apex ( Fig. 48–50 View Figures 42–50 ), dorsum of scape with basal half excavate ( Fig. 54–56 View Figures 51–56 ), tarsomeres of hind
62 63 64 65 66 67 68 tarsi narrow and elongate, and first tarsomere subequal to the following two tarsomeres together ( Fig. 66–68 View Figures 62–68 ). Frequently, the last tarsomere with the claw (e.g., Crioprosopus amoenus Jordan, Crioprosopus chiriquiensis Eya and Crioprosopus servillei Audinet-Serville ) is much more elongated than in Stenaspis . The prosternal intercoxal process of Stenaspis is protuberant or ridged behind the coxae and vertical or concave behind ( Fig. 42–47 View Figures 42–50 ), the scape is without a dorsal excavation ( Fig. 51–53 View Figures 51–56 ), and the hind tarsomeres are triangular and explanate with the first tarsomere slightly shorter than tarsomeres two and three together ( Fig. 62–65 View Figures 62–68 ). In general, the integument of Crioprosopus and Callistochroma is more glabrous and the punctures are finer than in Stenaspis . Also, the dorsum of antennomeres III–V is frequently canaliculate or impressed in many species of Crioprosopus (i.e., C. amoenus , C. chiriquiensis and C. servillei , and in males of Crioprosopus thoracicus (White) (i.e., Crioprosopus basileus Bates ), Crioprosopus championi Bates , Crioprosopus gaumeri Bates , Crioprosopus hondurensis Eya , Crioprosopus iridescens White , Crioprosopus nieti Chevrolat , Crioprosopus rimosus (Buquet) and Crioprosopus wappesi Eya ) ( Fig. 158–160 View Figures 155–160 ), while such sculpturing of antennomeres III–V is not found in Stenaspis .
Etymology of the name Stenaspis is Greek: στενὸς, narrow and άσπίς, shield referring to the elongated scutellum as described by Audinet-Serville (1834: 51) and Tavakilian (2020).
Key to Group III-Stenapses with Abruptly Separated Anteocular Space (partially adapted from Linsley 1962: 91 and modified from Eya 2015: 360)
1. Elytra distinctly margined at sides ( Fig. 69–74 View Figures 69–77 )................................................ 2
– Elytra obtusely margined at sides ( Fig. 75–77 View Figures 69–77 )................................................. 6
2(1). Dorsal surface of head, pronotum and elytra densely, coarsely, contiguously punctate (e.g., Fig. 13–14 View Figures 13–14 )... 3
– Dorsum glabrate, if pronotum or head densely punctate then elytra glabrous or rugulose, punctures fine and well separated..................................................................... 4
3(2). Elytral disc, femora, tibiae and ventral surface covered with long, erect, pale hairs, dorsal surface shining; ventral surface of femora and tibia densely, deeply punctate; lateral spine on pronotum obtuse; lateral margin of pronotum and elytra narrowly reddish; 12–24 mm ( Fig. 13–14 View Figures 13–14 )....... Pilostenaspis Eya View in CoL
– Elytra without long erect hairs (or with very short hairs obscurely covering surface); femora and tibiae finely punctate, not covered with long, erect hairs, elytra black with broad yellowish or reddish bands or maculae, lateral spine on pronotum prominent, acute, recurved; 26–36 mm ............................................................................. Megapurpuricenus Eya View in CoL
4(2). Basal half of scape excavate or scarred; prosternum with intercoxal process arcuate at apex; tarsomeres of hind tarsi elongate, narrow, 1 st tarsomere subequal in length to following two together ( Fig. 54–56 View Figures 51–56 , 48–50 View Figures 42–50 ; 66–68 View Figures 62–68 )......................................................................... 5
– Basal half of scape not excavate; prosternum with intercoxal process protuberant or ridged between coxae, vertical or concave behind; mesosternum not protuberant, about level with top of coxae; tarsomeres of hind tarsi short, explanate, 1 st tarsomere shorter than following two together ( Fig. 51–53 View Figures 51–56 , 42–47 View Figures 42–50 , 62–65 View Figures 62–68 ).................................................. Stenaspis Audinet-Serville View in CoL
