Podocoryna loyola, Haddad, Maria Angélica, Bettim, Ariane Lima & Miglietta, Maria Pia, 2014
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3796.3.5 |
|
publication LSID |
lsid:zoobank.org:pub:36C41D4D-CEB4-464F-8F03-CB5EEEFE8593 |
|
DOI |
https://doi.org/10.5281/zenodo.5671967 |
|
persistent identifier |
https://treatment.plazi.org/id/3B3B87D7-FF98-FFFC-87DA-CE22BC89B6EB |
|
treatment provided by |
Plazi |
|
scientific name |
Podocoryna loyola |
| status |
sp. nov. |
Podocoryna loyola View in CoL , n. sp.
(Pl. 1; Table 2)
Holotype: MUZUSP 1907, one fragment of colony (formalin 4%), growing on a polyethylene plate held at the marina of the Paranaguá Yacht Club (25.5S, 48.5W), Brazil, Paraná, Paranaguá, coll. A.L.Bettim, January 11 st, 2012.
Paratypes: MUZUSP 1908, fragment of colony (alcohol 96%), same place as holotype, coll. A.L.Bettim, July 6th, 2012 —MUZUSP 1915, three specimens of medusae (formalin 4%), released in aquarium, from colonies on polyethylene plates held at same place as holotype, coll. A.L.Bettim, September,14th, 2010—MUZUSP 1916 fragment of colony (formalin 4%), same place as holotype, coll. A.L.Bettim, July 6th, 2012 —MUZUSP 1917, three specimens of medusae (formalin 4%), realized in aquarium, from colonies on polyethylene plates held at same place as holotype, coll. A.L.Bettim, January 11 st, 2012—MUZUSP 1918, fragment of colony (alcohol 96%), same place as holotype, coll. A.L.Bettim, August 2nd, 2012 —MUZUSP 1919, fragment of colony (alcohol 96%), same place as holotype, coll. A.L.Bettim, November 21st, 2012 —MUZUSP 1920, fragment of colony (formalin 4%), same place as holotype, coll. M.A.Haddad, October 24th, 2012 —MUZUSP 1921, three medusae (alcohol 96%), released from colonies of same place as holotype, coll. A.L.Bettim, September,18th, 2010 (date of colony collection; medusae 1 to 4 days after)—MUZUSP 1922, three specimens of medusae (formalin 4%), realized in aquarium, from colonies on polyethylene plates held at same place as holotype, coll. A.L.Bettim, January 11 st, 2012—MUZUSP 1923 fragment of colony (formalin 4%), same place as holotype, coll. A.L.Bettim, September 29th, 2011 —DZoo-Cn 0 172, fragment of colony (alcohol 96%), same place as holotype, coll. A.L.Bettim, January 11 st, 2012—DZoo-Cn 0 173, fragment of colony (formalin 4%), same place as holotype, coll. M.A.Haddad, January 27th, 2012 —DZoo-Cn 0 174, five medusae (formalin 4%), released from colonies of same place as holotype, coll. A.L.Bettim, September,15th, 2010—DZoo-Cn 0 175, five medusae (alcohol 96%), released from colonies of same place as holotype, coll. A.L.Bettim, January 11 st, 2012.
Etymology. The species is named in honor of Dr. Jayme de Loyola e Silva, eminent crustacean researcher and professor of Zoology at the Universidade Federal do Paraná. He was Professor of Zoology during the Biology Graduation Course of the first author (M.A.H.) in the 1970s, her adviser through the MS degree, and most importantly he inspired and encouraged her to explore the biology of invertebrates.
Diagnosis. Polymorphic Hydractiniidae colonies with reticular stolons or encrusting hydrorhiza not covered by periderm; spines smooth, chitinous. Colonies growing on artificial substrates and adhering fouling organisms (especially barnacles). Newly-released medusae with eight tentacles and four small interradial gonads. Mature medusae with eight tentacles, unbranched oral lips, no gastric peduncle and no medusa-budding on manubrium.
