Alpheus pontederiae de Rochebrune, 1883
|
publication ID |
https://doi.org/10.11646/zootaxa.5282.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:DF418763-8F0E-44DD-97C4-B123A81A8DB4 |
|
DOI |
https://doi.org/10.5281/zenodo.7921865 |
|
persistent identifier |
https://treatment.plazi.org/id/2A26026D-4B12-FFD1-E7B8-FD1FFF2BFA51 |
|
treatment provided by |
Plazi (2023-05-09 07:04:28, last updated 2024-11-24 20:27:28) |
|
scientific name |
Alpheus pontederiae de Rochebrune, 1883 |
| status |
|
Alpheus pontederiae de Rochebrune, 1883 View in CoL View at ENA
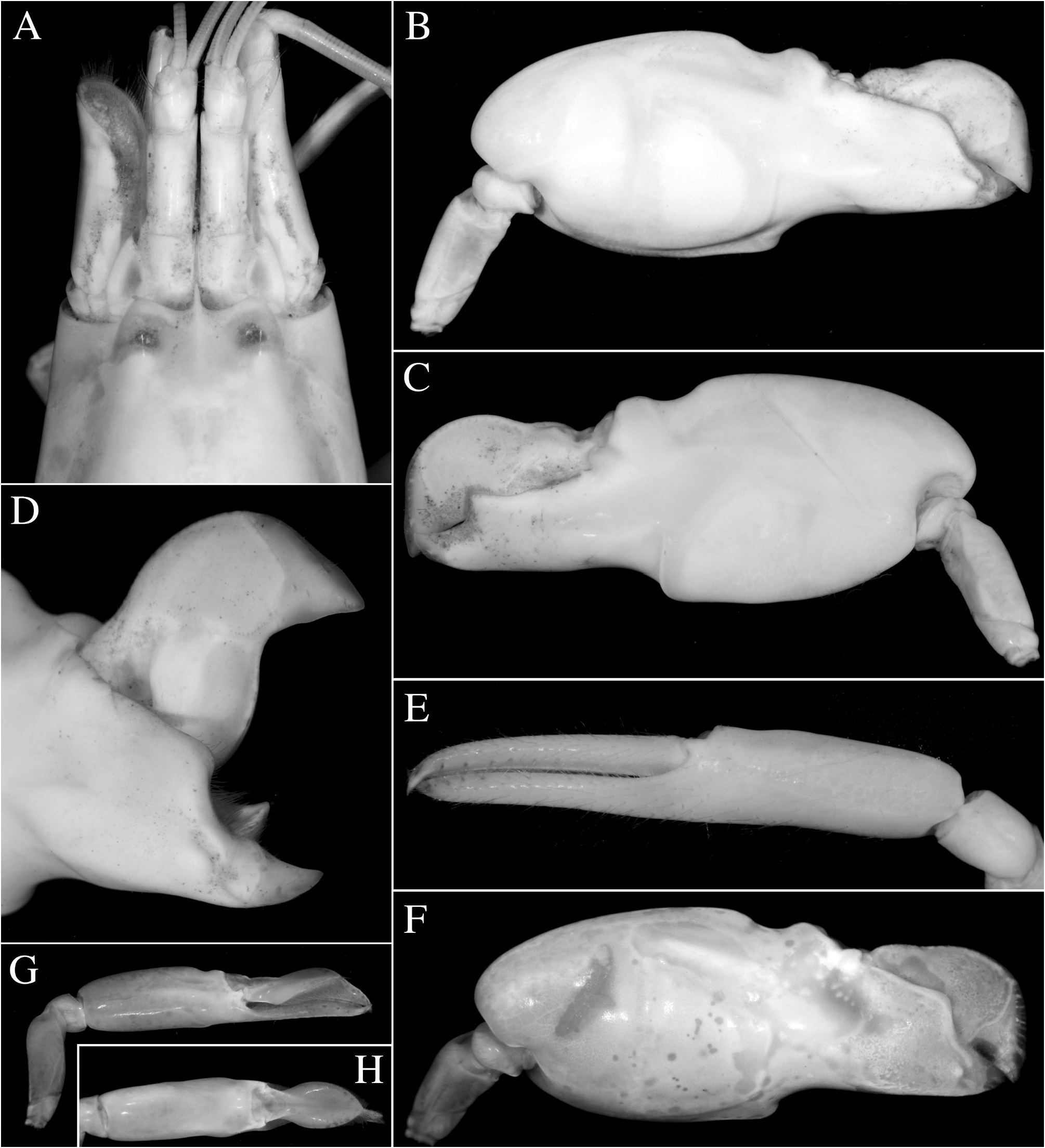
( Figs. 3J–M View FIGURE 3 , 47 View FIGURE 47 , 48A–D View FIGURE 48 , 52E View FIGURE 52 )
Alpheus Pontederiae de Rochebrune 1883: 174 View in CoL .