5(4). Mesosternal intercoxal process obtusely tuberculate and extending well below the plane of prosternal intercoxal process ( Fig. 50b View Figures 42–50 ); prothoracic sculpture of male usually confined to limited area towards anterior angle of disc ( Fig. 56 View Figures 51–56 ); apices of elytra obliquely angulate, with outer armature, sutural angle rounded or acute; antennae with dorsal surfaces of antennomeres III–IV flattened but not canaliculate; abdominal segments usually glabrous............................... Callistochroma Eya View in CoL
– Mesosternal intercoxal process without obvious projections or subtuberculiform ( Fig. 48b, 49b View Figures 42–50 ); prothoracic sculpture of male with disc finely, densely punctate throughout ( Fig. 158–160 View Figures 155–160 ); apices of elytra rounded or subtruncate, with or without outer armature; antennae with dorsal surfaces of antennomeres III–V frequently canaliculate from apical 2/3 of III; abdominal segments usually densely clothed with silken pubescence............................... Crioprosopus Audinet-Serville View in CoL
6(1). Pronotum armed with a lateral spine......................................................... 7
– Pronotum subcylindrical or rounded, without lateral spine; elytra with a pair of transverse yellowish bands; antennal tubercles prominent, vertex distinctly concave; body finely pubescent.................................................................... Aethecerinus Fall and Cockerell View in CoL
7(6). Body glabrate, dorsum opaque, elytra coarsely punctate at base................ Purpuricenus Dejean View in CoL
– Body pubescent, elytra sulcate or with distinct threadlike costae......... Tragidion Audinet-Serville View in CoL
Arnett RHJ. 1962. The beetles of the United States (A manual for identification). The Catholic University of America Press; Washington, DC. xi + 1112 p.
Audinet-Serville J-G. 1834. Nouvelle classification de la famille des longicornes (Suite). Annales de la Societe Entomologique de France (3) 1: 5 - 110.
Aurivillius C. 1912. Cerambycidae: Cerambycinae. p. 457 - 458. In: Junk W, Schenkling S. (eds.). Coleopterorum Catalogus pars 39 [Vol. 22]. W. Junk; Berlin. 574 p.
Bates HW, 1880. Longicornia. p. 1 - 224, pls. I - XV. In: Bates HW, Sharp D. Biologia Centrali-Americana. Insecta, Coleoptera, Vol. 5. Porter RH.; London. xii + 526 p. + 26 pl.
Blanchard CE. 1845. Histoire des insectes, traitant de leurs moeurs et de leurs metamorphoses en general, et comprenant une nouvelle classification fondee sur leurs rapports naturels. Libraire de Didot Freres; Paris. 2: 1 - 524.
Bradley JC. 1930. A manual of the genera of beetles of America north of Mexico. Daw, Illston and Co.; Ithaca, NY. 360 p.
Casey TL. 1912. Studies in the Longicornia of North America. Memoirs on the Coleoptera 3: 215 - 376.
Castelnau L (Le Comte de). 1840. Histoire Naturelle des Insectes Coleopteres. Dumenil P. Paris. 2: 1 - 563, 36 pl.
Chenu JC. 1870. Coleopteres buprestiens, scarabeiens, pimeliens, curculioniens, scolytiens, chrysomeliens, etc. p. 1 - 360. In: Buffon, Daubenton, Lacepede, Cuvier G, Cuvier F, Saint-Hilaire G, Latreille, de Jussieu, Brongniart, etc., etc. (eds.). Encyclopedie d'histoire naturelle ou traite complet de cette science d'apres les travaux des naturalistes les plus eminents de tous les pays et de toutes les epoques. Vol. 7. Librairie de Firmin-Diodot et Cie; Paris 1884: vi + 360 p., 296 fig., 48 pl.
Dupont H. 1838. Monographie des trachyderides de la famille des longicornes. Magasin de Zoologie 8: i - xiii + 1 - 28, pl. 186 - 200 and p. 29 - 59, pl. 204 - 224.
