Mycale (Aegogropila) sulevoidea Sollas, 1902
|
publication ID |
https://doi.org/10.11646/zootaxa.4912.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:9536C1CF-4AEF-47F8-959B-48CD7A5392D8 |
|
DOI |
https://doi.org/10.5281/zenodo.4473158 |
|
persistent identifier |
https://treatment.plazi.org/id/361087A7-FFD3-FFBA-55AB-FECB5455CA6E |
|
treatment provided by |
Plazi (2021-01-19 19:56:07, last updated 2024-11-25 23:38:48) |
|
scientific name |
Mycale (Aegogropila) sulevoidea Sollas, 1902 |
| status |
|
Mycale (Aegogropila) sulevoidea Sollas, 1902 View in CoL
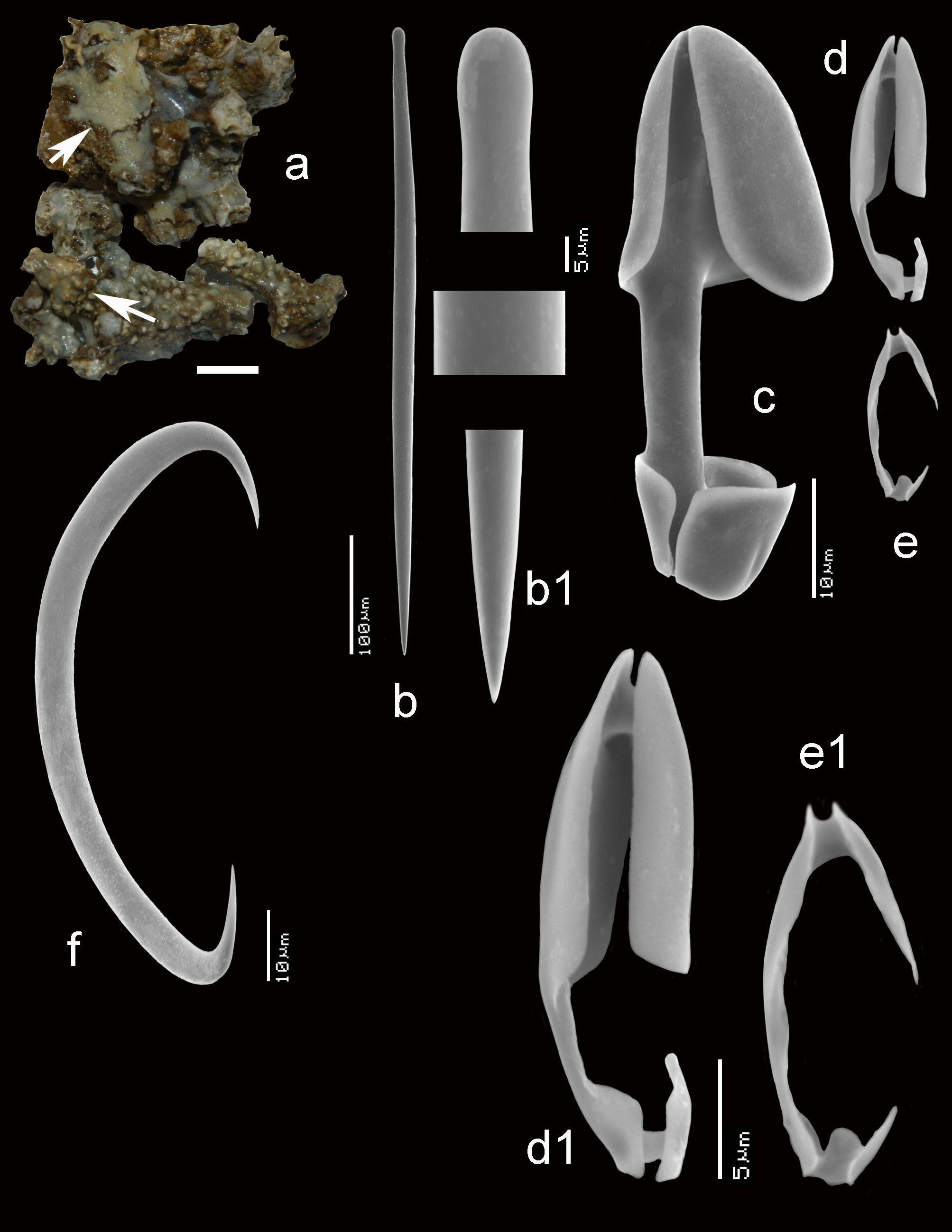
Figs 11 View FIGURE 11 a–e, 12a–g, 13a–g, 14, Table 2
Esperella sulevoidea Sollas, 1902: 213 View in CoL , pl. XV fig. 10.