Description of hydroid. Colonies polymorphic, stolonal. Hydrorhiza forming a network of stolonal tubes that normally coalesce into a mat lacking a visible perisarc covering. Some colonies with a thick, chitinous sheet or with chitinous portions under the hydrorhizal coenosarc. Spines conical, short (0.20 mm), smooth, distributed between the zooids and sometimes covered by coenosarc up to the apex (Pl. 1a). Small prickles also frequent. Base of zooid lacking a perisarc cup. Color of polyps varying from deep- to pale pink, to white.
PLATE 1. Podocoryna loyola n. sp.: a, chitinous spines and small granules in the midst of gastrozooids contracted (fixed colony); b, gastrozooids photographed alive. Zooids fixed: c, tentaculozooids (arrows); d, gonozooids with medusa buds. Undischarged nematocysts from gastrozooid tentacles: e, microbasic eurytele (left arrow) and desmonemes (right arrow); f, microbasic eurytele; g, basitricho isorhiza. Discharged nematocysts from tentaculozooid: h, microbasic eurytele (arrows); i, basitricho isorhiza (arrows). Medusae: j, newly liberated medusa photographed alive; k, mature fixed medusa. Undischarged nematocysts from medusae tentacles: l, desmoneme; m, microbasic eurytele. Undischarged nematocysts from manubrium of medusa; n, microbasic eurytele. Photomicrographs: (e, f, g) were obtained using motorized Axio Imager Z2 microscope (Carl Zeiss, Jena, DE), equipped with an automated scanning VSlide (Metasystems, Altlussheim, DE); (h, i) obtained with an Olympus® BX 40 optical microscope with an Olympus® DP 71 camera using the DP Manager program. Photomicrographs: (l, m, n) were obtained using motorized Axio Imager Z2 microscope (Carl Zeiss, Jena, DE), equipped with an automated scanning VSlide (Metasystems, Altlussheim, DE). Scale bars: (a–d) 5 mm; (e–i) 5 µm; (j, k) 0,0 5 mm; (l–n) 5 µm.
Life time in newly released 0 1 h 0 3 h 0 6 h 0 9 h 18 h days (d)
N m max min N m max min N m max min N m max min N m max min N m max min Dmax 4 0.73 0.85 0.52 5 0.73 1.00 0.83 5 0.99 1.05 0.92 5 1.09 1.35 0.81 5 1.12 1.23 1.01 5 1.08 1.20 0.95 Hmax 5 0.64 0.65 0.63 4 0.69 0.82 0.55 5 0.69 0.87 0.51 5 0.87 1.06 0.79 5 0.86 0.91 0.76 5 0.88 1.00 0.76 Dmar 0 0.65 0.73 0.57 3 0.77 0.82 0.75 4 0.89 0.94 0.82 5 0.98 1.30 0.67 5 0.98 1.05 0.90 5 0.97 1.10 0.85 Dape 0 0.00 0.00 0.00 2 0.74 0.85 0.63 5 0.59 0.65 0.45 5 0.77 0.95 0.61 5 0.82 0.87 0.75 5 0.68 0.75 0.60 Hmes 5 0.04 0.06 0.03 5 0.03 0.04 0.02 5 0.04 0.07 0.02 5 0.18 0.12 0.23 5 0.09 0.16 0.03 5 0.15 0.27 0.08 Hman 7 0.58 0.65 0.52 4 0.50 0.53 0.45 5 0.34 0.34 0.28 5 0.51 0.62 0.31 5 0.55 0.62 0.41 5 0.43 0.50 0.39 Thmes 5 0.06 0.09 0.03 5 0.03 0.04 0.02 5 0.03 0.05 0.02 5 0.07 0.11 0.03 5 0.06 0.09 0.04 5 0.09 0.11 0.05 Thg 2 0.08 0.08 0.08 5 0.11 0.17 0.07 5 0.17 0.20 0.15 5 0.18 0.23 0.15 5 0.13 0.21 0.06 5 0.17 0.20 0.10 continued.