Eya BK. 2015. Revision of the Genus Crioprosopus Audinet-Serville, and description of three new genera of Trachyderini (Coleoptera: Cerambycidae: Cerambycinae). Zootaxa 3914 (4): 351 - 405.
Gemminger M, von Harold E. 1872. Catalogus coleopterorum hucusque descriptorum synonymicus et systematicus. Sumptu E. H. Gummi (G. Beck) Monachii; Paris. Tom. 9: 2669 - 2988.
Lacordaire JT. 1869. Histoire naturelle des insectes. Genera des coleopteres ou expose methodique et critique de tous les genres proposes jusqu'ici dans cet ordre d'insectes. Famille des longicornes (suite). Librairie Encyclopedique de Roret; Paris. 9 (1): 1 - 409.
LeConte JL. 1850. An attempt to classify the Longicorn Coleoptera of the part of America North of Mexico. Journal of the Academy of Natural Sciences of Philadelphia (2) 2: 5 - 38.
LeConte JL. 1854. Descriptions of some new Coleoptera from Texas, chiefly collected by the Mexican Boundary Commission. Proceedings of the Academy of Natural Sciences of Philadelphia 6: 439 - 448.
LeConte JL. 1873 a. Classification of the Coleoptera of North America. Prepared for the Smithsonian Institution. Part II. Smithsonian Miscellaneous Collections 11 (265): 279 - 348.
LeConte JL, Horn GH. 1883. Classification of the Coleoptera of North America. Prepared for the Smithsonian Institution. Smithsonian Miscellaneous Collections 26 (507): i - xxvii + 567 p.
Leng CW. 1886. Synopses of Cerambycidae. Entomologica Americana 2 (3): 60 - 63, 2 pl.
Monne MA. 2005. Catalogue of the Cerambycidae (Coleoptera) of the Neotropical Region. Part I. Subfamily Cerambycinae. Zootaxa 946: 1 - 765.
Monne MA. 2012. Catalogue of the type-species of the genera of the Cerambycidae, Disteniidae, Oxypeltidae and Vesperidae (Coleoptera) of the Neotropical Region. Zootaxa 3213: 1 - 183.
Strauch A. 1861. Catalogue systematique de tous les Coleopteres decrits dans les Annales de la Societe Entomologique de France depuis 1832 jusqu'a 1859. Schmidt HW, Libraire-Editeur; Halle. i - iv + 160 p.
Tavakilian G. 2020. Base de donnees Titan sur les Cerambycides ou Longicornes. Institut de Recherche pour le Developpement; France. Webmaster: H. Chevillotte. Available at http: // madbif. mg / titan / aff _ genre. php (Last accessed October 2020).
Zajciw D. 1960. Novos Longicorneos Neotropicos (Col., Cerambycidae) - II. Revista Brasileira de Entomologia 9: 129 - 149, 9 fig.
Zajciw D. 1961. Novos Stenaspini (Col., Cerambycidae, Cerambycinae) neotropicos. Anais da Academia Brasileira de Ciencias 33 (3 - 4): 399 - 407, 6 fig.
Figures 1–12. Characteristics of the head, sternum and elytra of Stenaspis. 1–3) S. veritcalis, male, Puebla, MEX. 4–6) S. verticalis, female, Jalisco, MEX. 7, 10) S. castaneipennis, male, Oaxaca, MEX. 8, 11) S. solitaria, male Cochise Co., AZ, USA). 9, 12) S. verticalis, male, Nuevo Leon, MEX. 1–6) Characteristics of the head, i.e “frons large, square, perpendicular, abruptly separated from anteocular space,” 1, 4) Gena large, quadrate; mandibles well separated from lower eye lobe. 2: 2a; 5: 5a) Dorsal anterior margins of genae ridged. 2: 2b; 5: 5b) Frons perpendicular to vertex and recessed between “anteocular space” (i.e., recessed between the dorsal anterior margins of genae). 3, 6) Apex of mandibles acute, simple. 7–9: 7a, 8a, 9a) Prosternal intercoxal process with apex protuberant, ridged between coxae, abruptly declivous behind. 7–9: 7b, 8b, 9b) Mesosternum not protuberant, level with coxae. 10–12) Elytra with apices unarmed.