Mycale sulevoidea View in CoL ; Hentschel 1912: 325, pl. 13 fig. 6, pl. 18 fig. 14; Barnes & Bell 2002 a: table 1 (listed only).
? Mycale sulevoidea View in CoL ; Burton 1934: 548; Burton 1959: 228; Vacelet & Vasseur 1971: 86 (no adequate descriptions).
Not: Lévi (1961a: 16); Pulitzer-Finali (1993: 290) = M. (Ae.) orientalis View in CoL .
Carmia sulevoidea View in CoL ; Thomas 1968: 256, figs 3–4.
Mycale (Aegogropila) sulevoidea View in CoL ; Lim et al. 2008: 102.
Material examined. ZMA Por. 01594, Indonesia, Indonesia, Nusa Tenggara, Solor Islands, Lamakera , 8.4237°S 123.1502°E, depth 20 m, coll. Siboga Expedition, stat. 061, field nr. SE 527 GoogleMaps II, 1 May 1899 ; ZMA Por. 02891, Indonesia, Papua, Aru Islands, Pearl Banks , anchorage off Pulau Jedan , 5.4134°S 134.6677°E, depth 13 m, coll. Siboga Expedition, stat. 273, field nr. SE 144.1, 23 December 1899 GoogleMaps ; ZMA Por. 07989, Indonesia, Nusa Tenggara, Sumba, NE coast, reef flat off Melolo , 9.16668°S 120.75°E, depth 1–4 m, coll GoogleMaps . R. W.M. van Soest, snorkeling, Indonesian-Dutch Snellius II Expedition stat. 052, field nr. 052 / II/34 , red crust on Acropora , 14 September 1984 ; ZMA Por. 08964, Indonesia, Sulawesi, SE Sulawesi, SW Salayar, reef NE of Pulau Bahuluang, 6.45°S 120.43°E, depth 10–15 m, coll GoogleMaps . R. W.M. van Soest , SCUBA, Indonesian-Dutch Snellius II Expedition stat. 169, field nr. 169 / IV/35 , 30 September 1984 (red crust) ; ZMA Por. 09555, Singapore, Raffles Lighthouse , 1.2884°N 103.7563°E, littoral, depth 0–2 m, coll. H. Moll, snorkeling, 5 January 1978 (blood-red crust) GoogleMaps ; ZMA Por. 12459, Indonesia, Sulawesi, SE Sulawesi, Salayar anchorage and surroundings, 6.0963°S 120.4481°E, depth 0–35 m, coll. Siboga Expedition, stat. 213, field nr. SE 1753I, 26 September 1899 GoogleMaps ; ZMA Por. 17808, Kenya, Shimoni Channel , 4. 6667°S 39.3833°E, depth 8–11, coll. Y. Benayahu GoogleMaps , SCUBA, field nr. PO 25395 , 3 February 2003 ; ZMA Por. 21702a, Singapore, Pulau Hantu , coll. H. Moll, 21 September 1978 .
