Litoria quiritatus, Rowley & Mahony & Hines & Myers & Price & Shea & Donnellan, 2021
|
publication ID |
https://doi.org/ 10.11646/zootaxa.5071.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:E695DE38-387E-41E0-8188-532A907C3BB1 |
|
DOI |
https://doi.org/10.5281/zenodo.5725376 |
|
persistent identifier |
https://treatment.plazi.org/id/3464AB2A-5713-FFCD-FF3E-F9C7FCAC7B90 |
|
treatment provided by |
Plazi |
|
scientific name |
Litoria quiritatus |
| status |
sp. nov. |
Litoria quiritatus sp. nov.
Screaming Tree Frog
Figs 13 View FIGURE 13 , 14 View FIGURE 14
Holotype. AMS R185759 . An adult male collected from Coalcliff, New South Wales (-34.247, 150.975) by Richard Major on 6 October 2017. GoogleMaps
Material examined. See Supplementary Table S1 View TABLE 1 for details of all material examined.
Dimensions of holotype (mm). SVL 39.8 ; HL 10.8; HW 11.6; IND 2.5; EN 3.3; ED 3.4; IOD 7.7; TD 1.4; FLL 6.9; Fin3L 11.9; TL 17.1; Toe4L 16.2.
Diagnosis. Litoria quiritatus sp. nov. is distinguished from all species in the Litoria rubella group by a combination of (1) adult body size 36–43 mm in males and 34–46 mm in females, (2) relatively robust build, (3) the presence of a single, continuous, irregularly edged, dark brown dorsal band, (4) the absence of light spots on the dorsum, (5) lack of a well-defined pale mid-dorsal stripe, (6) absence of distinctive pale markings above the groin, vent and along lower leg, (7) a dorsolateral line diffusing above insertion of the arm, and (8) adult males having a vocal sac that is yellow when deflated and when inflated.
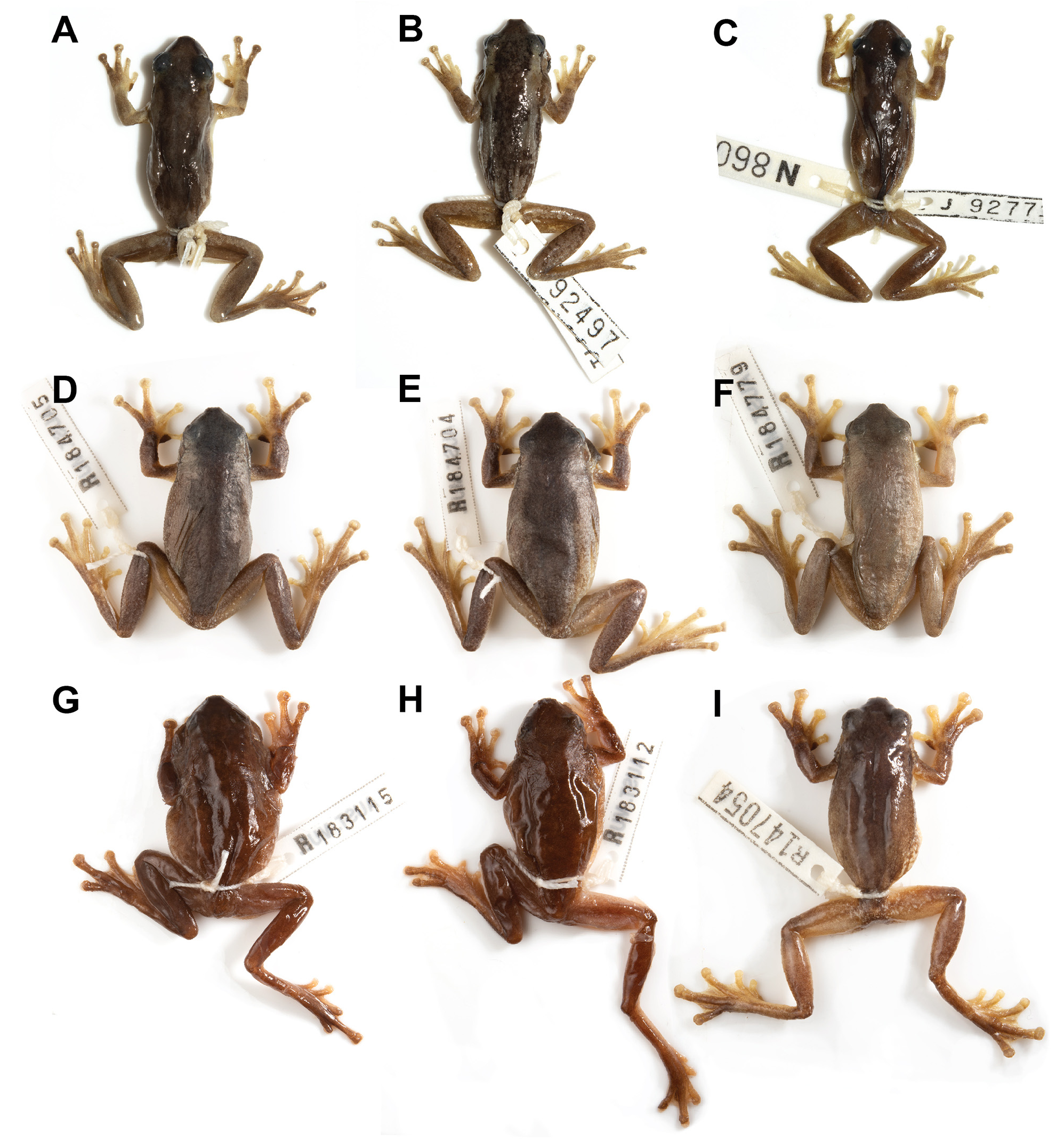
Description of holotype. Habitus relatively robust; head widest at eyes, slightly wider than long (HW/HL 1.08); snout bluntly rounded in profile and obtusely pointed in dorsal view; nostrils prominent in dorsal profile; vomerine teeth in single row running laterally anteriorly to choanae; tympanum circular and clearly visible and twothirds the diameter of the eye (TD/ED 0.67).
Fingers and toes with prominent terminal discs; fingers with basal webbing; toes half webbed. Relative lengths of fingers 3>4>2>1; of toes 4>5=3>2>1. Sub-articular tubercles present under fingers and toes but not prominent. Inner metatarsal tubercles present and prominent, approximately one third the length of first toe. Nuptial pad oval, restricted to dorsal surface of proximal half of first finger, comprised of small granules. Legs short (TL/SVL 0.43).
