Alpheus eurydactylus De Man, 1920
|
publication ID |
https://doi.org/ 10.11646/zootaxa.5282.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:DF418763-8F0E-44DD-97C4-B123A81A8DB4 |
|
DOI |
https://doi.org/10.5281/zenodo.7921843 |
|
persistent identifier |
https://treatment.plazi.org/id/2A26026D-4B53-FFA9-E7B8-FEE7FAEAFF68 |
|
treatment provided by |
Plazi |
|
scientific name |
Alpheus eurydactylus De Man, 1920 |
| status |
|
Alpheus eurydactylus De Man, 1920 View in CoL
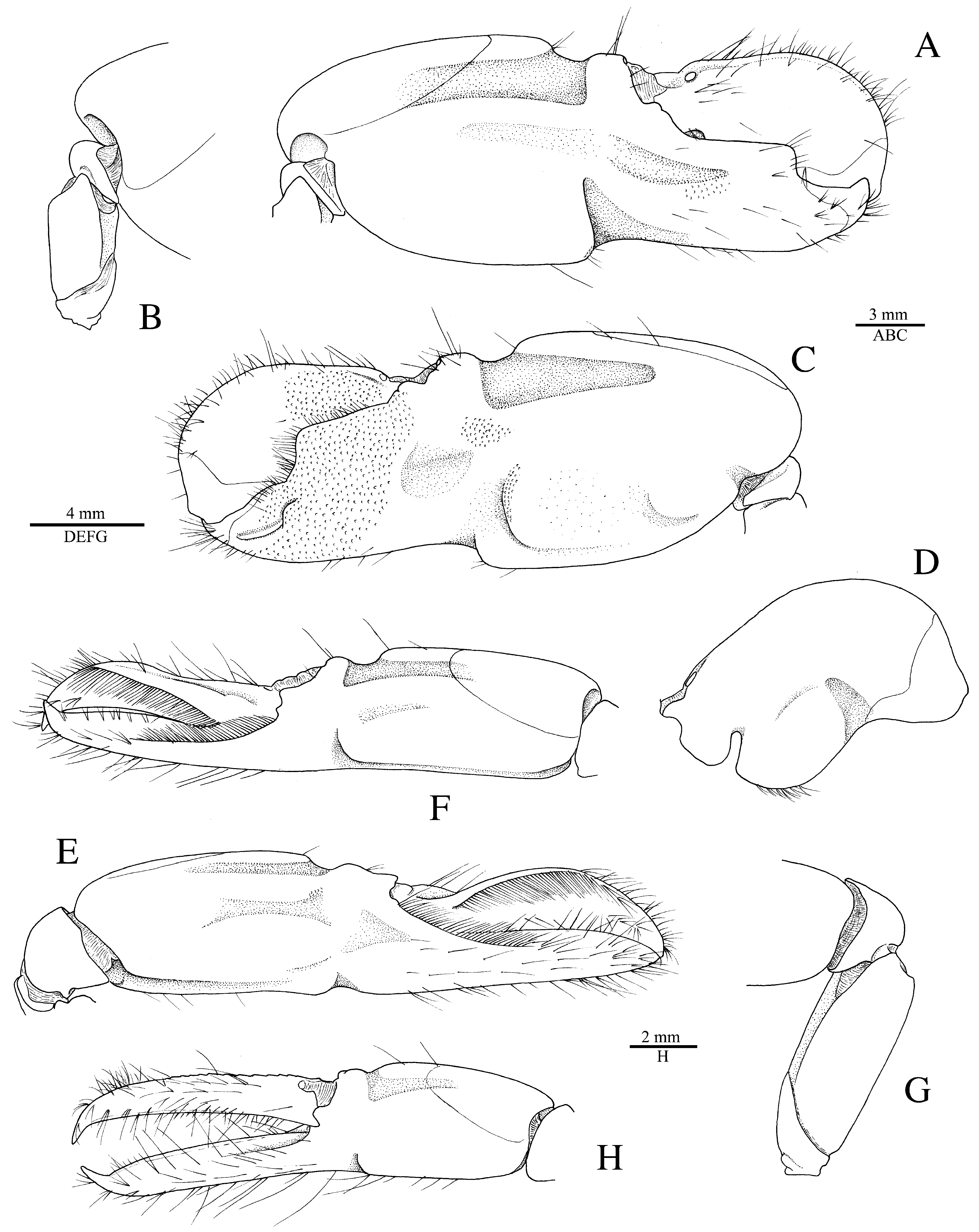
( Figs. 3H, I View FIGURE 3 , 11–16 View FIGURE 11 View FIGURE 12 View FIGURE 13 View FIGURE 14 View FIGURE 15 View FIGURE 16 , 51B View FIGURE 51 )
[see also Fig. 53B, C View FIGURE 53 for A. cf. eurydactylus ]
Alpheus eurydactylus De Man 1920: 109 View in CoL ; De Man 1924: 48, fig. 17.
Alpheus euphrosyne View in CoL .— Banner & Banner 1966: 130 (part.?), fig. 49; Johnson 1976: 37 (part.) [not A. euphrosyne De Man, 1897 View in CoL ].
(?) Alpheus euphrosyne euphrosyne View in CoL .— Banner & Banner 1981: 23, 92 (part.?); Banner & Banner 1982: 232, fig. 73 (part.?); Banner & Banner 1983: 30 (part.?); Banner & Banner 1985: 16 (part.?); Chace 1988: 27 (part.?) [not A. euphrosyne De Man, 1897 View in CoL ].
Alpheus cf. euphrosyne View in CoL . — Marin 2021: 381, fig. 1a [not A. euphrosyne De Man, 1897 View in CoL ].
(?) Alpheus sp. cf. audouini View in CoL .— Johnson 1962: 53 (part.?) [not A. audouini Coutière, 1905 View in CoL ].
Type material. Lectotype, male (cl 13.8 mm, tl ~40.0 mm, chl 21.0 mm), paralectotype, ov. female (cl 15.0 mm, tl ~44.0 mm, chl 21.5 mm), RMNH Crus.D.49670 = ZMA Crus.D.102536, Indonesia, Java, Surabaya , leg. P. Buitendijk, no date.
Additional material examined. Indonesia: 1 male (cl 19.4 mm), MNHN-IU-2018-5738, Papua, Kamora, mangrove, leg. A. Darmawan et al., 07.08.2012; 1 female (cl 12.6 mm), 1 ov. female (cl 17.3 mm), OUMNH. ZC. 2019.06.44, Papua, Ajkwa Island , mangrove, leg. A. Darmawan et al., 20.07.2012; 1 female (cl 21.5 mm), ZMA Crus.D.202791, Siboga Expedition sta. 4, “Strand” (beach), no further details; 1 ov. female (cl indet.), RMNH Crus. D.49671, Madura Island , leg. P. Buitendijk , 03.1930; 2 males, 1 ov. female (cl indet.), RMNH Crus.D.49660, Java, Tanjong Priok (near Jakarta), leg. P. Buitendijk , 1907; 4 males, 1 ov. female (cl indet.), RMNH Crus.D.49666, Java, Tanjong Priok (near Jakarta), leg. P. Buitendijk, 07.1927; 1 ov. female (cl indet.), RMNH Crus.D.49667, Java, Probolinggo, leg. P. Buitendijk , 04.1928; 1 male, 2 females (cl indet.), RMNH Crus.D.49661, Sumatra, Pulau Weh, leg. P. Buitendijk , 1910; 1 male (cl indet.), RMNH Crus.D.49668, East Java, Passeroean, leg. P. Buitendijk , 1929; 1 male (cl indet.), RMNH Crus.D.49665, East Java, Panaroekan, leg. P. Buitendijk , 08.1927; 1 female (cl indet.), RMNH Crus.D.49408, West Java, Banten Baai, Pulau Pamudjan Besar , 5°56’S 106°12’E, “laboratorium voor Onderzoek der zee, Batavia”, no further details GoogleMaps ; 1 female (cl 21.3 mm), RMNH Crus.D.49664, Java, Surabaya, leg. P. Buitendijk, 02.1927; 3 chelipeds, RMNH Crus.D.49672, Java, Bay of Jakarta, Alkmaar Island , leg. P. Buitendijk , 1906.
