Microgobius urraca, Tornabene, Luke, Van, James L. & Robertson, Ross, 2012
|
publication ID |
https://doi.org/ 10.5281/zenodo.212312 |
|
DOI |
https://doi.org/10.5281/zenodo.5671664 |
|
persistent identifier |
https://treatment.plazi.org/id/4021879B-5C52-EE21-FF44-953DFEEEFE96 |
|
treatment provided by |
Plazi |
|
scientific name |
Microgobius urraca |
| status |
sp. nov. |
Microgobius urraca View in CoL sp. nov.
Dark-finned sand goby (Figures 1,2)
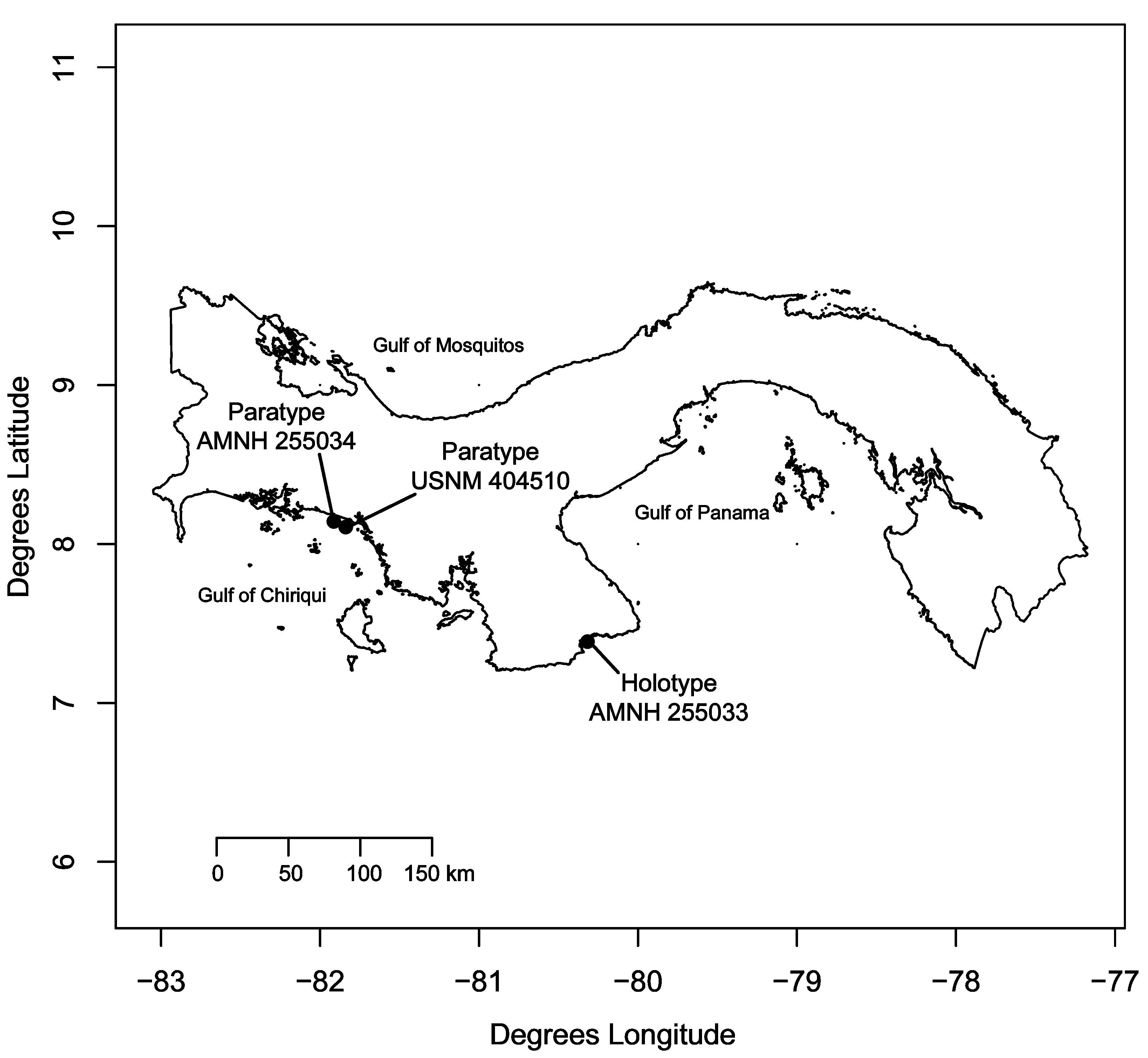
Material examined: Holotype: AMNH 255033, field number JVT-03-238, 69.4 mm SL, female, east of Isla Raya, Panama (Pacific), 7.3966o, -80.2991o, trawled specimen, collected by J.L. Van Tassell and D.R. Robertson, R/V Urraca , 20 June 2003, 12 m, sand substrate.