All dorsal surfaces dark brown, contrasting strongly with ventral surface of body and limbs ( Fig. 13 View FIGURE 13 ). Continuous, irregularly edged, dark-brown dorsal band from snout to vent, extending laterally to the dorsolateral margin. Flanks light brown. Dorsum very weakly granular, upper surfaces of legs, arms and distal lower surfaces of limbs smooth. Broad irregular, lighter brown bands on each side, from back of eye. A dark brown stripe from snout, through eye, onto tympanum and continuing laterally above arm and along lateral ventral margin of body, diffusing above insertion of the arm. Groin with yellowish orange wash and distinct white patches. White bar directly under eye and tympanum and immediately posterior to tympanum. Ventral surface immaculate light cream. Single vocal sac and chin dark brownish-grey and yellow; lower lip cream.
Variation. Male SVL 36–43 mm; female SVL 34–46 mm. Summary of variation in morphometric variables for each sex is presented in Table 5 View TABLE 5 .
Variation in colour is described from images taken in life ( Fig. 14 View FIGURE 14 ). Dorsal colouration varies from cream (e.g. Fig. 14E View FIGURE 14 ) to warm medium-brown (e.g. Fig. 14F View FIGURE 14 ), with a distinct darker brown patch across the head and down the back, narrowing in width over the axilla and then mid-dorsum, more diffuse posteriorly. Very narrow, diffuse paler brown mid-dorsal line present in some individuals (e.g. Fig. 14D View FIGURE 14 ). Darker brown dorsolateral line running from snout, through eye, over tympanum, and down the side of the body, diffusing above insertion of the arm, more diffuse posteriorly; distinctness of this line varies. Bright white patch on upper lip between lower margin of eye and insertion of arm. Dorsal surface of limbs brown, finger and toe tips may be slightly paler brown (e.g. Fig. 14F View FIGURE 14 ). White or pale yellowish patches in the groin (e.g. Fig. 14C View FIGURE 14 ) almost always present but vary in size and number. Back of thighs transparent pinkish-orange or yellowish-orange with varying amounts of darker brown pigment and opaque, creamy yellow flecks. Belly white; vocal sac in males dark yellow when deflated and pale yellow when inflated ( Fig. 14B, C, F View FIGURE 14 ). Iris copper-brown (e.g. Fig. 14D View FIGURE 14 ) to reddish-copper (e.g. Fig. 13A View FIGURE 13 ). During the breeding season males become yellowish (e.g. Fig. 14A, B View FIGURE 14 ).