Malaysia: 1 male (cl 11.2 mm), 1 ov. female (cl 17.5 mm), OUMNH. ZC. 2019.06.45, Penang (mainland part), Nibong Tebal, mudflat, leg. Z. Jaafar, 22.11.2001 ; 2 males (cl 11.5, 15.2 mm), 2 females (cl 10.3, 11.7 mm), MNHN-IU-2018-5680, same collection data as for previous specimens ; 1 male (cl 15.1 mm), MNHN-IU-2018-5599, Johor, Muar , mudflat, soggy mud, leg. N.K. Ng & J.C.Y. Lai, 10.02.2009 ; 1 ov. female (cl indet.), MNHN-IU-2018-5598, same collection data as for previous specimen ; 1 male (cl 15.1 mm), 1 ov. female (cl 13.8 mm), ZRC 2009.0306 View Materials , Terengganu, Telok Tebrau , mudflats, with gobies, leg. Z. Jaafar, 05.11.2002 ; 1 male (cl 13.9 mm), ZRC 1979.4 View Materials .3.7, Selangor, Port Klang ( Port Swettenham ), mudflats, leg. S. Kumar, 27.08.1968 ; 1 male (cl 14.7 mm), MNHN-IU-2018-5595, Selangor, Port Klang, Taman Sri Sementa , leg. N.K. Ng & J.C.Y. Lai, 10.02.2009 .
Singapore: 1 male (cl 18.0 mm), ZRC 2000.1220 View Materials , East Coast , mudflat, leg. P.K.L. Ng, 18.04.1996; 1 female (cl 10.6 mm), ZRC 1996.0463 View Materials , Sungei Punggol , sta. D1, mudflat, leg. R.E.S.T., 28.06.1995; 1 female (cl 11.0 mm), ZRC 1996.47 View Materials , Mandai , mangrove, burrow in soft mud, leg. P.K.L. Ng, 18.05.1984; 1 male (cl 10.8 mm), ZRC 2000.2145 View Materials , Sungei Buloh , mangrove, mud, leg. NUS Zoology Class, 28.07.1992; 1 male (cl 15.0 mm, missing minor cheliped), ZRC 1979.4 View Materials .4.14, Geylang River, sta. B71, mud bottom, depth: 2 m, leg. S.R.F.R.S., 03.03.1955 [J6851, det. D.S. Johnson as A. microrhynchus ]; 1 female (cl 10.7 mm, identification tentative), ZRC 1991.10140 View Materials , Singapore River , mud, leg. R.E.S.T., 03.10.1988 .
Philippines: 2 males (cl 13.8, 14.2 mm) , 1 female (cl 18.3 mm), ZRC 1999.0235 View Materials , Manila (?) fish market, leg. S.H. Tan, 26.03.1996 ; 1 female, RMNH Crus.D. 49750, Panay, Ilo Ilo, leg. J.C. Miquel, 19.06.1979 ; 1 female (cl 16.9 mm), ZRC 2014.0725 View Materials , Panay, Ilo Ilo, market near fishing port, leg. N.K. Ng & J.C.Y. Lai, 22.01.2005 ; 1 female? (cl 21.4 mm, pleopods damaged, missing minor cheliped), USNM 252028 About USNM , Palawan, Malampaya River , 26.12.1908 ; 1 male (cl 18.4 mm), USNM 1199121 About USNM , Luzon, Manila Bay, Malolos , Guzman Island , Bulacan, fish pond, 02.09.1927 .
Thailand: 1 male (cl 20.3 mm), OUMNH. ZC. 2019.06.46, Songkhla Lake (Thale Sap), leg. S. Hajisamae, 17.08.2013 ; 2 males (cl 13.3, 15.6 mm), 2 females (cl 10.1, 13.2 mm), ZRC 2014.0675 View Materials , same collection data as for previous specimen ; 1 male (cl 16.8 mm), ZRC 2014.0672 View Materials , Pattani Bay , Pattani, leg. S. Hajisamae, 01.2013 ; 1 male (cl 16.0 mm), OUMNH. ZC. 2019.06.47, same collection data as for previous specimen ; 4 females (cl 16.8–20.8 mm), USNM 65478 About USNM , Tachin River , leg. H.M. Smith, 30.10.1923 ; 1 female (cl 20.0 mm), USNM 65557 About USNM , Paknam, Menam Chao Phraya near Bangkok, leg. H. Smith, 21.07.1925 ; 1 female (cl 22.0 mm), NHM 1976.559, Phuket, leg. N. Ngoc-Ho, 1973 [det. D.M. Banner as A. euphrosyne ] ; 2 males (cl 21.6, 24.0 mm), 2 females (cl 22.5, 24.5 mm), NHM 1976.560, Phuket, leg. N. Ngoc-Ho, no date (possibly 1973) [det. A. Anker as A. euphrosyne ?] .
Cambodia: 1 male (cl 18.4 mm), 3 ov. females (cl 16.0– 20.7 mm), MNHN-IU-2014-10287, locality not specified, “Sac 200 / 7C”, leg. M. Fily; 2 males, 3 ov. females (cl indet.), MNHN-IU-2018-5727, locality not specified, “Sac 200 / 7B”, leg. M. Fily.
Vietnam: 1 female (cl 11.9 mm), OUMNH. ZC. 2019.06.48, Haiphong, Cam River , back swamp, leg. K. Wada, 12.09.1995 [YMP-1557]; 1 female (cl 17.8 mm), MNHN-IU-2018-5737, Haiphong, Cam River , back swamp, leg. K. Wada, 12.09.1995 [YMP-1558]; 3 males (cl 13.0– 14.2 mm, pinned dried specimens), MNHN-IU-2000-1124, Tonkin, locality not specified, leg. H. Muhllk / coll. J. Bourgeois , 1911; 1 ov. female (cl 18.7 mm), MNHN-IU-2018-5729, Ha Tien, Sau , 20–30 m (?), leg. H. Duy, 21.08.1995 .
China: 2 females (cl 18.0, mm, 18.5 mm), 1 ov. female (cl 13.4 mm), USNM 62108 About USNM , locality unknown, don. S.F. Light .
Australia: 1 ov. female (cl 16.2 mm, missing minor cheliped), AM P 28130 , Queensland, Cairns, Barron River , 16°55’S 145°46’E, among crab burrows ( Sesarma sp. ), deep within riverbank, leg. J.C. Yaldwyn, 02.11.1963 GoogleMaps .
Tentative identification. Alpheus cf. eurydactylus . Singapore: 1 male (cl 10.5 mm, tl 28.0 mm, rostrum damaged, missing minor cheliped), ZRC 2014.0724 View Materials , Lim Chu Kang, mangrove, sta. Y595, 04.04.1989 ; 1 female (cl 15.7 mm, missing both chelipeds), ZRC 2014.0674 View Materials , Jurong prawn pond, no further data [J8048] ; 1 female (cl 9.6 mm, tl 26.0 mm), ZRC 1979.4 View Materials .4.6, Jurong , prawn ponds, leg. S. R.F. R.S., 1– 2.06.1954 [J8096]; 1 female (cl 12.5 mm, tl 35.0 mm, missing major cheliped), ZRC 1992.8064 View Materials , Sungei Buloh , mangrove swamp, leg. P.K.L. Ng, 03.04.1991 .
India: 1 ov. female (cl indet.), RMNH Crus.D. 49749 , Kochi (Cochin), leg. J.C. Miquel, 28.03.1979 (det. Y. Miya as A. euphrosyne ) .