Alpheus pontederiae View in CoL .— Coutière 1899: 37; Holthuis 1951: 85, fig. 17; Longhurst 1958: 31, 46, 47, 49, 91; Rossignol 1962: 131; Crosnier & Forest 1965: 607; Crosnier & Forest 1966: 278, fig. 23a–j; Powell 1979: 127; Christoffersen 1980: 105, figs. 24, 25; Christoffersen 1984: 197, figs. 3, 4; Christoffersen 1998: 360; Almeida et al. 2006: 9; Almeida et al. 2012: 12; Soledade & Almeida 2013: 104, fig. 6D; Pachelle et al. 2016: 9; Soledade et al. 2017: 182, fig. 1; Almeida et al. 2018: 341.
Alpheus Edwardsi. — Aurivillius 1898: 30 [not Alpheus edwardsii ( Audouin, 1826) View in CoL ].
Alpheus megacheles .— Coutière 1899: 37 [not Alpheus megacheles Norman, 1868 ].
Alpheus macrocheles View in CoL .— Rathbun 1900: 312; Balss 1916: 20 [not Alpheus macrocheles ( Hailstone, 1835) View in CoL ].
Crangon langi Schmitt 1926: 20 View in CoL , fig. 63.
Alpheus Bouvieri. View in CoL — Monod 1927: 594 [not Alpheus bouvieri A. Milne Edwards, 1878 View in CoL ].
Alpheus Langi. View in CoL — Monod 1928: 252.
Alpheus heterochaelis var. orientalis Vilela 1949: 55 , fig. 3.
Alpheus euphrosyne langi .— Banner & Banner 1982: 238.
Material examined. Brazil: 1 female (cl 10.0 mm), MZUSP 21489 View Materials , Pará , Bragança , Praia de Ajuruteua, Furo do Meio, sta. 6, 0052,440’S 4939,000’W, mangrove, leg. M. Tavares et al., 03.02.2010; 2 ovig. females (cl 11.3, 16.4 mm), MCZ 96843 About MCZ , Pará, Thayer Expedition 1865, leg. Agassiz & Bouget, 1865 (det. as Crangon lutarius ) ; 1 male, (cl indet.), MPEG, Pará, near Bragança, leg. R.R.R. Vieira, 2016 .
Venezuela: 1 female (cl 19.3 mm), OUMNH. ZC. 2011.06.4, Orinoco Delta , sta. XI-52-24, leg. G. Pereira et al., date unknown ; 1 male (cl 11.7 mm, missing minor cheliped), OUMNH. ZC. 2011.06.2, Orinoco Delta , sta. XI-50-37, leg. G. Pereira et al., date unknown ; 1 male (cl 7.2 mm), OUMNH. ZC. 2011.06.010, north of Maracaibo , El Nazareth, leg. A. Godoy, date unknown ; 1 male (cl indet.), 1 female (cl indet.), MZUSP, Orinoco Delta, further details not recorded .