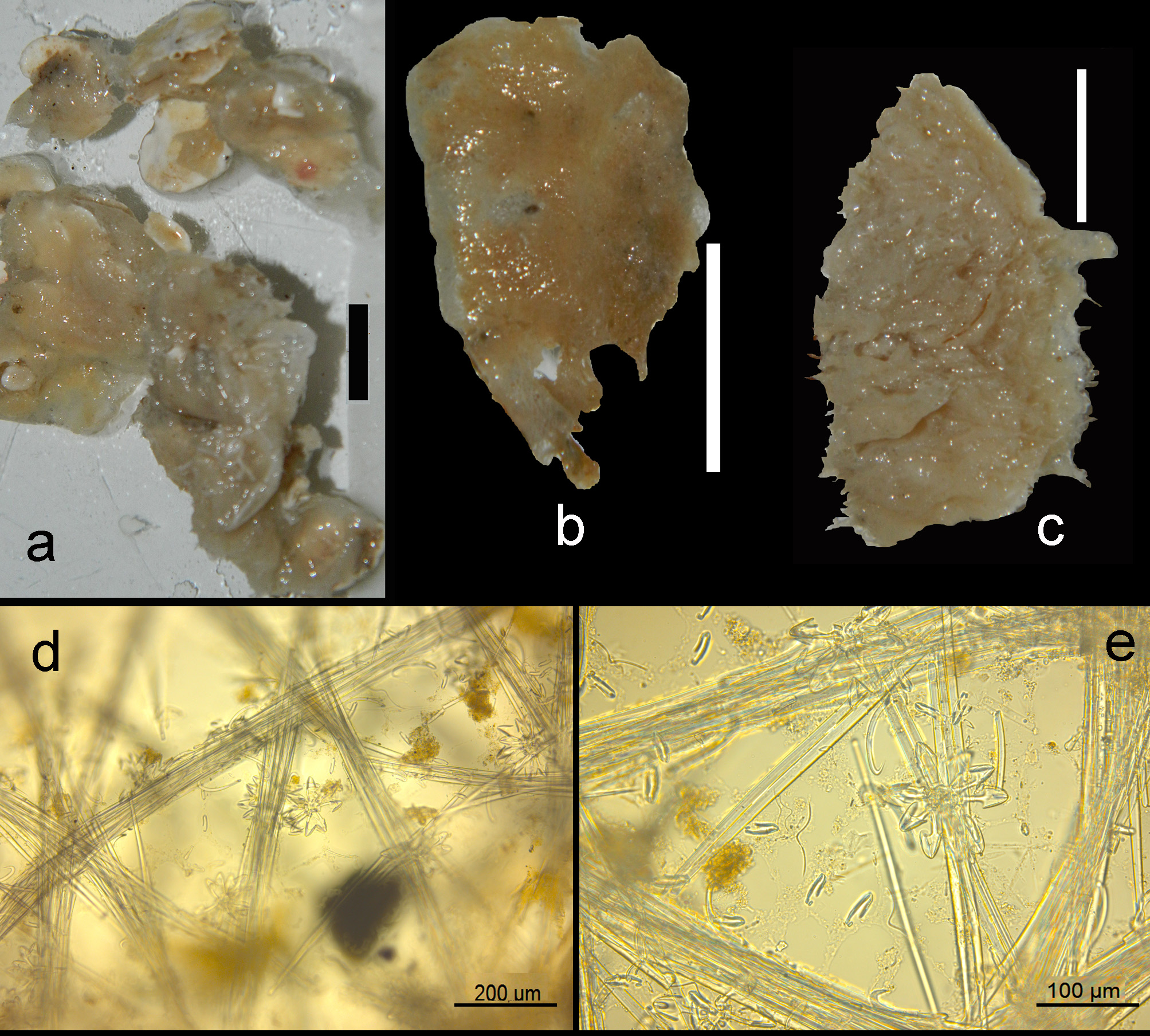
Description ( Figs 11 View FIGURE 11 a–c). Encrusting, consolidating dead coral rubble. The type was described as creeping with disc-like attachment. Our specimens are similarly patchy, but may also be somewhat thicker forming cushions. Oscules are usually slightly raised above the surface. Sizes usually do not exceed several cm 2. Surface smooth but irregular. Consistency soft. Color where recorded red or blood-red (cf. photo in Lim et al. 2008).
Skeleton ( Figs 11 View FIGURE 11 d–e). The ectosomal aegogropila-type skeleton is fairly robust, with intercrossing spicule tracts 30–70 µm in diameter made up of 5–8 spicules in cross section, delimiting triangular meshes of 300–600 µm in size. Variability among the various specimens is large. Several specimens tend to have the ectosomal tracts aligned with sigma I microscleres, but this is not always the case. Rosettes of anisochelae I measure 95–150 µm in diameter and are concentrated near the crossing points of the spicule tracts. Choanosomal tracts in the inner parts of the sponge are separated at approximately 500–800 µm and have a maximum thickness of 120–180 µm. Towards the surface they subdivide into thinner tracts as thin as 40 µm, carrying the surface skeleton. Microscleres are evenly distributed in the ectosomal region, but become scarcer in the interior.
Spicules ( Figs 11e View FIGURE 11 , 12 View FIGURE 12 a–g, 13a–g). Mycalostyles, anisochelae in three categories, sigmas in two categories, toxas.