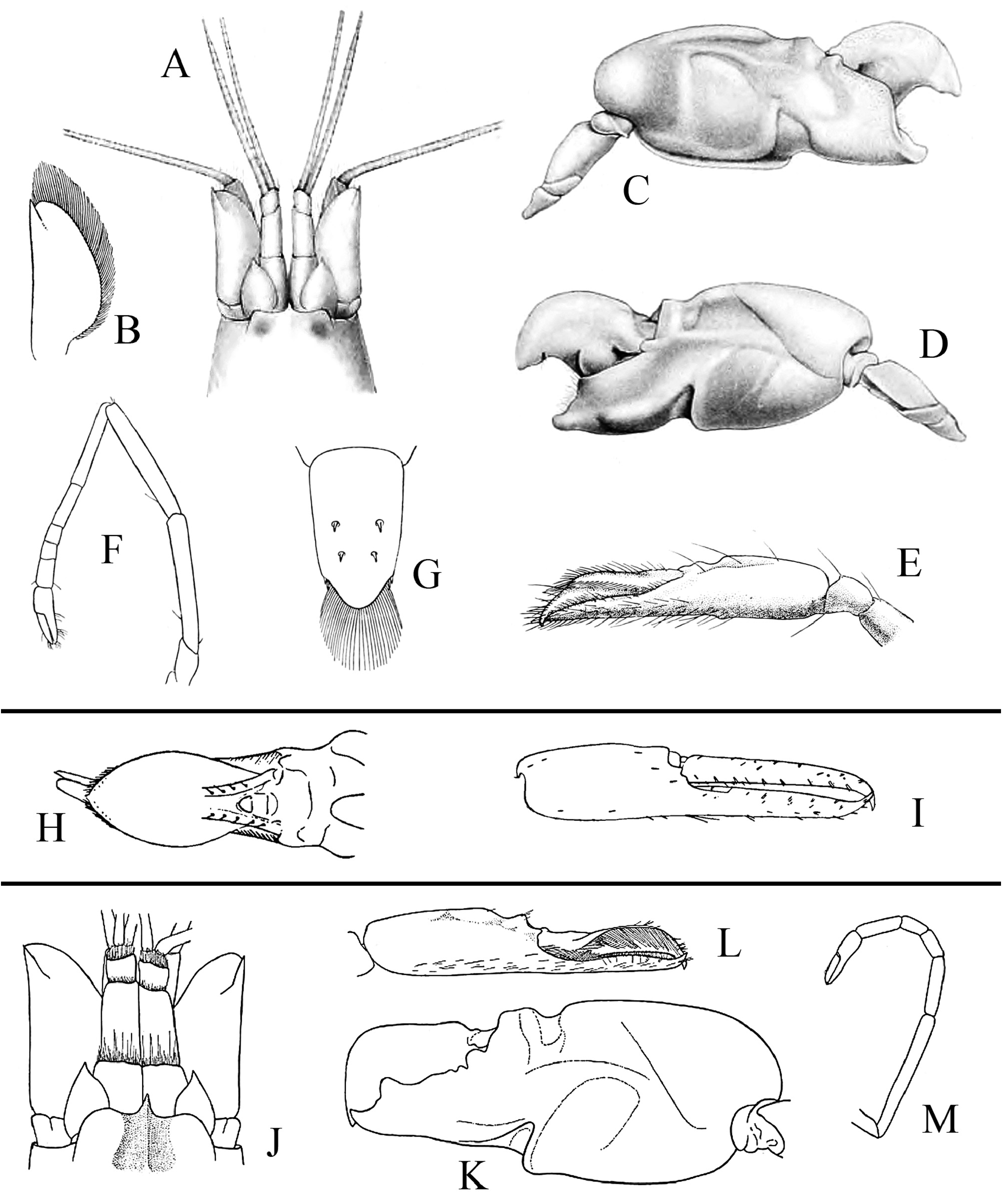
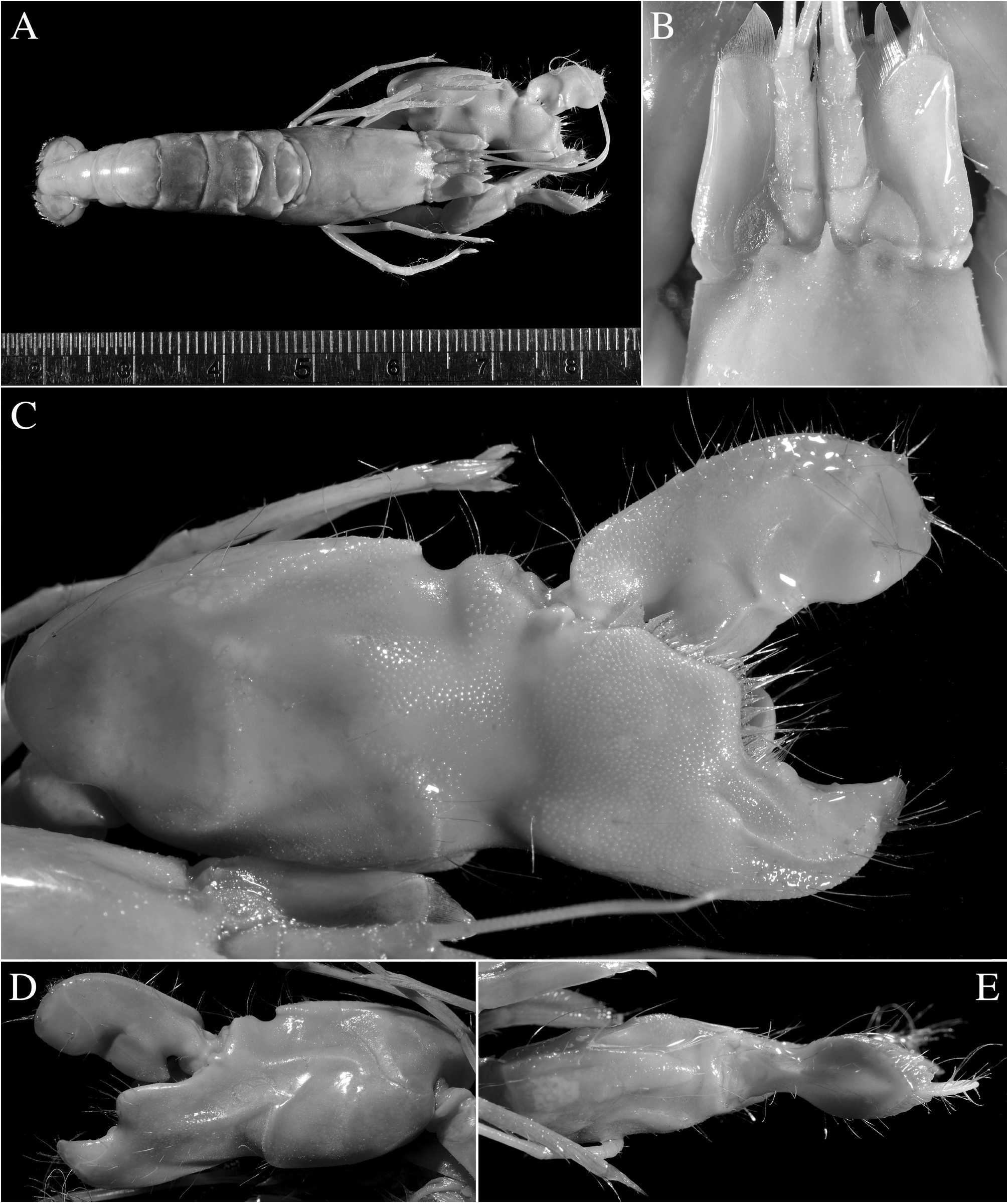
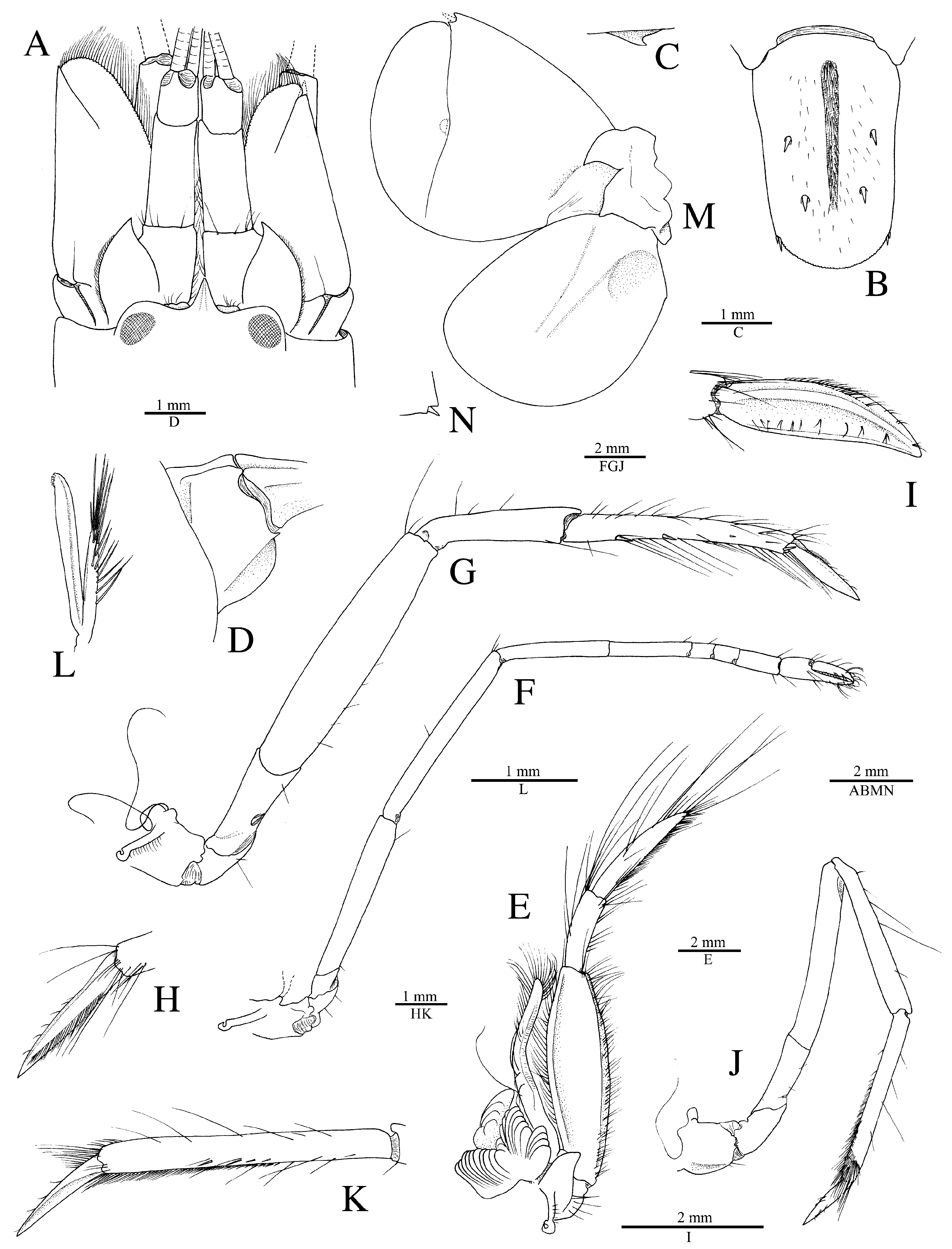
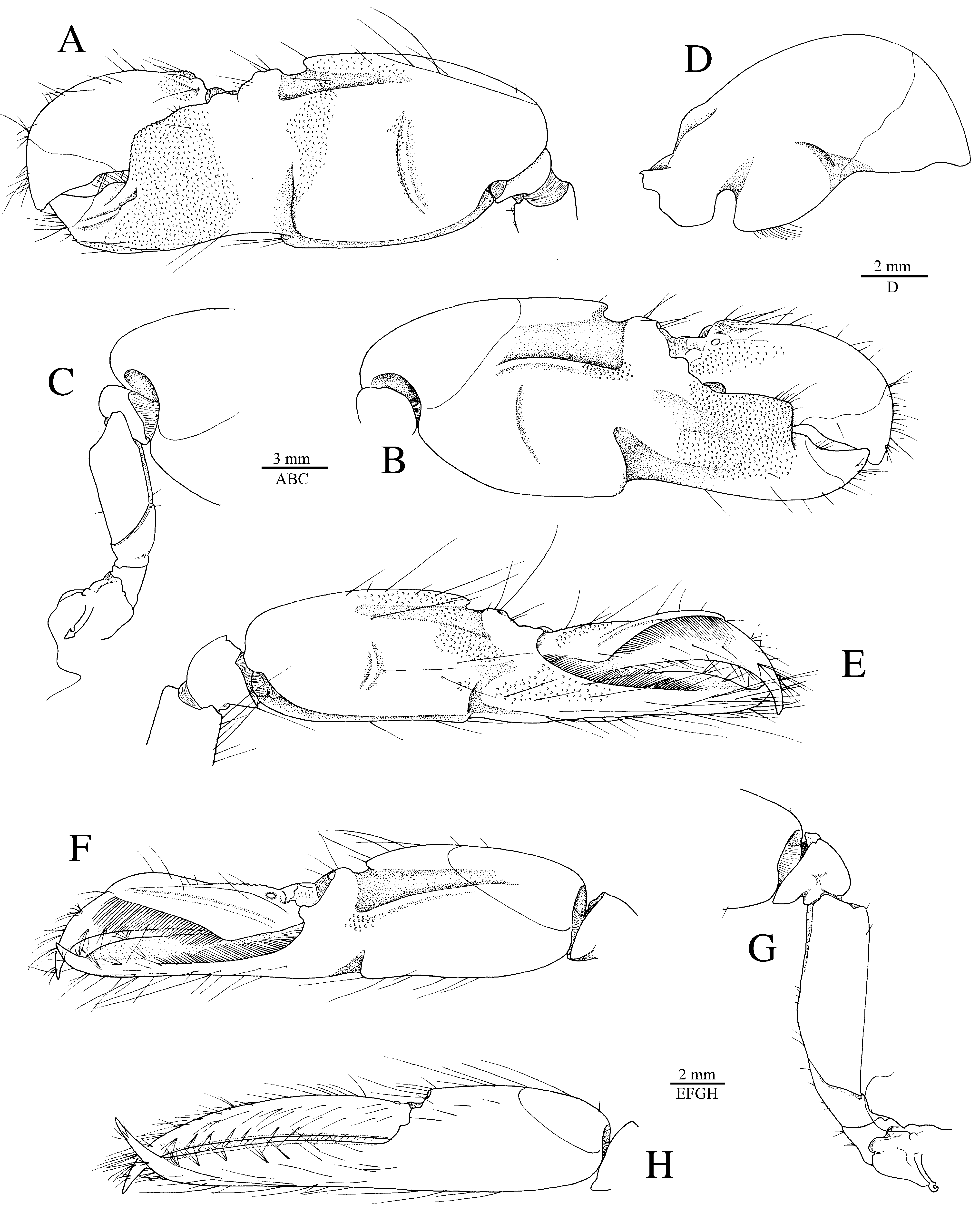
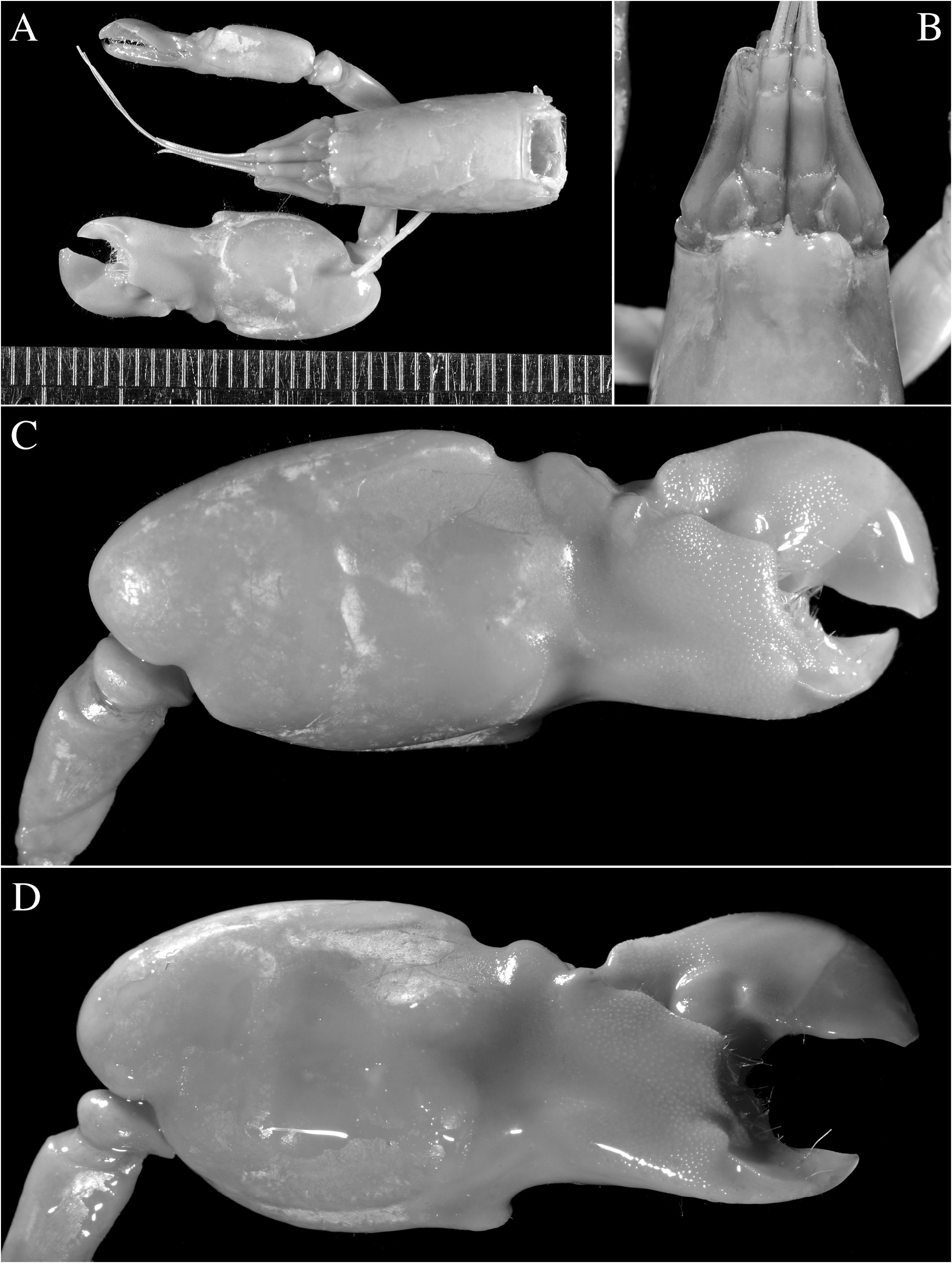
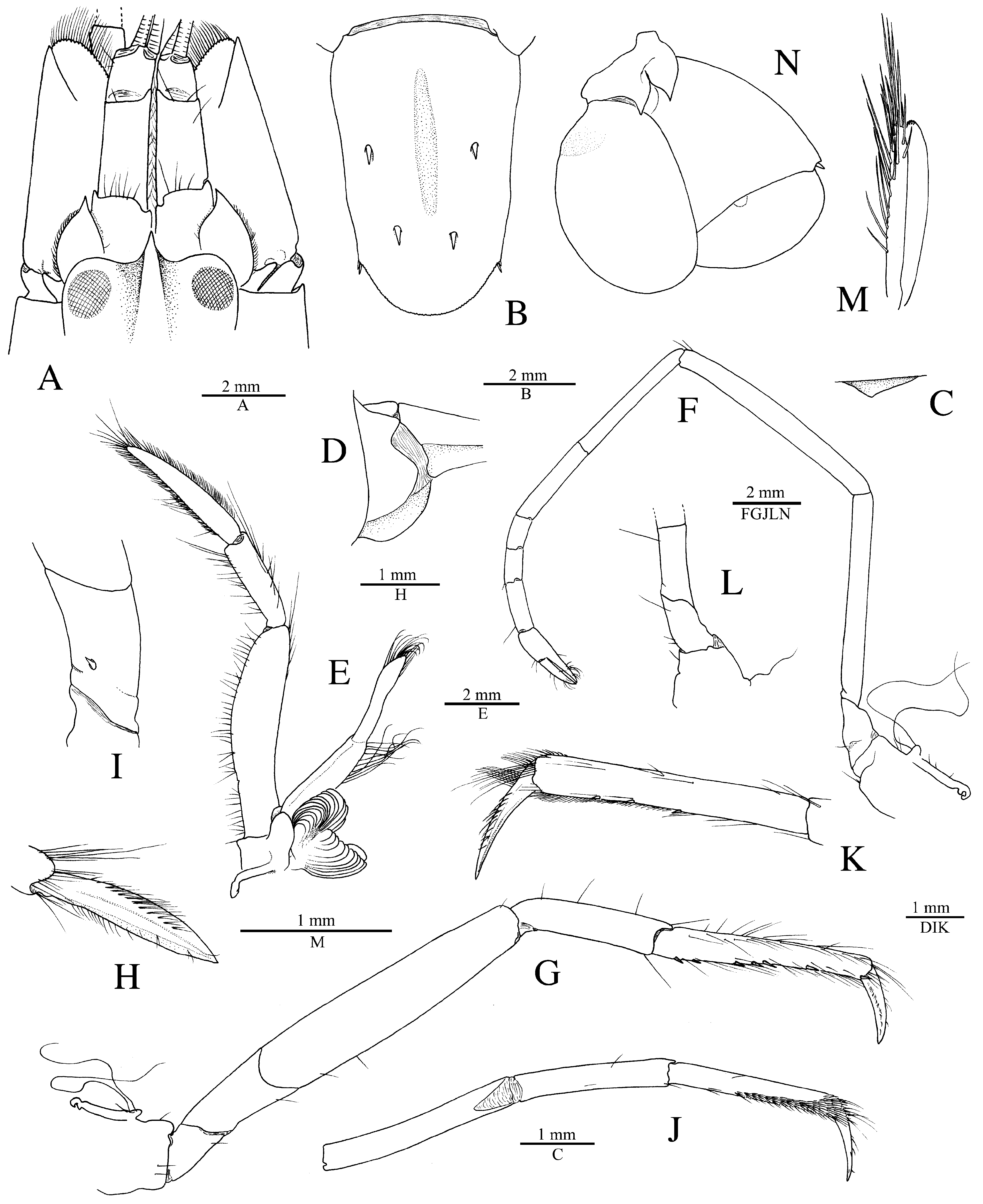
Redescription. See Figs. 11–15 View FIGURE 11 View FIGURE 12 View FIGURE 13 View FIGURE 14 View FIGURE 15 . Large-sized species of Alpheus (maximal cl 20.3 mm, tl ~65.0 mm). Carapace glabrous, finely pitted, especially around cardiac region, without pubescence, with slight grooves. Rostrum short, subtriangular, narrowing towards apex, distally acute, typically falling short of mid-length of first article of antennular peduncle; rostral carina feebly developed, low, flattening posterior to orbital hoods; rostro-orbital furrows distinct, shallow; anterior margin of orbital hoods shallowly concave. Pterygostomial angle broadly rounded; cardiac notch deep.