São Tomé and Príncipe: 1 male (cl ~6.0 mm), OUMNH. ZC. 2011.06.8 , S„o Tomé Island , near Porto Alegre, mangrove, in mud under rocks, leg. A. Anker, 05.02.2006 [06-154].
Description. See de Rochebrune (1883) for original description (without illustrations), Schmitt (1926, as Crangon langi ), Holthuis (1951), Crosnier & Forest (1966), Christoffersen (1984) and Soledade et al. (2017) for additional accouts and taxonomic remarks, all with illustrations; see also Fig. 47 View FIGURE 47 for complementary illustrations.
Colour pattern. Body uniform brownish to brown-green or green-blue; pleon with weak pale yellow markings; posterior-most part of each pleonite somewhat darker, resulting in impression of dark transverse banding; antennular and antennal flagella pale greenish or yellowish; mesial face of major greenish or bluish brown with some blue-grey, purplish and paler yellow areas; fingers darker, more olive-green, with contrasting pink fingertips; mesial subdistal ridge on pollex conspicuously blue; dactylar plunger whitish with green-yellow tinge and blue spot; minor chela greenish or bluish, with darker fingers; walking legs pale reddish; telson marbled with whitish; uropods whitish proximally, mottled with brown or grey distally, exopod darker brown with blue tinge posterior to transverse suture ( Fig. 48A–D View FIGURE 48 ); see also Soledade & Almeida (2013: fig. 6D).