Mycalostyles ( Figs 12a,a View FIGURE 12 1 View FIGURE 1 , 13a,a View FIGURE 13 1 View FIGURE 1 ), robust, with elongated head and only slightly constricted neck, 286– 370.9 – 465 x 7– 9.2 – 17 µm.
Anisochelae I ( Figs 12b View FIGURE 12 , 13b View FIGURE 13 ), well-developed alae, including broad upper and lower frontal alae, with shaft approximately 40% of its length free, in side view, the upper frontal alae are flaring outwards, 39– 51.1 – 63 µm.
Anisochelae II ( Figs 12c,c View FIGURE 12 1 View FIGURE 1 ,c 2 View FIGURE 2 , 13c View FIGURE 13 ), characteristically duck-bill shaped in side-view (terminology cf. Hajdu et al. 1995 for similar anisochelae in their species Brazilian M. (Ae.) escarlatei ); narrow elongated upper alae, with upper frontal alae tapering from distally broad to proximally narrower, curved outwards at the lower rim, causing a curved duckbill-like outline in side view (this varies somewhat among the specimens); free part of the shaft quite short; in dorsal view the spicule is violin-shaped due to upper lateral alae narrow from being expanded distally to become barely thinner than the shaft proximally and subsequently swelling again in the lower alae; spicules robust in comparison with anisochelae II of M. (Ae.) orientalis and they are on average longer in the present species; length quite variable within and among specimens, 24– 34.4 – 47 µm.
Anisochelae III ( Figs 12d View FIGURE 12 , 13d,d View FIGURE 13 1 View FIGURE 1 ), possibly occurring in two overlapping shapes and sizes; both types are narrow in shape, the larger may be resembling small anisochelae II, the smaller approaching the compact shape found in M. (Ae.) gravelyi and M. (Ae.) orientalis with slightly longer shaft; separation in two distinct spicule types is not quite clear, so we treat them both as growth forms of the same anisochela III , length 11– 16.2 – 24 µm.
Sigmas I ( Figs 12e View FIGURE 12 , 13e View FIGURE 13 ), robust, with strongly curved apices, length variable among specimens, 76– 102.3 – 130 µm, thickness up to 12 µm.
Sigmas II ( Figs 12f View FIGURE 12 , 13f View FIGURE 13 ), thin, strongly curved, 17– 25.8 – 35 µm.
Toxas ( Figs 12g View FIGURE 12 , 13g View FIGURE 13 ), predominantly deeply curved, but more shallow-curved shapes also occur; large variation in size, 22– 93.5 – 240 µm, thickness up to 1.5 µm, almost invariably thinner than the toxas of M. (Ae.) orientalis ; in some specimens the toxas were rare.
Distribution and ecology. ( Fig. 14 View FIGURE 14 ) Indonesia, Singapore, Malaysia, India, Kenya, possibly Mozambique, Madagascar and Red Sea, from shallow water down to 20–35 m depth.
Remarks. The assignment to I. Sollas’ Esperella sulevoidea is based especially on the small drawing she presented in pl. XV fig. 10 of the middle-sized category of anisochelae. The type description does not mention presence of sigmas II, but these spicules may often be easily overlooked. Hentschel’s (1912) description of material from the Aru Islands does contain measurements of sigmas II as well as a good description of the characteristic anisochela II. His measurements of the mycalostyles exceed those of Sollas’ and our own measurements (up to 576 µm), but overlaps with our data, and we are confident Hentschel’s material belongs to the present species. This also applies to Thomas’ (1968) record from Palk Bay, India, who provides an excellent description of a ‘brick-red’ specimen collected at 1–2 m depth.
We refrained from distinguishing different discrete types of anisochelae III, which was suggested by Hajdu et al. 1995, as anisochelae III and IV, for the present species. Although we acknowledge that shapes of anisochelae III in M. (Ae.) sulevoidea are more variable than is usual in other species of Mycale , we could not confirm the clear distinctness. In various specimens the variation usually overlapped, both in size and shape. These tiny spicules may likely depend for their shape of a number of factors such as silica content of the environment or growth process.
Burton’s (1934) record of three ‘flagelliform’ specimens of 35 x 1.5 cm from the Great Barrier Reef remains dubious without further information. Such habitus forms have not been encountered in our material. Their shape reminds of Mycale (Zygomycale) parishi (q.v.). Likewise, Burton’s (1959) record of the species from the Red Sea is uncertain due to lack of description.