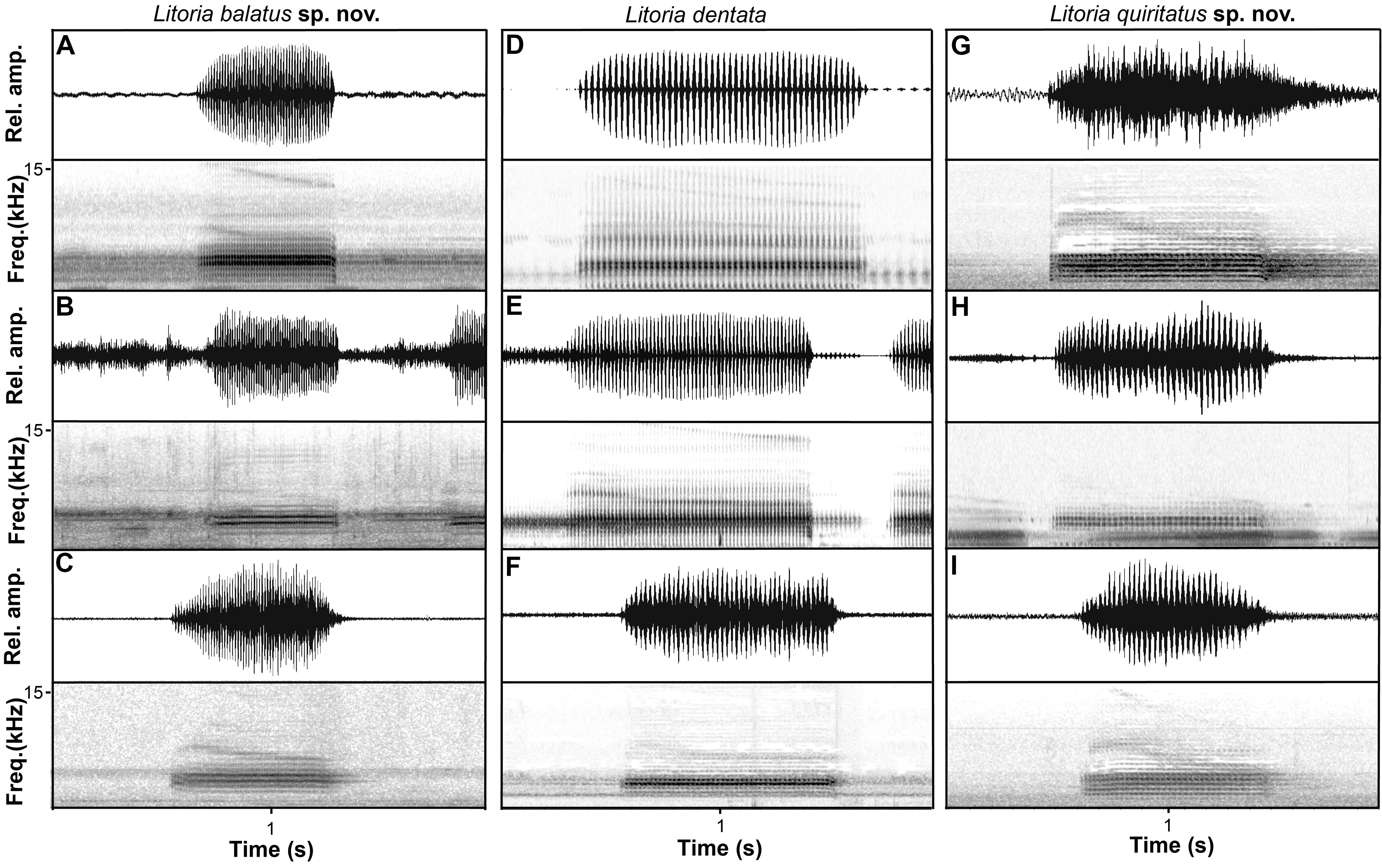
Advertisement call. Call descriptions are based on the calls of 16 individuals, including the holotype ( Table 6 View TABLE 6 , Figs. 6 View FIGURE 6 , 8 View FIGURE 8 ). The advertisement call of L. quiritatus sp. nov. comprises a single, highly-pulsed note. Individuals had a mean call duration of 0.70– 1.31 s and an average of 24–51 uniformly spaced pulses repeated at a rate of 30–53 pulses/s. Calls were amplitude modulated, and highly variable in the timing of their peak amplitude with respect to call duration. The dominant frequency was 3.1–4.1 kHz.