Type locality. Mouth of the rivers Leybar , Thiank and Dakar-Bango, Senegal .
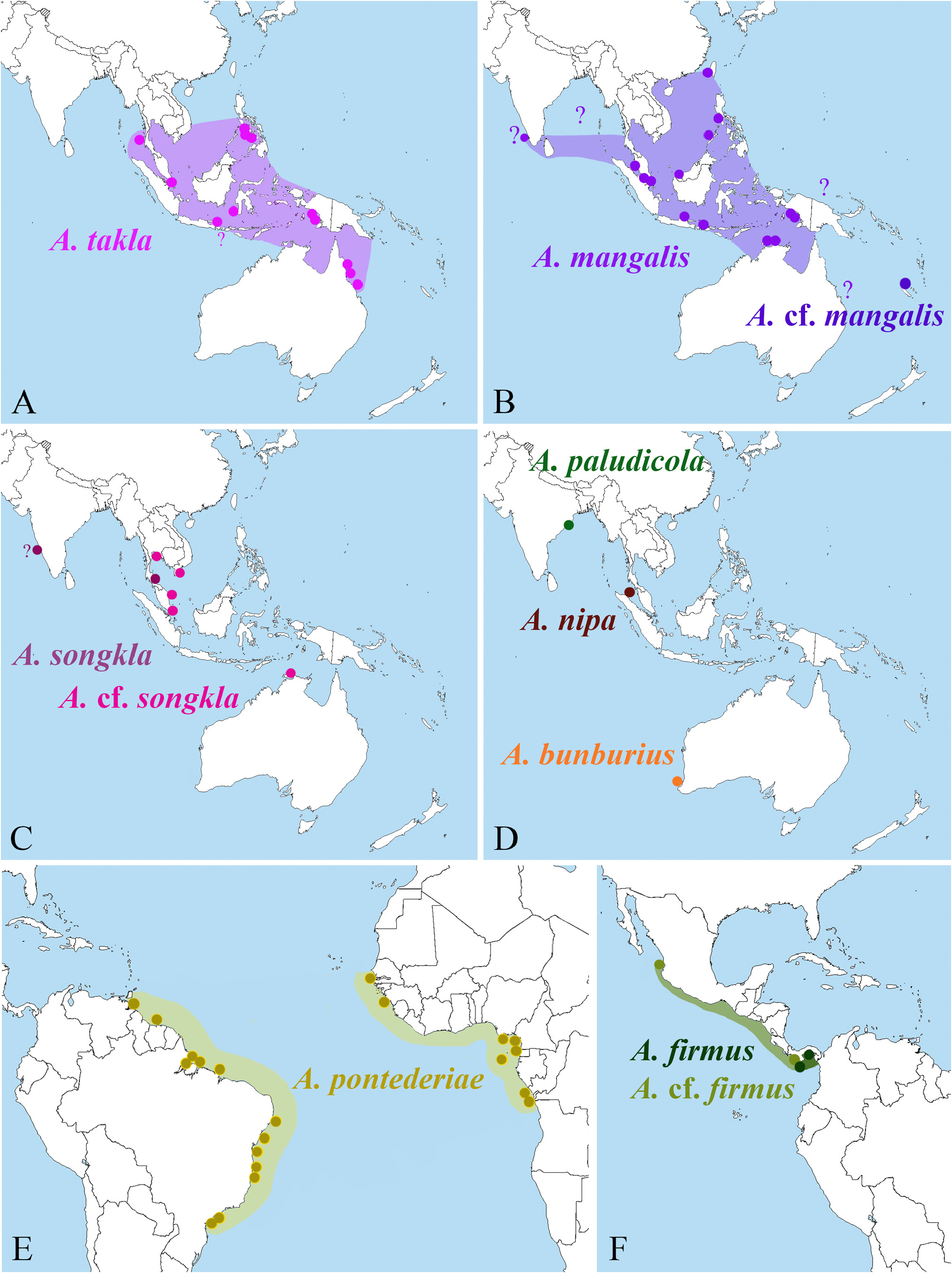
Distribution. Eastern and western Atlantic, from Senegal to Congo, and from Venezuela to southern Brazil ( Fig. 52E View FIGURE 52 ); specific localities include, in the eastern Atlantic: Senegal (Leybar, Thiank, Dakar-Bango), Guinea (Conakry), Nigeria ( Niger delta), Cameroon (Bibundi, Souelaba, Malimba Bay), Pop. Rep. Congo (Pointe Noire), Dem. Rep. Congo (Banana), Equatorial Guinea (Passo, Canchungo, Rubane Island), S„o Tomé ( Schmitt 1926; Vilela 1949; present study); in the western Atlantic: Venezuela (Orinoco Delta), Suriname, Brazil (Pará, Maranh„o, Ceará, Paraíba, Alagoas, Bahia, S„o Paulo, Paraná) ( Christoffersen 1984, 1998; Almeida et al. 2006, 2012; Soledade & Almeida 2013; Pachelle et al. 2016; Soledade et al. 2017; present study).
Common name proposed. Rochebrune’s snapping shrimp.
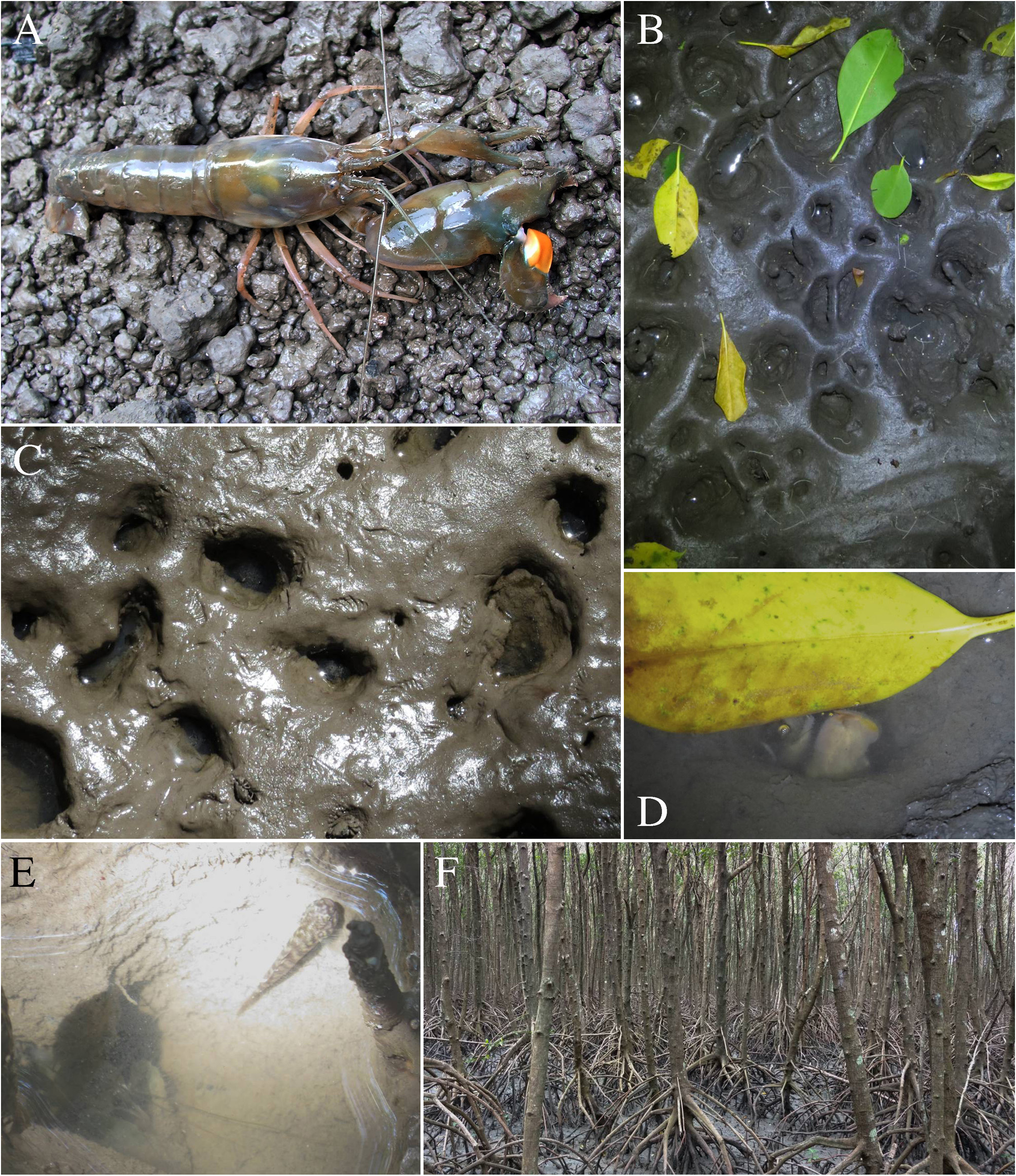