We made SEM images of 6 specimens, but a detailed comparison of regional specimens is not presented as our specimens were all from the West Pacific except the single specimen from Kenya. This has smaller and thinner mycalostyles, some diversity in shape of anisochelae III, and the toxas were rare. However, the single specimen precludes regional conclusions .
The present species is very similar to M. (Ae.) orientalis , and we compared the two species including spicule sizes (cf. Table 2). The comparison yielded the following differences: (1) the subtly different shape of the upper alae of the anisochelae II (cf. Figs 12c,c View FIGURE 12 1 View FIGURE 1 ,c 2 View FIGURE 2 ), which in the present species are curved abruptly outwards in the lower part of the median alae, whereas these are gradually curved in M.(Ae.) orientalis ; moreover in dorsal view the anisochelae differ in the violin-shaped narrowing of the lateral alae in M. (Ae.) sulevoidea opposed to the normal shaped lateral alae in M. (Ae.) orientalis (cf. Figs 5d,d View FIGURE 5 1 View FIGURE 1 ), (2) in the larger size of the anischelae II ( Table 2), and (3) in the longer and thicker mycalostyles ( Table 2). Less clear is the difference in color. M. (Ae.) orientalis is reported to have an orange live color, against the present species reported as red, blood-red or brick-red, but there are currently no live images to further prove this. The here cited alleged differences require further confirmation.
Additional Mycale (Aegogropila) species from the region
Remark. Most of the species listed here have not been examined by us (excepting Mycale (Aegogropila) cavernosa Bergquist, 1965 ), so their properties can only be derived from the original description and if present subsequent records. It is not certain that they are all valid species. As stated above, we limit the listing to the considered region stretching from the West Pacific Islands in the east, the coasts of East Africa in the west, South China and the Philippines in in the north and northeastern, northern and southwestern parts of Australia.
Barnes, D. K. A. & Bell, J. J. (2002) Coastal sponge communities of the West Indian Ocean: taxonomic affinities, richness and diversity. African Journal of Ecology, 40, 337 - 349. https: // doi. org / 10.1046 / j. 1365 - 2028.2002.00387. x
Bergquist, P. R. (1965) The Sponges of Micronesia, Part I. The Palau Archipelago. Pacific Science, 19 (2), 123 - 204.
Burton, M. (1934) Sponges. Scientific Reports of the Great Barrier Reef Expedition 1928 - 29, 4 (14), 513 - 621, pls. 1 - 2.
Burton, M. (1959) Sponges. Scientific Reports John Murray Expedition 1933 - 34, British Museum (Natural History), London, 10 (5), 151 - 281.
Hajdu, E., Zea, S., Kielman, M. & Peixinho, S. (1995) Mycale escarlatei n. sp. and Mycale unguifera n. sp. (Demospongiae) from the Tropical-Western Atlantic. Beaufortia, 45 (1), 1 - 16.
Hentschel, E. (1912) Kiesel- und Hornschwamme der Aru- und Kei-Inseln. Abhandlungen herausgegeben von der Senckenbergischen naturforschenden Gesellschaft, 34 (3), 293 - 448. https: // doi. org / 10.5962 / bhl. title. 85325
Levi, C. (1961 a) Resultats scientifiques des Campagnes de la ' Calypso'. Campagne 1954 dans l'Ocean Indien (suite). 2. Les spongiaires de l'Ile Aldabra. Annales de l'Institut oceanographique, 39 (1), 1 - 32, pls. 1 - 2.
Lim, S. C., De Voogd, N. J. & Tan, K. S. (2008) A guide to sponges of Singapore. Science Centre, Singapore, 173 pp.
Pulitzer-Finali, G. (1993) A collection of marine sponges from East Africa. Annali Museo Civico Storia Naturale Giacomo Doria, 89, 247 - 350.
Sollas, I. B. J. (1902) On the Sponges collected during the ' Skeat Expedition' to the Malay Peninsula 1899 - 1900. Proceedings of the Zoological Society of London, 72 (3), 210 - 221. https: // doi. org / 10.1111 / j. 1469 - 7998.1902. tb 08232. x
Thomas, P. A. (1968) Studies on Indian Sponges-III. Two species of siliceous sponges of the famliy Ophlitaspongiidae de Laubenfels (Class: Demospongiae Sollas, Order: Poecilosclerida Topsent). Journal of the Marine Biological Association of India, 10 (2), 255 - 259.