Life time in 1 d 2 d 3 d 4 d 5 d 6 d days (d)
N m max min N m max min N m max min N m max min N m max min N m max min Dmax 5 1.09 1.23 1.00 5 1.20 1.27 1.03 5 1.35 1.55 1.10 5 0.96 1.13 0.89 5 0.90 0.96 0.80 5 1.25 1.37 1.16 Hmax 5 1.05 1.77 0.76 5 0.84 0.91 0.79 5 1.08 1.18 1.00 5 0.74 0.90 0.67 5 0.77 0.90 0.57 5 0.83 1.05 0.61 Dmar 5 1.04 1.17 0.99 5 1.16 1.24 1.00 5 1.21 1.28 1.00 5 0.83 0,93 0.77 5 0.81 0.85 0.75 5 1.09 1.28 0.95 Dape 5 0.76 0.90 0.60 5 0.75 0.86 0.60 5 0.85 1.04 0.70 5 0.59 0.80 0.37 5 0.69 0.80 0.60 5 0.92 1.10 0.80 Hmes 5 0.04 0.15 0.01 5 0.15 0.30 0.04 5 0.21 0.36 0.14 5 0.19 0.35 0.12 5 0.26 0.30 0.24 5 0.16 0.23 0.08 Hman 5 0.62 0.68 0.56 5 0.52 0.59 0.42 4 0.59 0.68 0.53 5 0.42 0.54 0.28 5 0.50 0.55 0.43 5 0.54 0.68 0.43 Thmes 5 0.03 0.04 0.02 5 0.04 0.06 0.02 5 0.14 0.17 0.06 5 0.15 0.20 0.11 5 0.14 0.19 0.09 5 0.15 0.16 0.12 Thg 5 0.14 0.17 0.13 5 0.11 0.18 0.08 3 0.18 0.25 0.13 5 0.12 0.16 0.09 4 0.10 0.09 0.11 5 0.09 0.23 0.01 continued.
Life time 7 d 8 d 9 d 10 d 11 d 13 d in days (d)
N m max min N m max min N m max min N m max min N m max min N m max min Dmax 5 1.09 1.25 0.92 5 1.02 1.10 0,9 5 1.13 1.33 0.97 5 0.96 1.18 0.75 5 0.73 0.77 0.69 3 0.83 0.97 0.70 Hmax 5 0.95 1.17 0.81 5 0.85 1.06 0,7 5 0.87 1.05 0.66 5 0.85 0.95 0.70 5 0.91 0.95 0.90 3 0.80 0.93 0.67 Dmar 5 0.92 1.04 0.80 5 0.91 1.03 0,8 5 1,0 1 1.26 0.86 5 0.86 1.10 0.61 5 0.60 0.65 0.52 3 0.72 0.81 0.60 Dape 5 0.95 1.20 0.76 5 0.67 0.80 0,5 5 0.68 0.75 0.52 3 0.58 0,70 0.43 3 0.42 0.50 0.30 3 0.49 0.56 0.40 Hmes 5 0.23 0.27 0.16 5 0.20 0.40 0,1 5 0.17 0.21 0.12 5 0.13 0.16 0.08 5 0.11 0.12 0.10 3 0.11 0.15 0.07 Hman 5 0.44 0.52 0.37 5 0.40 0.66 0,3 5 0.39 0.61 0.29 5 0.35 0.47 0.26 5 0.34 0.36 0,32 3 0.38 0.49 0.30 Thmes 5 0.17 0.27 0.13 5 0.12 0.17 0,1 5 0.14 0.17 0.12 5 0.09 0.11 0.07 5 0.11 0.12 0.10 3 0.12 0.15 0.10 Thg 3 0.10 0.12 0.07 4 0.09 0.15 0,1 3 0.10 0.16 0.06 0 - - - 4 0.09 0.08 0.10 1 0.10 0.10 0.10 Gastrozooids cylindrical, 0.19 to 0.55 mm long (average 0.34 mm), wide (average 0.10 mm) (Pl. 1b). Hypostome extensible, dome-shaped, white, with a layer of contiguous nematocysts; oral tentacles filiform, 6–13, with swollen distal ends, variable lengths, often reaching just over half the body length; arranged in two alternating rows around upper third of hypostome. Nematocyst rings surround entire tentacle. Sparse nematocysts in the column.
Tentaculozooids (Pl. 1c) with wide base, tapering toward rounded distal end, sometimes much longer than other polyps, 0.7 to 2.0 mm long (average 1.07 mm), 0.04 to 0.09 mm wide (average 0.06 mm); commonly occupying peripheral zone of colony or border area close to other colonies of the same species.