Figures 15–23. Pronotal and prosternal characteristic of Stenaspis verticalis: 15–17) S. verticalis, male, Jalisco, MEX with sides of pronotum rounded. 15) Disc coarsely punctate with 5 vague calli (red arrows). 16: 16a) Proepisternum inflated and finely punctate (dark reddish area). 16: 16b) Demarcation of proepisternal finely punctate area from coarsely punctate dorsum of disc. 17: 17a) Subrectangular impressed area. 17: 17b) Vague demarcation dividing proepisternum from the subrectangular impressed area. 18, 20) S. verticalis, male, Nuevo Leon, MEX with side of pronotum with distinct lateral tubercles. 19) S. verticalis, male, Puebla, MEX with proepisternum inflated and finely punctate clearly demarcated from dorsum. 21–22) S. verticalis, female, Guerrero, MEX with side of pronotum distinctly tuberculate. 21: 21a) Anterior angles obtusely tuberculate. 22) Pronotal disc and proepisternum coarsely punctate. 23) S. verticalis, female, Jalisco, MEX with prosternum coarsely punctate.
Figures 24–32. Pronotal and prosternal characteristic of Stenaspis castaneipennis. 24–26) S. castaneipennis, male, Oaxaca, MEX with sides of pronotum rounded, disc coarsely punctate with 5 vague calli. 25) Proepisternum inflated and finely punctate (dark reddish area) clearly demarcated from coarsely punctate dorsum (black area). 27–29) S. castaneipennis, male, Oaxaca, MEX with sides of pronotum with distinct lateral tubercles. 28) Proepisternum inflated and finely punctate (reddish area) clearly demarcated from dorsum (black area). 29: 29a) Subrectangular finely punctate area of prosternum. 29: 29b) Demarcation line dividing proepisternum from finely punctate subrectangular area of prosternum. 30–32) S. castaneipennis, female, Oaxaca, MEX with side of pronotum distinctly tuberculate. 30: 30a) Anterior angles obtusely tuberculate. 31–32) Pronotal disc, proepisternum and prosternum coarsely punctate; proepisternun not demarcated from dorsum and prosternum.
Figures 33–41. Pronotal and prosternal characteristic of Stenaspis solitaria and Stenaspis superba. 33–35) S. solitaria, male, Cochise Co., AZ, USA with disc coarsely punctate with 5 vague calli (yellow arrows). 33: 33a) Pronotum with prominent lateral tubercle. 33: 33b) Rounded anterior angles, which does not extend and merge over lateral tubercles. 34: 34a) Proepisternum finely punctate. 35: 35a) Prosternum with finely densely punctate transverse subrectangular area on each side of middle. 36–38) S. solitaria, female, Cochise Co., AZ, USA 36: 36a) Anterior angle of pronotum obtusely callused and obliquely tapering to the apex of pronotum. 37) Coarsely punctate proepisternum of female. 38) Coarsely punctate prosternum of female. 39–41) S. superba, holotype, female, Mojos, BOL (photos provided from J. Bergsten, NHRS). 39) Coarsely punctate pronotal disc with two prominent discal calli in anterior half. 40) Convex pronotal disc with coarsely punctate proepisternum. 41) Coarsely punctate prosternum and broadly callused anterior angle of S. superba.