Comparison with other species. The distribution of L. quiritatus sp. nov. is parapatric with L. dentata but is allopatric with the other members of the L. rubella species group ( L. balatus sp. nov. in south-eastern Queensland, L. electrica in north-western Queensland, L. congenita and L. pygmaea in New Guinea and L. capitula on the Tanimbar Islands, Indonesia). Litoria quiritatus sp. nov. can be distinguished from L. rubella by the presence of continuous, irregularly edged, dark brown dorsal band (versus absence) and less robust body (versus more robust). It can be distinguished from L. electrica by the presence of continuous, irregularly edged, dark brown dorsal band (versus two dark chocolate-coloured bars across the dorsum). It can be distinguished from the New Guinean species L. congenita and L. pygmaea by absence of light spots (usually large and conspicuous spots in L. pygmaea , smaller and more variable in L. congenita ) on dark dorsal background. It can be distinguished from L. capitula by the absence of distinctive pale markings above the groin, vent and along lower leg that are present in L. capitula .
Litoria quiritatus sp. nov. can be distinguished from L. dentata by males having a vocal sac that is yellow when deflated and inflated (versus a vocal sac that is black or very dark yellowish black when deflated and yellowish brown when inflated). It can be distinguished from L. balatus sp. nov. by males having a vocal sac that is yellow when deflated and inflated (versus males having a vocal sac that is black) and having a robust build (versus slender build) ( Fig. 5 View FIGURE 5 ). From a genetic perspective, apomorphic nucleotide states at 28 sites in the mitochondrial ND4 gene reliably diagnose L. quiritatus sp. nov. from L. balatus sp. nov. and L. dentata ( Table 7 View TABLE 7 ).
Etymology. The specific epithet, quiritatus , is a masculine Latin 4 th declension noun based on the verb quirito, meaning a shriek or scream, used as a noun in apposition to the genus name.
Distribution. In the south from the Genoa River, 10 km NW Mallacoota, Victoria along the coast and eastern fall of the Great Dividing Range north to Mernot State Forest, NSW. The ranges of L. dentata and L. quiritatus sp. nov. approach to within 60 km of each other between Taree and Woko National Park respectively. The known elevation range of the species is from sea level to ~ 1100 m on the Newnes Plateau, Blue Mountains.
Ecology. Litoria quiritatus sp. nov. calls from the ground or emergent vegetation associated with permanent or ephemeral water courses and ponds in both natural and disturbed habitats. Anstis (2018) described oviposition, egg and larval morphology, and development (as L. dentata ) from Nowra, Darkes Forest, Watagan Mountains and the Blue Mountains. The species is the 24 th most commonly recorded species in the FrogID database (>3400 records from 10 November 2017 – 30 June 2021) and appears relatively tolerant of disturbed areas, with 42% of records documented as being in suburban or urban habitats and 37% in rural areas. L. quiritatus sp. nov. has been detected via the FrogID project calling from August to May, with peak calling activity from September to February.
Conservation status. L. quiritatus sp. nov. is a relatively widespread frog species, with an estimated Extent of Occurrence of 145,000 km 2. There are no documented or suspected population declines, so the species likely meets IUCN Red List criteria ( IUCN 2012) for Least Concern.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.