Pleon smooth, finely pitted, without pubescence. Telson broad, ovate-rectangular, slightly tapering towards posterior margin, about 1.3–1.4 times as long as maximal width near proximal margin; lateral margins very shallowly concave in proximal half almost straight in distal half; dorsal surface finely pitted, with shallow, longitudinal, median depression (without setae on edges) and two pairs of stout spiniform setae inserted in deep pits at some distance from lateral margins, first pair near telson mid-length, second pair at about 0.7 of telson length; posterior margin broadly rounded, with two pairs of small spiniform setae at posterolateral angles, lateral shorter than mesial (sometimes broken or missing).
Antennular peduncle with stylocerite broad, moderately convex laterally, with acute tip, latter not overreaching distal margin of first article; ventromesial carina with anteriorly rounded tooth, without acute point; second article about 2.2–2.3 times as long as wide. Antennal peduncle with distoventral margin of basicerite unarmed; carpocerite not reaching beyond scaphocerite; scaphocerite with lateral margin straight to very shallowly concave; blade very broad, separated from broad distolateral tooth by deep cleft; anterior margin of blade broadly rounded and reaching distinctly beyond distolateral tooth.
Third maxilliped with antepenultimate article not particularly broadened; penultimate article about 2.5 times as long as wide, with long setae on ventrolateral margin, latter surpassing ultimate article; ultimate article with dense transverse rows of short serrulate setae on mesial surface and some longer setae on dorsal and lateral surfaces, especially near or on apex; coxal lateral plate bluntly produced dorsally; exopod fleshy, broad, reaching end of antepenultimate article.