Paratypes: AMNH 255034, field number JVT-03-208, GenBank accession number JX139737 View Materials (partial cytochrome c oxidase I sequence), 62.2 mm SL, male, north of Isla Secas near main land, Panama (Pacific), 8.1435o, -81.8775o, trawled specimen, collected by J.L. Van Tassell and D.R. Robertson, R/V Urraca , 16 June 2003, 15- 18 m, sand and leaf litter substrate; USNM 404510, field number JVT-03-210, 59.8 mm SL, male, north of Islas Secas, Panama (Pacific), 8.1135o, -81.8336o, trawled specimen, collected by J.L. Van Tassell and D.R. Robertson, R/V Urraca , 16 June 2003, 20 m, sand and leaf litter substrate.
Diagnosis. The new species is morphologically similar to M. erectus , but differs from that species in possessing fewer lateral scale rows, lacking a patch of ctenoid scales under the pectoral fin, having unpigmented epaxial myosepta, a distinct oval-shaped dark blotch on the first dorsal fin, and possessing three blue-white stripes on a dark caudal fin. Differences in both the number and type of scales, dorsal and anal fin ray counts, the poor development of a fleshy dorsal crest, and overall color pattern further distinguish this species from the remaining eastern Pacific congeners.
Description. Morphometric data and summary of meristics given in Table 1. Counts of the holotype indicated by an asterisk, followed by number of specimens with each count in parentheses.
Median and paired fins: first dorsal VII*(3); first dorsal spines not highly filamentous, free tips of spines extending only a short distance beyond interspinal membrane; second dorsal I,14*(3); anal I,14*(3); second dorsal and anal fin rays extending slightly posterior to origin of caudal fin rays when laid flat; pectoral rays 21(1), 22*(1), 23(1); pectoral fin length 17.3–20.2 % SL; caudal fin long and lanceolate, 35.3–40.0 % SL; segmented caudal rays 17*(2); branched caudal rays 15*(2); pelvic fin I,5*(3); pelvic fins united to form oval-shaped disk with welldeveloped frenum; pelvic frenum with smooth posterior margin.
Scales: trunk covered with cycloid scales; predorsal region, cheek, operculum, pectoral fin base and pelvic fin base without scales; pre-anal region with small embedded cycloid scales; scales on anterior and ventral portions of trunk small, partially imbedded, and in irregular rows, becoming larger and arranged in distinct rows dorsally and posteriorly; no small patch of ctenoid scales beneath pectoral fin; lateral scale rows 67*(1), 75(1), 76(1); transverse scale rows 15*(1), 17(1), 18 (1).
ABLE 1. Microgobius urraca morphometrics and counts. Measurements are in % SL.