Topsent, E. (1897) Spongiaires de la Baie d'Amboine. (Voyage de MM. M. Bedot et C. Pictet dans l'Archipel Malais). Revue suisse de Zoologie, 4, 421 - 487, pls. 18 - 21.
Vacelet, J. & Vasseur, P. (1971) Eponges des recifs coralliens de Tulear (Madagascar). Tethys, Supplement 1, 51 - 126.
FIGURE 1. A. Numbers of available specimens for the present study from the collections of the Naturalis Biodiversity Center distributed over Indo-West Pacific Marine Ecoregions (MEOWS, cf. Spalding et al. 2007). B. Ditto for type specimens and additional specimens from other institutions consulted for this study.
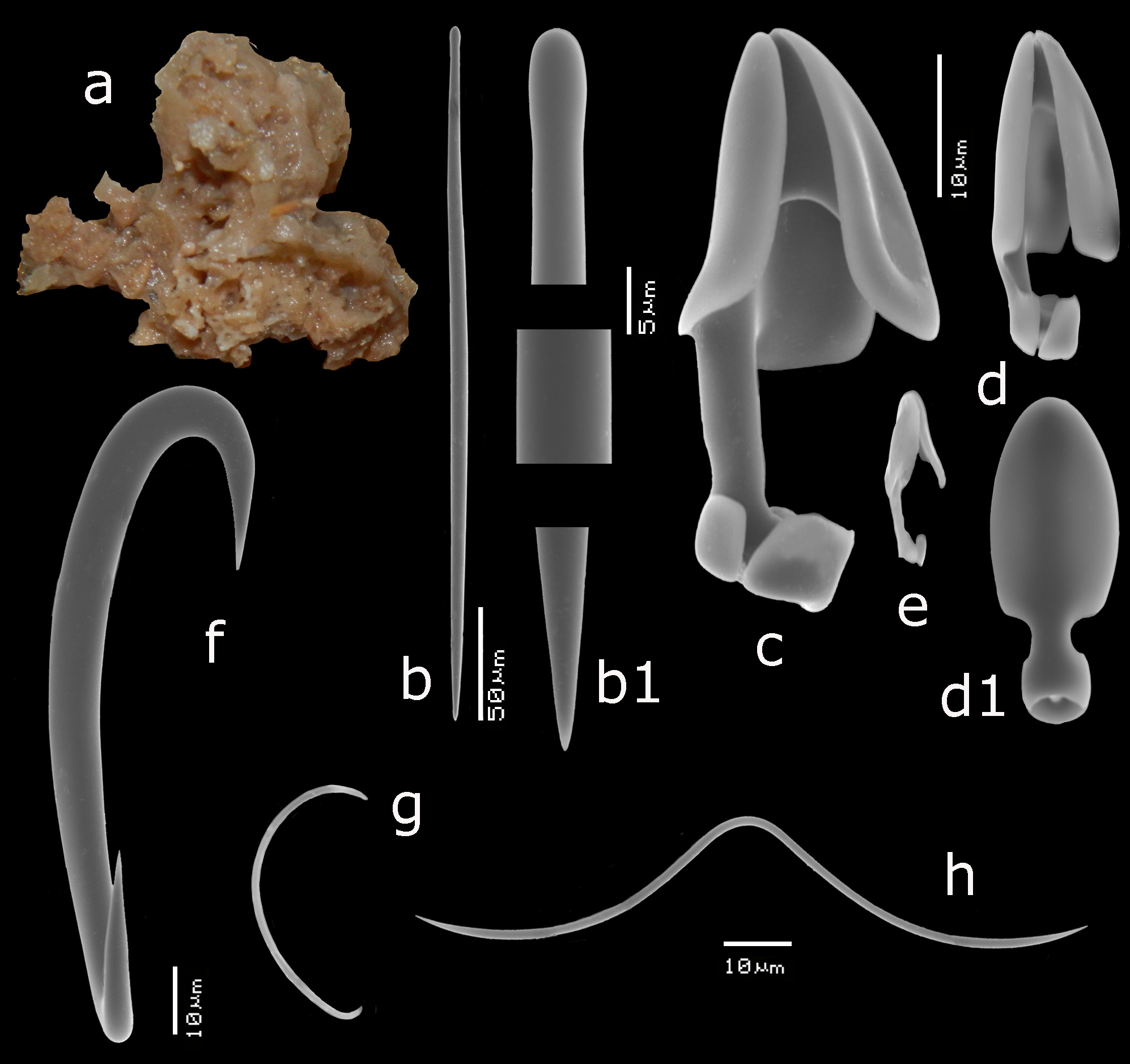
FIGURE 2. Mycale (Aegogropila) gravelyi Burton, 1937, ZMA Por. 07864 from Ambon Bay, Indonesia, a, habitus encrusting on coral rubble (arrows) (scale bar = 1 cm), b–f, SEM images of spicules, b, mycalostyle, b1, details of mycalostyle, c, anisochela I in side view, d, anisochela II, d1, anisochela II enlarged, e, anisochela III, e1, anisochela III enlarged, f, sigma I.