Figures 42–50. Prosternal and mesosternal intercoxal process of Stenaspis compared to Crioprosopus and Callistochroma. 42–43) S. verticalis, male, Jalisco, MEX with uniformly sloping prosternal intercoxal process to apex that is abruptly declivous behind. 42) Lateral profile. 43) lateral-tilted profile showing gradual sloping of intercoxal process to apex. 44–45) S. verticalis, male, Puebla, MEX with prosternal intercoxal process concaved and abruptly acuminate apex. 44) Lateral profile. 45) Lateral-tilted profile showing inverted “U-shaped” sloping of intercoxal process to apex. 46–47 S. castaneipennis, male, Oaxaca, MEX with prosternal intercoxal process gradually sloping and then abruptly acuminate at apex. 46) Lateral profile. 47) Lateral-tilted profile showing gradual sloping and abrupt acumination at apex. 42–47 Mesosternal intercoxal process not protuberant, about level with coxae, and abruptly declivous in front. 48) Crioprosopus servillei Audinet-Serville, female, Tegucigalpa, HND. 48: 48a) Prosternal intercoxal process arcuate at apex. 48: 48b) Mesosternal intercoxal process without obvious projection below coxal cavity. 49) Crioprosopus wappesi Eya, male, Baja Verapaz, GTM. 49: 49a) Prosternal intercoxal process arcuate at apex. 49: 49b) Mesosternal intercoxal process level with coxae. 50) Callistochroma lampros (Bates), male, Panama Prov., PAN. 50: 50a) Prosternal intercoxal process arcuate at apex. 50: 50b) Mesosternal intercoxal process protuberant, obtusely tuberculate and extending well below plane of prosternal intercoxal process.
Figures 51–56. Scape of Stenaspis compared to Crioprosopus and Callistochroma. 51) Stenaspis verticalis, male, Puebla, MEX. 52) S. solitaria, male, Cochise Co., AZ, USA. 53) S. castaneipennis, male, Oaxaca, MEX. 54) Crioprosopus servillei, female, Tegucigalpa, HND. 55) Crioprosopus baldwini, female, Chiriquí Prov., PAN. 56) Callistochroma lampros, male, Panama Prov., PAN. 51–53) Scape of Stenaspis without excavated area in basal half. 51–52) Scape glabrate, sparsely punctate. 53) Scape coarsely, separately punctate. 54–56) Scapes of Crioprosopus and Callistochroma with excavated area in basal half.
Figures 57–61. Antennae of Stenaspis. 57) S. verticalis, male, Nuevo Leon, MEX. 58) S. castaneipennis, male, Oaxaca, MEX. 59) S. lingafelteri sp. nov., holotype, male, Chiapas, MEX. 60) S. solitaria, male, Cochise Co., AZ, USA. 61) S. superba, holotype, female, Mojos, BOL. 57–59) Antennae bicolored, antennomeres III–V, glabrate, sparsely punctate except apically darker, densely punctate and pubescent. 60) Antennomeres concolorous, densely punctate except basal half of III sparsely punctate. 61) Antennomeres black to brown, scape glabrate, sparsely punctate, scape to antennomere IV sparsely punctate.
Figures 62–68. Hind tarsomeres of Stenaspis compared to Callistochroma and Crioprosopus with red brackets showing lengths of tarsomere 1 (T1) and tarsomeres 2+3 (T2+3). 62–65) Hind tarsi of Stenaspis with tarsomeres triangular, explanate, T1 slightly shorter than following two together (T 2+3). Approximate ratio of length of tarsomere 1 over tarsomeres 2+3 (T1/T2+3) are as follows: 62) Stenaspis castaneipennis, female, Oaxaca, MEX (T1/T2+3: 1/1.3); 63) S. verticalis, female, Nuevo Leon, MEX (T 1 /T 2+3: 1/1.4); 64) S. solitaria, female, Cochise Co., AZ, USA (T1/T2+3: 1/1.3); and 65) S. lingafelteri sp. nov., holotype, male, Chiapas, MEX (T1/T2+3: 1/1.4). 66–68) Hind tarsi of Callistochroma and Crioprosopus with 1st tarsomere narrow, elongate, and subequal in length to following two tarsomeres together. Approximate ratios of T1 /T 2+3 are as follows: 66) Callistochroma viridipennis, male, Esmeraldas Prov., ECU (T1/T2+3: 1/1.1); 67) Crioprosopus chiriquiensis, female, Chiriquí Prov., PAN (T1/T2+3: 1/0.9); and 68) Crioprosopus rimosus, female, Hidalgo, TX, USA (T 1 /T 2+3: 1/1.1).