Ecology and biology. Alpheus pontederiae is mainly an estuarine snapping shrimp, inhabiting mudflats and mangrove channels close to river mouths and deltas, often in brackish water (salinity range: 30 / 00 – 200 / 00) (see also Fig. 48E View FIGURE 48 ). The species lives in deep galleries built in mud or muddy sand, sometimes under rocks, decaying wood or other debris, usually in the intertidal zone, but also in deeper water down to 30 m ( Schmitt 1926; Monod 1927; Holthuis 1951; Christoffersen 1984; Almeida et al. 2012; Soledade & Almeida 2013; Pachelle et al. 2016). In West Africa, A. pontederiae is often found close to burrows of mangrove-dwelling crabs, viz. Panopeus africanus A. Milne-Edwards and Sarmatium curvatum H. Milne Edwards , whilst young shrimps occasionally dwell in decomposing mangrove wood, together with the mud shrimps Upogebia furcata (Aurivillius) ( Aurivillius 1898; Schmitt 1926). According to de Rochebrune (1883), the type specimen was found “between floating clusters of the marine herb Eichhornia natans (P. Beauv.) [= Pontederia natans ] in rivers of Senegal at the time when their water is saltish” ( Holthuis 1951). In some areas of eastern Brazil, A. pontederiae also occurs in mangrove oyster [ Crassostrea rhizophorae (Guilding) ] beds ( Almeida et al. 2012). The large number of eggs and their small size suggest that this species has an extended larval development.