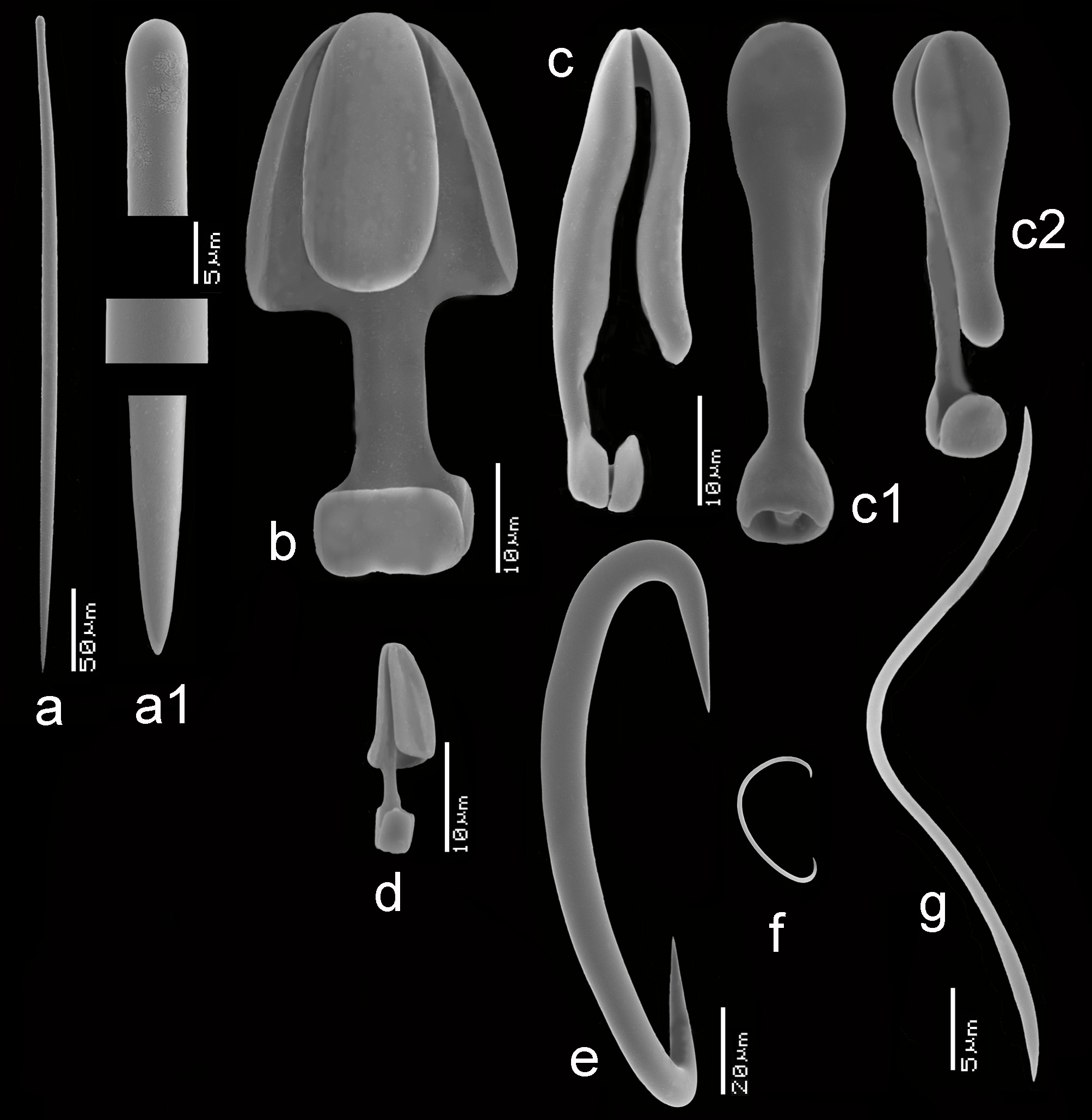
FIGURE 5. Mycale (Aegogropila) orientalis (Topsent, 1897), ZMA Por. 01612 from Indonesia, a, habitus encrusting coral rubble (scale bar = 1 cm), b–h, SEM images of spicules, b, mycalostyle, b1, details of mycalostyle, c, anisochela I in side view, d, anisochela II in side view, d1, anisochela II in back view (compare with anisochelae II of M. (A.) sulevoidea), e, anisochela III in side view (all anisochelae in same magnification), f, robust sigma I, g, sigma II, h, toxa.