Figures 155–160. Images of dorsum and antennomeres I–IV of Crioprosopus. 155) Crioprosopus plagiatus (Waterhouse), male, holotype, Guatemala City, GTM (BMNH) (Bezark 2020, id:18552). 156) C. wappesi, male, Baja Verapaz, GTM. 157) C. nieti, male, Veracruz, MEX. 158) C. plagiatus with impressed or canaliculate basal half of scape and canaliculate dorsum of antennomeres III–IV (red arrows) (Bezark 2020, id: 18553). 159–160) C. wappesi and C. nieti with canaliculated scape and dorsum of antennomeres III–IV (red arrows) as found in C. plagiatus. 160) C. nieti used as an example to show arrangement of glabrate maculae on pronotal disc (yellow arrows) as found in C. plagiatus and C. wappesi. 160: a) Black glabrate macula at middle post-medially. 160: b–d) Six black, glabrate maculae on either side. 160: b) Antemedial pair. 160: c) Postmedial pair on each side of middle macula (160a). 160: d) Pair of maculae on each side in a depressed area adjacent to lateral tubercles.
Figures 69–77. Epipleural margin of elytra of Group III-Stenaspes. 69–74) Elytra distinctly margined at side with acute lateral epipleural margin below humerus (red arrow) in Stenaspis, Callistochroma and Crioprosopus. 69) Stenaspis castaneipennis, male, Oaxaca, MEX. 70) S. lingafelteri sp. nov., holotype, male, Chiapas, MEX. 71) Callistochroma viridipennis, male, Esmeraldas, ECU. 72) Crioprosopus chiriquiensis, female, Chiriquí Prov., PAN. 73) Crioprosopus rimosus, female, Hidalgo, TX, USA. 74) Callistochroma lampros, male, Panama Prov., PAN. 75–77) Elytra obtusely margined at side without acute lateral margin (red arrows) as in Aethecerinus, Purpuricenus and Tragidion. 75) Epipleural margin of elytra broadly rounded below humerus in Aethecerinus wilsoni, female, Bexar Co., TX, USA. 76) Epipleural margin narrowly rounded in Purpuricenus linsleyi, male, Comal Co., TX, USA. 77) Epipleural margin obtusely angulated in Tragidion coquus, male, Terrell Co., TX, USA.
Figures 13–14. Prosternal and mesosternal intercoxal process of Pilostenaspis. 13) Pilostenaspis lateralis (LeConte, 1884), female, dorsum, Pecos Co., TX, USA). 13: 13a–13b) P. lateralis, prosternal intercoxal process cuneiform, resembling Stenaspis. 13: 13c–13d) P. lateralis, mesosternal intercoxal process. 14) Pilostenaspis pilosella (Bates, 1892), female, dorsum, Mexico D.F., MEX. 14: 14a–14b) P. pilosella, prosternal intercoxal process convex, resembling Crioprosopus 14: 14c–14d) P. pilosella, mesosternal intercoxal process.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
Stenaspis Audinet-Serville, 1834
| Eya, Bryan K. 2021 |
Smileceras
| LeConte JL 1850: 8 |
Stenaspis
| Eya BK 2015: 361 |
| Monne MA 2012: 62 |
| Monne MA 2005: 642 |
| Arnett RHJ 1962: 863 |
| Zajciw D. 1961: 401 |
| Zajciw D. 1960: 144 |
| Bradley JC 1930: 241 |
| Casey TL 1912: 318 |
| Aurivillius C. 1912: 458 |
| Leng CW 1886: 60 |
| LeConte JL & Horn GH 1883: 299 |
| Bates HW 1880: 76 |
| LeConte JL 1873: 314 |
| Gemminger M & von Harold E. 1872: 2967 |
| Chenu JC 1870: 311 |
| Lacordaire JT 1869: 171 |
| Strauch A. 1861: 127 |
| LeConte JL 1854: 441 |
| Blanchard CE 1845: 145 |
| Castelnau L & Le Comte 1840: 419 |
| Dupont H. 1838: 50 |
| Audinet-Serville J-G. 1834: 51 |