Holotype Paratype Paratype
Catalog Number AMNH 255033 AMNH 255034 USNM 404510 Field number JVT-03-238 JVT-03-208 JVT-03-210 Sex female male male SL (mm) 69.4 62.2 59.8 Eye diameter 6.23 6.27 6.05 Upper jaw length 10.2 12.86 12.2 Head length 22.1 23.33 22.4 Post orbital length 10.95 10.93 11.2 Depth at DI origin 15.85 15.51 16.3 Least caudal peduncle depth 8.07 8.84 7.85 Pectoral fin length 18.7 17.25 20.2 Caudal fin length 35.3 38.7 40
Snout length 4.7 5.86 5.2
Interorbital width 3.2 3.4 3.7
Patch of ctenoid scales under pectoral fin absent absent absent
Head: head length 22.1–23.3 % SL; mouth angled upwards at approximately 45–55 from horizontal; upper jaw length 10.2–12.9 % SL; teeth in upper jaw in two to three rows near symphysis, becoming two distinct rows near midpoint of premaxilla, terminating as single row near end of upper jaw; teeth in lower jaw two to three rows anteriorly near symphysis, becoming a single row near midway of dentary; teeth in outer row of both jaws enlarged, elongate and with slightly curved canines; tongue bilobed with deep medial notch; eye diameter 6.1–6.3 % SL; interorbital width 3.2–3.7 % SL; snout short, 4.7–5.9 % SL; low fleshy dorsal crest anterior to first dorsal fin in both sexes; anterior nare on short erect tube; posterior nare small, adjacent to head pore B’; gill rakers 5+16; epibranchials present.
Genitalia: female papillae unpigmented, short and bulbous; papilla in males unpigmented, slightly elongate and conical.
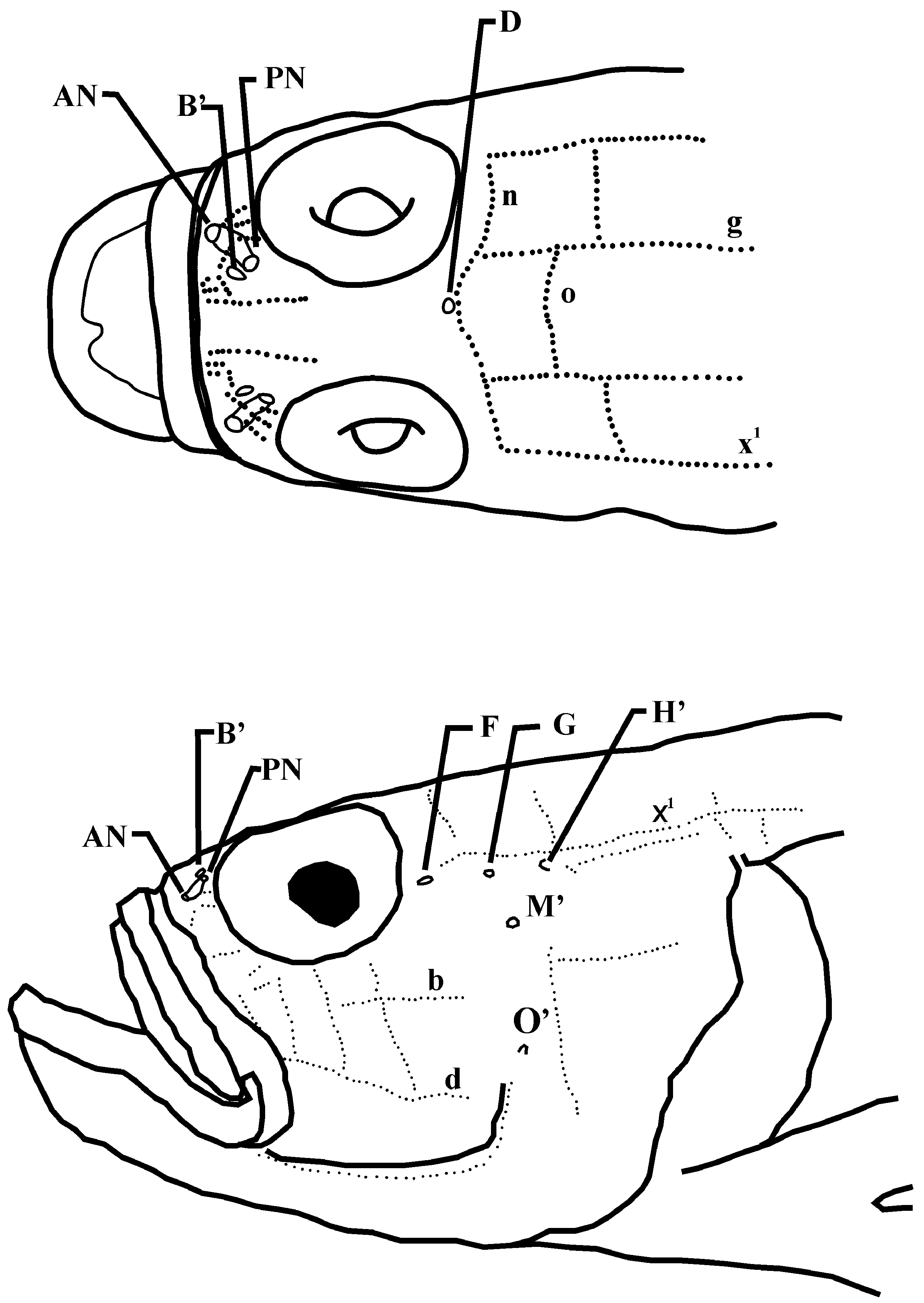
Sensory papillae and head pores ( Figure 2 View FIGURE 2 ): two interorbital canals anteriorly, joining posteriorly to become single interorbital canal at point in line with midpoint of eye; oculoscapular pores B’, D(s), F, G, H’ present; posterior canal (typically between pores K’ and L’) absent; preopercular pores M’, O’ present; sensory papillae on head in transverse pattern with three vertical rows below eye; transverse papillae row b beginning at vertical through midpoint of pupil, extending posteriorly, ending well short of posterior margin of preopercle; transverse papillae row d long, beginning anterior one third ofeye, extending posteriorly, ending well short of posterior margin of preopercle; papillae row n continuous across dorsal midline; papillae row x1 continuous from above pore F to above base of pectoral fin.
