Crocidura nigripes, G. S. Miller & Hollister, 1921
|
publication ID |
https://doi.org/ 10.5281/zenodo.6870843 |
|
DOI |
https://doi.org/10.5281/zenodo.6870082 |
|
persistent identifier |
https://treatment.plazi.org/id/3D474A54-A078-8715-FAFA-AC511337FA12 |
|
treatment provided by |
Felipe |
|
scientific name |
Crocidura nigripes |
| status |
|
Black-footed White-toothed Shrew
Crocidura nigripes View in CoL
French: Crocidure a pattes noires / German: SchwarzfulR-Weil 3zahnspitzmaus / Spanish: Musarana de pies negros
Other common names: Black-footed Shrew
Taxonomy. Crocidura nigripes G. S. Miller & Hollister, 1921 View in CoL ,
south-west from Tondano Lake , Temboan , north-eastern Sulawesi, Indonesia.
Unlike the other shrews endemic to Sulawesi, C. mnigripes is phylogenetically unrelated to the “Old Sulawesian” clade and is more closely related to species from the Sunda Shelf, suggesting a relatively recent (perhaps late Pliocene) colonization across the Makassar Strait. Its karyotype,
consisting of 38 oy supports such a recent biogeographic origin, as this formula is shared with most other species from the Sunda Shelf, while other Sulawesian Crocidura have a decreased chromosome count (2n = 30-34). Races differ in size and in a single fixed diagnostic locus over the 32 assayed allozyme loci; such a difference might reflect the large geographic distance separating the samples rather than having true taxonomic implications; there is a possible zone of overlap in east-central Sulawesi, supporting animals of intermediate size. Two subspecies recognized.
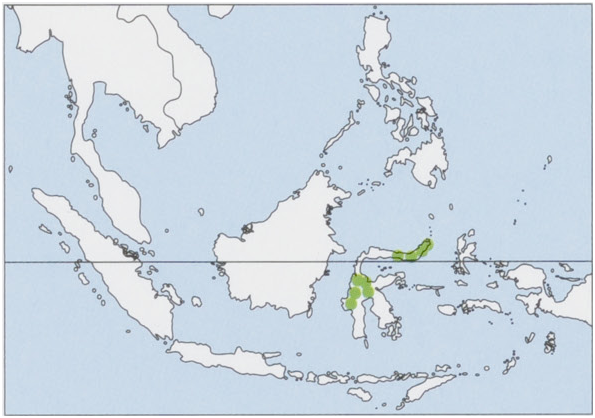
Subspecies and Distribution.
C.n.nigripesG.S.Miller&Hollister,1921—NEpeninsulaofSulawesiandLembehI.
C. n. ipara G. S. Miller & Hollister, 1921 — C Sulawesi. Its absence from the S peninsulas should be ascertained by adequate surveys. View Figure
Descriptive notes. Head—body 72-86 mm, tail 50-65 mm, ear 6 mm, hindfoot 13-1-15-5 mm; weight 8-12 g. The Black-footed White-toothed Shrew is a medium-sized shrew with dark brown to black pelage, but molting individuals show a browner tinge to the fur, particularly on the throat and upper venter, which can be chestnut in color.
Face, ears, feet, and tail also blackish. Tail cylindrical, relatively short (60-80% headbody length) and essentially naked; a few long bristles present only along its basal third. Skull slender with narrow rostral parts and typically parallel tooth rows. Subspecies lipara is distinctly larger, particularly in skull dimensions (mean greatest length of skull 22:9 mm vs. 21-7 mm). Chromosomal complement has 2n = 38 and FN = 56, with submetacentric X and subtelocentric Y chromosomes. Chromosomal complement very similar to those of most other Sundaland Crocidura but differs significantly from those of all other tested species from Sulawesi, stressing a different biogeographic origin of the Black-footed White-toothed Shrew.
Habitat. Lowland evergreen to montane rainforests. Known altitudinal range 30-2200 m. The nominate race apparently occurs exclusively in lowland rainforests; race lipara occupies a much broader elevational range of habitats, up to 2200 m, being most abundant at moderate altitudes (800-900 m).
Food and Feeding. Black-footed White-toothed Shrews feed on invertebrates but no precise data are available.
Breeding. In August, several pregnant females carried 1-2 near-term embryos.
Activity patterns. Black-footed White-toothed Shrews are terrestrial, being found among leaflitter on the forest floor, near logs or dead trees. They are essentially nocturnal or crepuscular.
Movements, Home range and Social organization. Black-footed White-toothed Shrews may be sympatric with all other shrew species present in Sulawesi, but no precise data on their natural history are available.
Status and Conservation. Classified as Least Concern on The IUCN Red List in view of its widespread occurrence in Sulawesi and expected large population size, but its relative tolerance of anthropic changes to its habitat is unknown. The Black-footed White-toothed Shrew is locally common as demonstrated by pitfall trapping. It occurs in protected areas such as Lore Lindu National Park and Bogani Nani Wartabone National Park.
Bibliography. Dubey, Salamin et al. (2008), Esselstyn & Brown (2009), Esselstyn et al. (2009), Hutterer (2005b), Lunde (2008), Miller & Hollister (1921), Musser (1987), Ruedi (1995, 1996), Ruedi & Vogel (1995), Ruedi et al. (1998).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.