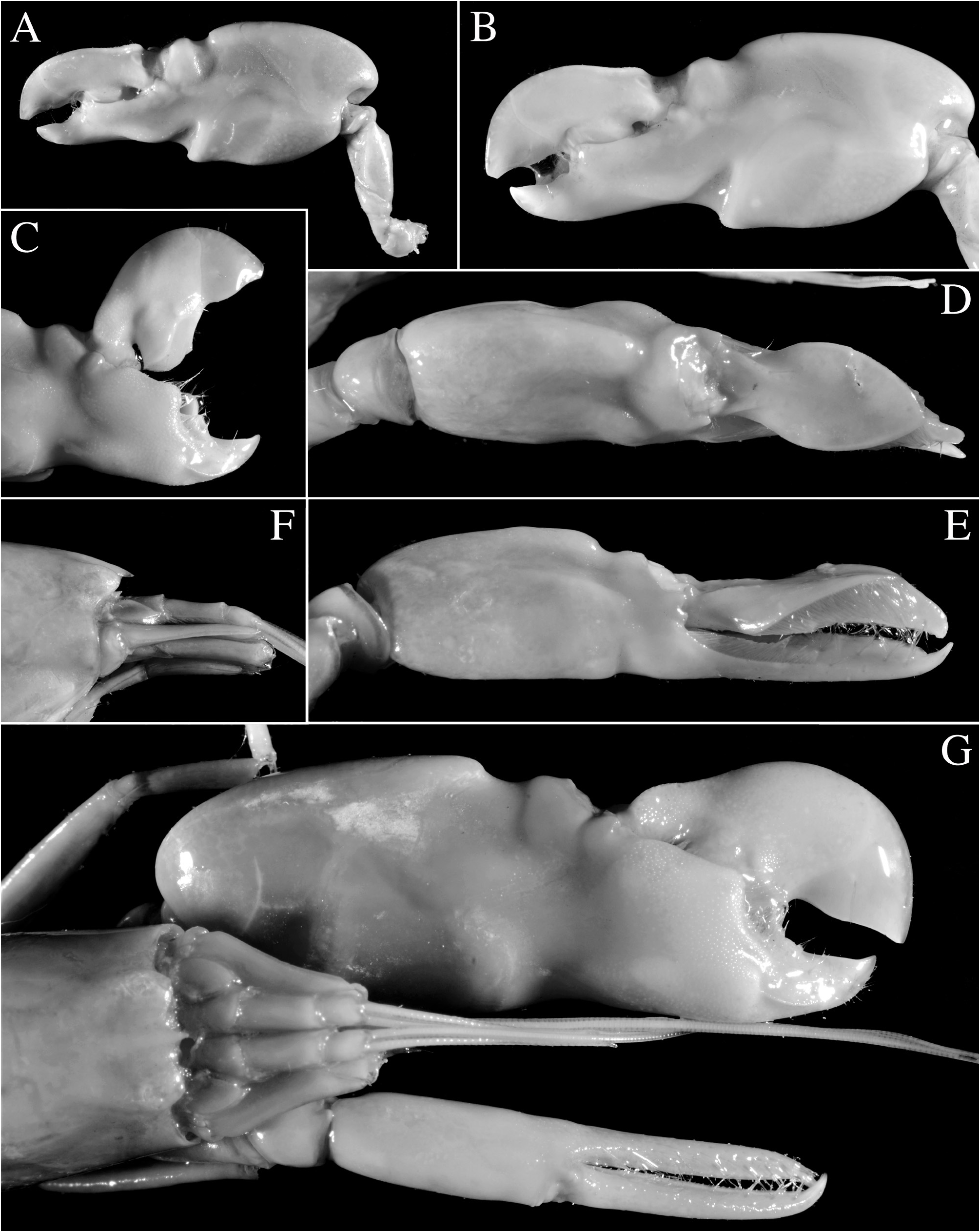
Major cheliped of A. edwardsii - type. Merus very stout, about 1.8 times as long as wide; ventromesial margin unarmed distally. Carpus very short, cup-shaped. Chela large, massive, with fingers about 0.6–0.7 length of palm. Mesial face of palm with low mesial transverse ridge in proximal half, extending from proximal edge of subtriangular mesial longitudinal groove to ventral surface of palm; surface smooth, largely without granules, except for very few near ventral shoulder. Lateral face of palm smooth, without granulation; lateral longitudinal groove deep, subrectangular; lateral half with low transverse ridge. Dorsal shoulder pronounced, rounded, sloping with angle of 45º–80º into broad transverse groove, not overhanging. Ventral shoulder pronounced, slightly protruding in lateral view, broadly rounded. Pollex with mesial face granulated over most of its surface, with prominent mesial subdistal ridge marked by conspicuous protuberance proximally and extending to subdistal angle of pollex; area ventral to mesial subdistal ridge conspicuously grooved; distomesial angle distinctly superior to 90º, blunt; lateral face smooth, not granulated; distolateral angle about 90º, broadly rounded. Dactylus slightly longer than pollex, with prominent dorsal ridge, latter twisted mesially and protruding proximally; mesial surface granulated in proximal half; lateral surface largely smooth; dactylar plunger large, stout, clearly distinct from ventral dactylar margin; distal surface of plunger with obliquely flattened ventromesial area, its mesial surface with feebly developed transverse channel. Adhesive discs small.
Male minor cheliped with chela strongly balaeniceps. Merus somewhat slenderer than that of major cheliped, about 2.5 times as long as wide; ventral margin with distomesial angle unarmed. Chela moderately swollen, with fingers about as long as palm. Palm subcylindrical, somewhat swollen; mesial face with surface largely smooth, except for very small field of granules on subdistal swelling below mesial longitudinal groove; mesial longitudinal groove well marked, deep; lateral longitudinal groove well marked, deep; dorsal shoulder pronounced, rounded, sloping with 45º or smaller angle into deep dorsal transverse groove; ventral transverse groove moderately deep, bordered by distinct ventral shoulder on lateral surface. Pollex with largely smooth surfaces; mesial surface with row of balaeniceps setae on low crest extending from base to slightly beyond mid-length of pollex; lateral surface with similar row of balaeniceps setae as on mesial surface. Dactylus with well-developed proximal balaeniceps expansion, about 2.0–2.2 times as long as maximal width; lateral and mesial dactylar ridges each with dense rows of balaeniceps setae. Cutting edges of both fingers shallowly excavated on each side of sharp, blade-like ridge.
Female minor cheliped simple, not balaeniceps. Chela slender, not particularly swollen, with fingers about 1.1–1.2 times length of palm. Palm surface smooth, without granulation (at most with some granules on distomesial surface), with shallow (sometimes traces of) mesial and lateral longitudinal grooves, and with shallow dorsal transverse groove and poorly demarcated ventral shoulder (sometimes not distinct). Fingers simple, with sharp cutting edges, not or slightly gaping when closed; fingertips curved and crossing.
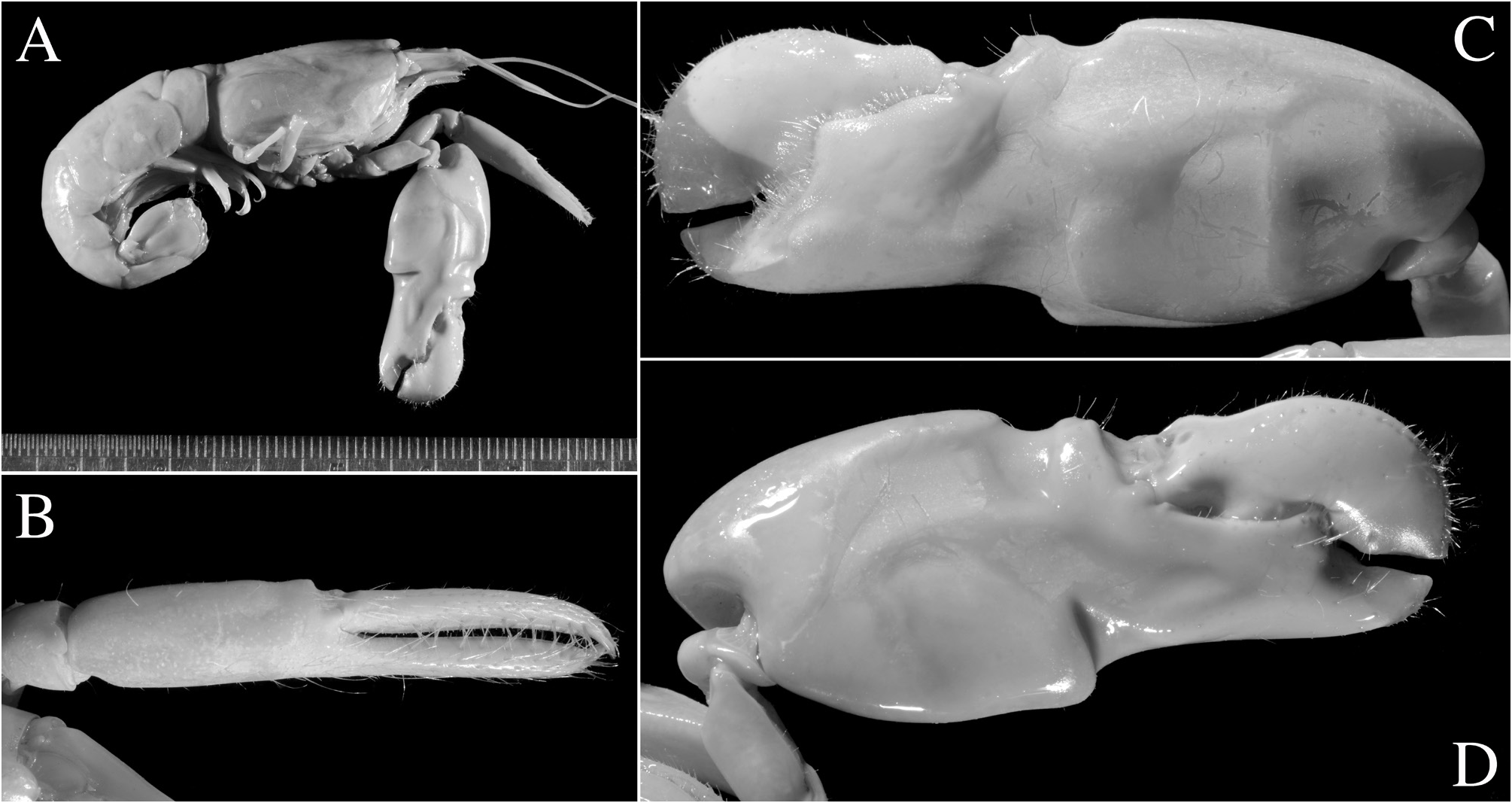
Second pereiopod with ratio of carpal subarticles approximately equal to 4.2: 2.5: 1: 1: 1.5. Third pereiopod fairly robust; ischium armed with small, stout spiniform seta on ventrolateral surface near proximal margin, sometimes unarmed (especially in larger individuals); merus about 4.2 times as long as maximal width, unarmed; propodus with five or six stout spiniform setae on ventral margin and one pair of spiniform setae (sometimes one seta) on distoventral margin adjacent to dactylus; dactylus about 0.4 and sometimes close to half-length of propodus, spatulate, expanded, as wide as propodus in dorsal view, with row of tufts of setae along mesial ridge. Fourth pereiopod generally similar to third pereiopod, slightly slenderer; merus about five times as long as maximal width; dactylus about 0.4 length of propodus, spatulate. Fifth pereiopod much slenderer than third and fourth pereiopods; ischium unarmed; propodus with three small spiniform setae and well-developed setal brush; dactylus subspatulate, about 0.4 length of propodus.