Vertebral skeleton: precaudal vertebrae 11; caudal vertebrae (including terminal element) 16; dorsal fin pterygiophore formula 3(221110); two anal fin pterygiophores preceding first haemal arch.
Pigmentation (based on freshly collected specimens unless otherwise noted): head brownish grey, with an indistinct silvery blotch at the lower corner of the operculum; iris creamy yellow; body uniformly light brownishgrey (pale grey to yellow in preservation); anterior half of trunk with four or five narrow, wavy, diffuse pale vertical “ Y ” shaped bars; epaxial myosepta unpigmented; first dorsal fin with a light grey-brown base, pale grey outer half, those two color areas separated by a conspicuous, horizontal oval blackish blotch across the center of the fifth to seventh spines; second dorsal fin darker posteriorly than anteriorly, with a dark grey base, grey-brown distal half, those two zones separated by a narrow blue-white stripe (stripe not apparent in preservation); anterior third of anal fin dark grey basal on basal quarter and paler grey on distal three-quarters; the posterior two-thirds of the fin is uniformly dark grey; caudal fin very dark, nearly black, with two blue-white stripes along its entire length above midline and one blue-white stripe below midline; pelvic and pectoral fins uniformly light grey; abdominal region pale; prepelvic region lightly pigmented with scattered melanophores; gular region pale.
Habitat. Microgobius urraca was collected in depths ranging from 12–20 m over sand or sand and leaf litter bottoms. In collections where leaf litter was present this species was collected with several specimens of Ptereleotris carinata and M. erectus . Microgobius urraca may be a burrowing species (see Discussion section below).
Distribution. Known only from the Pacific coast of western Panama ( Figure 3 View FIGURE 3 )
Etymology. The specific epithet urraca is in reference to the Smithsonian Tropical Research Institute’s research vessel the Urraca , which served the institute between 1994–2007. Microgobius urraca was collected on one of the many expeditions throughout the tropical eastern Pacific and Caribbean by the R/V Urraca , which contributed a wealth of information on fish diversity in the tropical Americas. The species name is to be treated as a noun in apposition. The common name “dark-finned sand goby is given in reference to the greyish-black second dorsal, anal and caudal fins and the sandy habitat over which it occurs.