FIGURE 11. Mycale (Aegogropila) sulevoidea (Sollas, 1902), a–c, habitus (preserved) (scale bars 1 mm), a, ZMA Por. 01594 from Indonesia, b, ZMA Por. 09555 from Singapore, c, ZMA Por. 17808 from Kenya, d–e, light microscopic images of surface skeleton of ZMA Por. 09555 from Singapore, d, aegogropila-type of surface skeleton and rosettes of anisochelae I, e, enlarged view of surface skeleton with anisochelae I, II and III, sigmas and toxas.
FIGURE 12. Mycale (Aegogropila) sulevoidea (Sollas, 1902), SEM images of spicules of ZMA Por. 01594 from Indonesia, a, mycalostyle, a1, details of mycalostyle, b, anisochela I in front view, c, anisochelae II in lateral view showing characteristic duck-bill shape, c1, ditto in back view showing ‘guitar-shaped’ outline, c2, ditto in front view, d, anisochela III (all anisochelae in same magnification), e, sigma I, f, sigma II, g, toxa.
FIGURE 13. Mycale (Aegogropila) sulevoidea (Sollas, 1902), SEM images of spicules of ZMA Por. 17808 from Kenya, a, mycalostyle, a1, details of mycalostyle, b, anisochela I in lateral view, c, anisochelae II in lateral view, d, anisochela III, larger spicule in front view, d1, smaller spicule in lateral view (all anisochelae in same magnification), e, sigma I, f, sigma II, g, toxa.
FIGURE 14. Mycale (Aegogropila) sulevoidea (Sollas, 1902), approximate distribution of specimens studied (blackish green squares) and literature records (pale green dots). Squares and dots may represent several close collecting localities. Question marks indicate uncertain literature records possibly concerning the similar species M. (Ae.) orientalis (cf. above).
| ZMA |
Universiteit van Amsterdam, Zoologisch Museum |
| R |
Departamento de Geologia, Universidad de Chile |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Mycale (Aegogropila) sulevoidea Sollas, 1902
| Van, Rob W. M., Aryasari, Ratih & De, Nicole J. 2021 |
Mycale (Aegogropila) sulevoidea
| Lim, S. C. & De Voogd, N. J. & Tan, K. S. 2008: 102 |
Carmia sulevoidea
| Thomas, P. A. 1968: 256 |
Mycale sulevoidea
| Vacelet, J. & Vasseur, P. 1971: 86 |
| Burton, M. 1959: 228 |
| Burton, M. 1934: 548 |
Mycale sulevoidea
| Hentschel, E. 1912: 325 |
Esperella sulevoidea
| Sollas, I. B. J. 1902: 213 |
1 (by plazi, 2021-01-19 19:56:07)
2 (by ExternalLinkService, 2021-01-19 20:27:22)
3 (by diego, 2021-01-25 17:46:50)
4 (by diego, 2021-01-25 18:09:16)
5 (by diego, 2021-01-25 18:56:44)
6 (by diego, 2021-01-27 13:57:57)
7 (by diego, 2021-01-27 16:13:01)
8 (by diego, 2021-01-27 16:26:52)
9 (by ExternalLinkService, 2021-01-27 16:47:25)
10 (by ExternalLinkService, 2021-01-27 17:19:26)
11 (by ExternalLinkService, 2021-09-20 00:43:26)
12 (by ExternalLinkService, 2021-11-11 05:07:22)
13 (by plazi, 2023-11-01 17:27:49)