Male second pleopod with appendix masculina ~0.8 length of appendix interna. Uropod with each protopodal lobe ending in sharp tooth distally; exopod and endopod broad, ovate; diaeresis of exopod complete, almost straight, with small subacute or blunt tooth near small distolateral spiniform seta and adjacent distolateral tooth; lateral margin of exopod broadly convex.
Eggs numerous (> 200 in large females), small; egg diameter less than 0.5 mm.
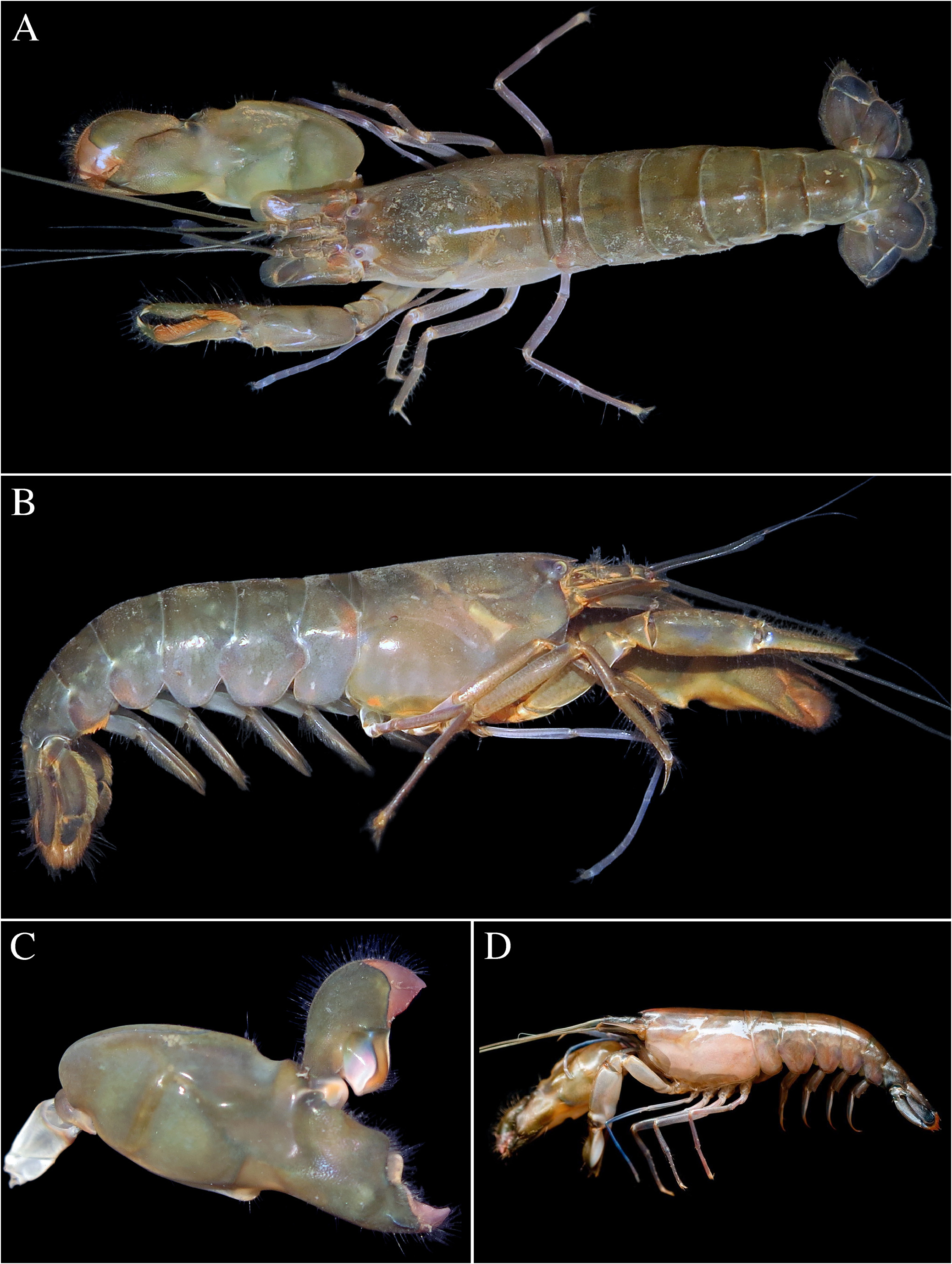
Colour pattern. Body uniform dark green, green-brown or brown, without marked banding on carapace or pleon; antennular flagella greenish, bluish proximally; antennal flagella olive-greenish with some bluish tinge proximally; mesial face of major chela olive or greenish brown with large pale orange area, especially near ventral transverse groove; fingers darker olive-green, distally purplish or pinkish; dactylar plunger with mesial side white and blue proximally and bright orange distally and anteriorly; minor chela olive-green with darker fingers; second pereiopods reddish proximally, bright blue distally; ambulatory legs reddish; pleopods pinkish; telson and uropods darker than pleon; uropods with dark, dusky blue patch distally ( Fig. 16 View FIGURE 16 ). One of the Singaporean females (ZRC 1996.47) was accompanied by a short colour note: “overall dark green, chela dactylus purple”.
Type locality. Surabaya , Java, Indonesia .
Distribution. Tropical Indo-West Pacific from the southern Andaman Sea and Malacca Straits to South-China Sea and northern Australia ( Fig. 51B View FIGURE 51 ), including coasts of Indonesia (Sumatra, Java, Papua), Singapore (East Coast, Mandai, Sungei Buloh, Punggol), Malaysia (Penang, Selangor, Johor), Thailand (Songkhla Lake, Pattani), Cambodia, Vietnam (Haiphong, Nha Trang, Ho Chi Minh City, Can Gio), China, Philippines (Manila Bay, Panay), and Australia (Queensland) ( De Man 1920, 1924; Marin 2021; present study); possibly extending to southern India ( Kochi, see discussion below and Fig. 51B View FIGURE 51 ).
Common name proposed. De Man’s mangrove snapping shrimp.
Ecology and biology. Alpheus eurydactylus is a largely intertidal and shallow subtidal snapping shrimp (maximal depth probably not exceeding 3 m) inhabiting estuarine, mangrove mudflats and brackish lagoons, where it lives in deep burrows dug in thick, soft mud, sometimes under large rocks or mangrove debris, among mangrove roots, or in the muddy banks of mangrove creeks. The unusual depth range (20—30 m) given for the female from Ha Tien, Vietnam (MNHN-IU-2018-5729), is somewhat questionable. Like most euryhaline decapods present in these habitats, A. eurydactylus is a resilient shrimp capable of tolerating very low salinities. The ovigerous females of A. eurydactylus carrying a large number of eggs of relatively small size suggest an extended larval development in this species.