Comparisons. Microgobius urraca differs from all other eastern Pacific species of Microgobius except M. erectus in having counts of I, 14 in both the second dorsal and anal fins (all other species typically have ≥ 15 rays in the second dorsal and anal fins). Microgobius urraca is most similar morphologically to M. erectus , but differs in possessing a higher lateral scale count (67–76 vs ≤ 48 in M. erectus ), scales entirely cycloid and not easily lost, a dark blotch on the first dorsal fin (vs a pale/dusky fin with dark distal margin in female M. erectus ), and in having unpigmented epaxial myosepta (darkly pigmented in preserved specimens of M. erectus ). The absence of ctenoid scales under the pectoral fin further distinguishes M. urraca from M. erectus , M. cyclolepis , M. curtus , M. crocatus , M. miraflorensis , and M. tabogensis . Like most species of Microgobius , M. urraca can also be distinguished from its congeners by color patterns in life. No other species of Microgobius possesses a prominent sooty blotch on the first dorsal fin, dark grey anal and second dorsal fins, and a blackish caudal fin with three blue-white stripes. Microgobius urraca replaces M. brevispinis as the largest species in the genus (69.4 mm SL vs 64.6 mm SL, maximum size of M. brevispinis , SIO62-719)
Discussion. Birdsong (1981) reported M. erectus from mud and broken shell bottoms from 15– 30 m. Many M. erectus were collected from the 2003 Urraca Panama expedition, several of which were collected with M. urraca in a trawl over sand and leaf litter (field number JVT-03-210, AMNH 255301 and field number JVT-03-209, AMHH 255297) and others were collected over sand and mud. Nearly all M. erectus specimens from these collections had lost most of their scales and fins were severely damaged. The ease at which scales were lost and the delicate fin membranes of M. erectus led Birdsong (1981) to conclude that M. erectus , unlike many other Microgobius species, likely did not burrow. Although the specimens of M. erectus collected in 2003 were heavily damaged, the specimens of M. urraca from the same trawls had not lost scales and fins were more or less intact. Following Birdsong’s rationale, it is possible that the partially imbedded nature of the scales and the integrity of the fins in M. urraca indicate a burrowing lifestyle. Burrowing behavior may also explain why, despite heavy sampling effort, so few specimens of M. urraca were collected in trawls in comparison to M. erectus , which were common in trawls in the same area.
The phylogenetic relationship between M. urraca , the morphologically similar M. erectus , and other Microgobius species remain unclear. Birdsong (1981) struggled greatly to generate even a broad phylogenetic hypothesis for the genus, noting inconsistent patterns of shared characters across species, difficulty in determining ancestral/derived character states, and complex and contradicting patterns of sexual dimorphism (i.e. female conditions in one species are the male conditions in other species, while yet other species are sexually monomorphic). The only phylogenetic hypothesis to date was a molecular phylogeny of the Gobiosomatini based on mtDNA by Rüber et al. (2003) which contained 6 named (plus one unidentified) Microgobius species. Although more than half of the known named species of Microgobius were missing from this study the relationships shown were statistically robust and can provide a temporary framework for assessing the evolution and distribution of some morphological characters. When several morphological characters (i.e. predominant scale type, presence/ absence of a patch of ctenoid scales under pectoral fin, second dorsal/anal fin counts, filamentous dorsal spines, presence/absence of a dorsal crest, head pores) are mapped onto the phylogeny of Rüber et al. (2003), many character states appear to have either arisen or been lost more than once, to which the overall confusion alluded to by Birdsong (1981) may be attributed. A comprehensive phylogenetic analysis that includes all 15 currently recognized species of Microgobius and incorporates morphological characters and additional molecular data (including nuclear loci) would help clarify the interspecific relationships of this diverse group.
continued. curtus emblematicus brevispinnis
continued.
crocatus miraflorensis tabogensis Ocean Pacific Pacific Pacific Distribution El Salvador to Ecuador West coast southern Baja California West coast of Baja
(Bahia Magdalene), central eastern California(n. Bahia
Gulf of California to northern Peru Magdalena), to Peru
(Puerto Pizarro)
Habitat mangrove slough with estuarine species, mud to silty sand mangrove ares with mud-
muddy detritus bottom bottom sand bottoms continued.
continued.
thalassinus carri gulosus Ocean Atlantic Atlantic Atlantic
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.