Gonozooids tubular, 0.24 to 0.40 mm long (average 0.30 mm), 0.04 to 0.09 mm wide (average 0.06 mm), with distal hypostome, resembling gastrozooids but smaller and more tapered, and with tentacles that are shorter and fewer in number (2–6) (Pl. 1d). Medusa buds of different developmental stages arising from small peduncles in a whorl just below tentacle insertions, surrounded by membranous perisarc, 3–10 in number.
Nematocysts observed on tentacles of gastrozooids included basitrichous isorhizas, microbasic euryteles and desmonemes (Pl. 1e–g). Only basitrichous isorhizas and microbasic euryteles were observed on tentaculozooids (Pl. 1h–i).
Description of medusa. Newly liberated medusae (Pl. 1j) campanulate, with thin mesoglea, 0.63 to 0.65 mm high, 0.52 to 0.85 mm wide ( Table 2); marginal tentacular bulbs generally eight, four perradial and four interradial, all of equal size; tentacles eight, of nearly equal length; apical process and ocelli absent. Velum well-developed, allowing a narrow opening to subumbrellar cavity, normally spanning 1/3 of its radius. Gonads four, interradial, on manubrium, in newly released medusae male gonads elongate, females rounded. Manubrium 0.52 to 0.65 mm long, square in cross section, reaching umbrella margin; mouth with four simple perradial lips each studded with a cluster of nematocysts; radial canals four; ring canal present.
Development of medusa. Newly liberated medusae with mature gonads, usually with remnants of umbilical canal and stalk that fixed them to gonozooid. Medusae increasing in size (Pl. 1k) until third day, gaining the wider than high campanulate form of umbrella. Numbers of tentacles and oral lips remaining unchanged during entire life ( Table 2). Manubrium length nearly equal that of newly liberated medusa, or distensible beyond umbrella margin. Gonads, in 12 individuals, 0.08 to 0.25 mm thick; thickest gonads (0.25 mm) observed in a three-day-old specimen. Older medusae with smaller or reduced gonads, mostly empty, indicating that gametes had already been spent. Medusa maturation completed in three days, under our laboratory conditions. From fourth day onwards, signs of degeneration and senescence clearly apparent. Degenerating medusae survived up to 13 days after release from colony.