This large snapping shrimp is occasionally seen on fish markets and crab / shrimp landings, for instance, in the Philippines (Manila). Interestingly, one of the specimens from Thailand (USNM 65557) was accompanied by a note: “kung deet = snapping shrimp, not eaten but used as bait for catfish and drum”.
Taxonomic remarks. Alpheus eurydactylus is morphologically very close to A. euphrosyne , as redefined above. The two species can be most easily separated from each other by the dorsal shoulder of the major chela sloping with an angle of>45º into the adjacent transverse groove in A. eurydactylus ( Figs. 11C, D View FIGURE 11 , 15A, C View FIGURE 15 ) vs. forming a rounded tooth overhanging the transverse groove in A. euphrosyne ( Figs. 5C, D View FIGURE 5 , 7A, B View FIGURE 7 ). In A. eurydactylus , the mesial palmar surface of the major chela is granulated only distally and subdistally ( Fig. 15C View FIGURE 15 ), whereas in A. euphrosyne , several fields of granules are also present on the central portion of the palmar surface ( Fig. 7A View FIGURE 7 ). The dactylar plunger of A. eurydactylus is larger, heavier and not continuous with the ventral edge of the dactylus, as in A. euphrosyne (cf. Figs. 7D View FIGURE 7 , 15D View FIGURE 15 ). The rostral carina and rostro-orbital furrows are more demarcated in A. eurydactylus than in A. euphrosyne , where they are both not or barely distinct (cf. Figs. 6A View FIGURE 6 , 14A View FIGURE 14 ). Some differences between the two species also exist in the sculpture and proportions of the female minor chelae (cf. Figs. 7H View FIGURE 7 , 15H View FIGURE 15 ). The colour patterns of A. eurydactylus ( Fig. 16 View FIGURE 16 ) and A. euphrosyne ( Fig. 8–10 View FIGURE 8 View FIGURE 9 View FIGURE 10 ) are markedly different. In addition, A. eurydactylus is a largely intertidal and shallow subtidal species commonly encountered in soggy mud in mangroves and on estuarine mudflats, whereas A. euphrosyne appears to be a subtidal snapping shrimp preferring sandy-muddy bottoms in deeper water (8–27 m), in brackish lagoons and near estuaries. For separation of A. eurydactylus from other morphologically similar species (especially A. takla sp. nov.) see below or refer to Table 1 View TABLE 1 .
Some previous records of A. euphrosyne or A. euphrosyne euphrosyne , for instance those in Banner & Banner (1966, 1981, 1982, 1983) and Sha et al. (2019), may actually refer to A. eurydactylus . At least some specimens from Australia identified by D.M. Banner & A.H. Banner as A. euphrosyne euphrosyne were reexamined by the author and reidentified as A. eurydactylus . At least part of the material reported as A. euphrosyne by Sha et al. (2019), collected at various localities in southern and eastern China, could also represent A. eurydactylus , at least judging from the detailless illustrations provided by these authors.
Banner & Banner’s (1981, 1983) records of A. euphrosyne euphrosyne from the northern and western Indian Ocean cannot be confirmed as A. eurydactylus without examination of their material, which unfortunately remains untraceable. The photograph of a snapping shrimp identified as A. malabaricus from Vembanad Lake, Kerala ( Harikrishnan & Kurup 2008: fig. 1, specimen on the right side), superficially resembles A. eurydactylus . However, a short correspondence between the present author and Dr. M. Harikrishnan in 2014 (accompanied by photographs of some morphological details of the preserved material) led to the conclusion that it could be A. mangalis sp. nov. or another closely related taxon (see below). On the other hand, a colour photograph of a large male from Kochi, India, sent by Dr. Anil Kumar in 2021 (A. Anker, pers. obs.) shows a snapping shrimp with essentially the same colour pattern as A. eurydactylus from Vietnam and Thailand ( Fig. 16 View FIGURE 16 ; see also Marin 2021: fig. 1a). In particular, the general shape and the diagnostic colour of the dactylar plunger of the Indian and the Vietnamese specimens are identical. The only noticeable difference is the distally blue second pereiopod in the specimens from Vietnam and Thailand; this appendage is entirely reddish in the Indian specimen. Although the presence of A. eurydactylus in India, or more generally in the Indian Ocean west of Sumatra, cannot be confirmed at this stage, the above observations strongly suggest that the geographical range of A. eurydactylus may extend to south-western India (as indicated in Fig. 51B View FIGURE 51 ). In any case, a more thorough examination of the Indian material (unavailable for the present study) and its molecular comparison with the South-East Asian material will be needed for clarification of the actual distribution of A. eurydactylus in the Indian Ocean.
The material herein tentatively identified as A. cf. eurydactylus from Singapore is morphologically heterogeneous and possibly contain two different species. The specimens from Jurong, Sungei Buloh and Lim Chu Kang are different from the typical A. eurydactylus in several details. For instance, in the incomplete male from Lim Chu Kang (ZRC 2014.0724), the third pereiopod merus is about 3.5 times as long as wide, i.e., is relatively broader than in A. eurydactylus . The ischia of the third and fourth pereiopods have no trace of a spiniform seta on the ventrolateral margin, as in A. microrhynchus (see below), and unlike the ischia of A. eurydactylus , which are usually armed with a stout spiniform seta. The third pereiopod dactylus is proportionally longer, at least 0.6 as long as the propodus. In the second pereiopod, the relative length ratio of the carpal subarticles (4.5: 2: 1: 1: 2) is slightly different from that of the typical A. eurydactylus (4.2: 2.5: 1: 1: 1.5). Unfortunately, the major cheliped of the male from Lim Chu Kang appears to be regenerated, judging from the somewhat unusual proportions of the chela. Nevertheless, it can be characterised by the ventromesial margin of the merus distally unarmed; the dorsal shoulder gently sloping into the adjacent transversal groove; the mesial face of the pollex with some granules, but without mesial subdistal ridge; and the moderately developed, obliquely “sliced” plunger. The rostro-orbital region is aberrant in the absence of a rostrum, probably resulting from an injury (also indicated by the much smaller, clearly regenerated right antennular peduncle). The antennal basicerite is unarmed on both sides, as in the typical A. eurydactylus , but the carpocerite is longer than in A. eurydactylus , reaching far beyond the distal margin of the scaphocerite, as seen in A. paludicola ( Yeo & Ng 1996) . The females from Jurong and Sungei Buloh are similar to the male from Lim Chu Kang in the proportions of the third and second pereiopods, antennules, and antennae. The major cheliped of the complete female from Jurong (ZRC 1979.4.4.6) appears to be regenerated and is generally similar to that of the male, except for the absence of granulation. The frontal margin of the carapace of this specimen bears a minute rostrum, similar to that of A. microrhynchus or A. paludicola . The minor chelipeds of the females from Jurong and Sungei Buloh (ZRC 1992.8064) are characterised by the palm being smooth on both sides and bearing a slight ventral notch or sinus, and with the fingers slightly longer than the palm; in these features, they are generally concordant with the female minor cheliped of A. eurydactylus . However, the relatively broad merus of the third pereiopod, the absence of a median dorsal groove on the telson and the very short rostrum of these females are features that do not allow the identification of the Jurong and Sungei Buloh specimens as A. eurydactylus . In many respects, the female from Sungei Buloh, for instance, appears to be intermediate between A. eurydactylus and A. microrhynchus . The ovigerous female from Kochi, India (RMNH Crus.D.49749), is also problematic, largely for the same reason as the female from Jurong, i.e., in the absence of marked granulation on the mesial face of the major cheliped. A molecular comparison (e.g., using the barcoding segment of the mitochondrial COI gene) of the material herein assigned to A. cf. eurydactylus with the typical A. eurydactylus and A. microrhynchus is needed to confirm its identity.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Alpheus eurydactylus De Man, 1920
| Anker, Arthur 2023 |
Alpheus cf. euphrosyne
| Marin, I. N. 2021: 381 |
Alpheus euphrosyne
| Johnson, D. S. 1976: 37 |
| Banner, A. H. & Banner, D. M. 1966: 130 |
Alpheus eurydactylus
| De Man, J. G. 1924: 48 |
| De Man, J. G. 1920: 109 |