Nematocysts of medusae include microbasic euryteles and desmonemes (Pl. 1l–m). Microbasic euryteles thinner than those of polyps were observed on manubrium (Pl. 1n).
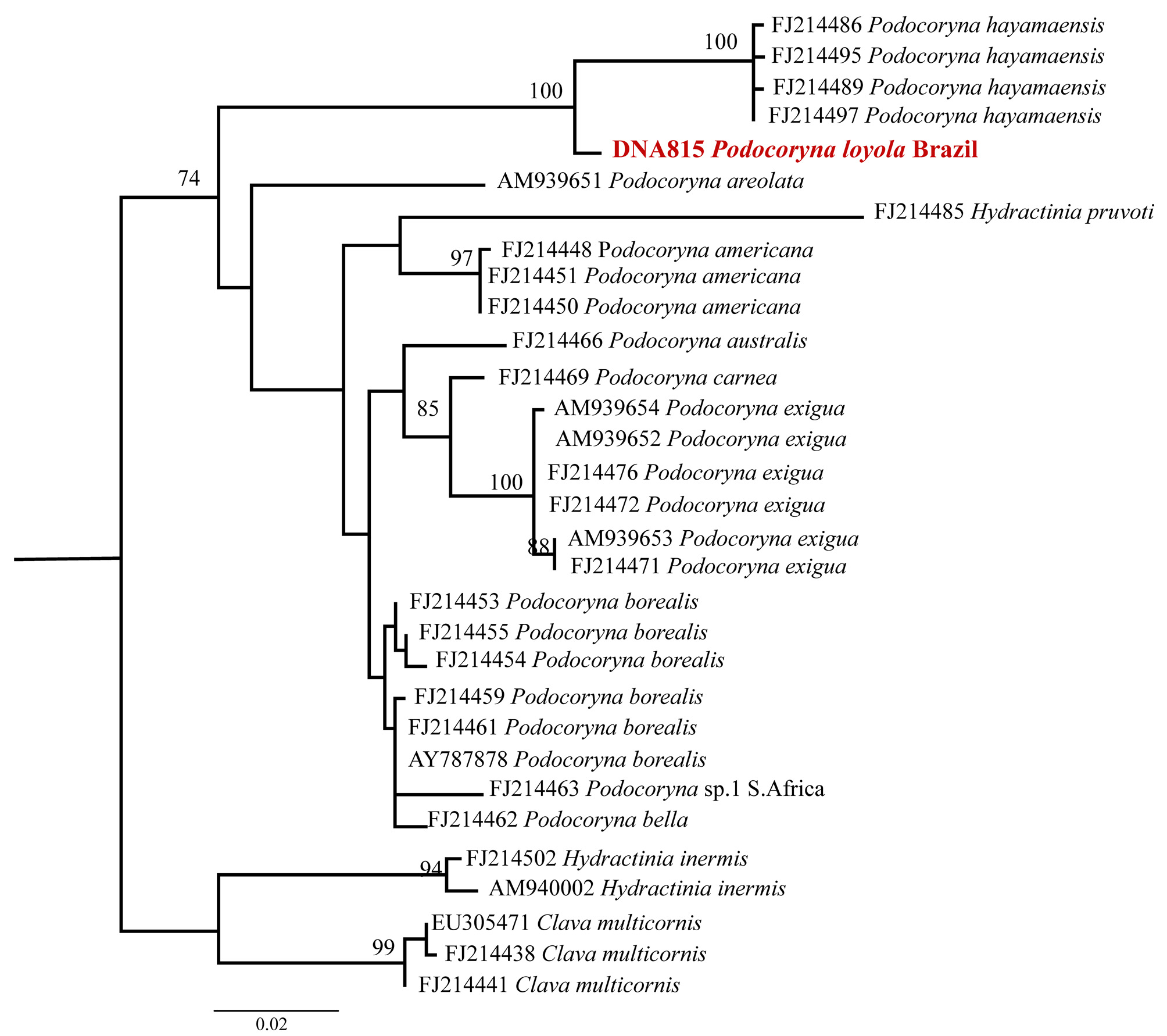
Molecular and phylogenetic analysis. One 16S sequence of Podocoryna loyola obtained from medusae (GenBank KJ418373 View Materials ) was aligned with 26 Podocoryna sequences, three Clava multicornis sequences, and three Stylactaria inermis sequences, all obtained from GenBank. Clava and Stylactaria were used as Podocoryna outgroups (Miglietta et al. 2009). The 23 Podocoryna sequences from GenBank belong to the species P. hayamaensis Hirohito, 1988 , P. areolata ( Alder, 1862) , P. americana ( Mayer, 1910) , P. exigua ( Haeckel, 1879) , P. carnea , P. australis Schuchert, 1996 , P. borealis ( Mayer, 1900) , and P. bella Hand, 1961 . Podocoryna loyola from Brazil nests within the Podocoryna clade, as expected ( Fig. 3 View FIGURE 3 ). Podocoryna loyola is distinct from all other species of Podocoryna in the phylogeny and is the sister species of P. hayamaensis (= Hydractinia hayamaensis Hirohito, 1988 ) from Japan, with a bootstrap support of 100% ( Fig. 3 View FIGURE 3 ).
Abundance. Colonies of Podocoryna loyola have been found on polyethylene plates, deployed at the marina of the Paranaguá Yacht Club, since May 2007. From February 2007 to February 2008, studies of community succession on the plates have been carried out by Altvater (2009) and Cangussu et al. (2010). During this period, 37 of 48 submerged plates were colonized by P. l o y ol a. The greater abundance of colonies occurred from the middle of autumn to the end of winter (May to September), when more than 75% of the plates were full of colonies. During spring and summer, colonies were found on only 25% of the plates. On quarterly sampling plates of the exotic species monitoring program, conducted from April 2009 to April 2012, colonies appeared on 95 of 314 polyethylene plates. The species was found mostly during spring 2009, summer and autumn 2010, and summer 2012, when colony coverage surpassed 55% of the total area of the plates.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
SubClass |
Hydroidolina |
|
Order |
|
|
Family |
|
|
Genus |