Taxonomic remarks.Several morphological features link A.pontederiae to the A.euphrosyne — A.microrhynchus complex. These are the general shape of the chelipeds, including the presence of a well-developed mesial subdistal ridge on the major chela pollex ( Fig. 47B, D View FIGURE 47 ; Christoffersen 1984: fig. 4a) and the male minor chela with strongly balaeniceps fingers ( Fig. 47G, H View FIGURE 47 ; Christoffersen 1984: fig. 4c, d); and the third and fourth pereiopods with broadly spatulate dactyli ( Christoffersen 1984: fig. 4g). The Indo-West Pacific species, which are morphologically closest to A. pontederiae , are A. eurydactylus and A. takla sp. nov. The Atlantic species differs from A. eurydactylus by the mesial face of the major chela non-granulated (vs. granulated on the distal portion of palm and pollex in A. eurydactylus ) and the antennal basicerite distolaterally armed with a strong tooth (vs. unarmed in A. eurydactylus ); and from A. takla sp. nov. by the slenderer third pereiopod, with its merus almost seven times as long as wide (vs. five times in A. takla sp. nov.) and ischium armed with a stout spiniform seta (vs. usually unarmed in A. takla sp. nov.). In addition, A. pontederiae , A. eurydactylus and A. takla sp. nov. have differently coloured plungers of the major chela dactylus: whitish, tinged with green-yellow and a blue spot in A. pontederiae ; white and blue proximally and orange-yellow distally in A. eurydactylus ; and bright red-orange in A. takla sp. nov. (cf. Figs. 16C View FIGURE 16 , 32A View FIGURE 32 , 35A View FIGURE 35 , 48C View FIGURE 48 ).
Alpheus pontederiae has a disjunct amphi-Atlantic distribution ( Fig. 52E View FIGURE 52 ), being absent from the islands of the Central Atlantic, probably due to the lack of suitable habitats there. Interestingly though, A. pontederiae also seems to be absent from the Caribbean Sea and Gulf of Mexico / Florida region, where estuarine and mangrove habitats are plentiful; the western-most record of this species is the Orinoco Delta in Venezuela. A genetic comparison of the western and eastern Atlantic populations of A. pontederiae , similar to the one recently performed for A. intrinsecus Spence Bate, 1888 ( Cunha et al. 2017), is highly desirable. In this context, it is noteworthy that A. pontederiae was reported to be variable in the relative length of the antennal scaphocerite blade, which ranges from being much shorter than the adjacent distolateral tooth to reaching distinctly beyond it ( Crosnier & Forest 1966; Christoffersen 1984).
Almeida, A. O. de, Coelho, P. A., Santos, J. T. A. dos & Ferraz, N. R. (2006) Crustaceos decapodos estuarinos de Ilheus, Bahia, Brasil. Biota Neotropica, 6 (2), bn 03406022006, 1 - 24. https: // doi. org / 10.1590 / S 1676 - 06032006000200024
Almeida, A. O. de, Boehs, G., Araujo-Silva, C. L. & Bezerra, L. E. A. (2012) Shallow-water caridean shrimps from southern Bahia, Brazil, including the first record of Synalpheus ul (Rios & Duffy, 2007) (Alpheidae) in the southwestern Atlantic Ocean. Zootaxa, 3347 (1), 1 - 35. https: // doi. org / 10.11646 / zootaxa. 3347.1.1
Audouin, V. (1826) Explication sommaire des planches de Crustaces de l'Egypte et de la Syrie, publiees par Jules-Cesar Savigny, Membre de l'Institut; offrant un expose des characteres naturels des genres avec la distinction des especes. Animaux invertebres. In: Savigny, J. C. (Ed.), Description de l'Egypte ou receuil des observations et des recherches qui ont et faites en Egypte pendant l'expedition de l'armee francaise, publie par les orders de sa Majeste l'Empereur Napoleon le Grand. Imprimerie Imperiale, Paris, pp. 77 - 98.
Aurivillius, C. W. S. (1898) Krustaceen aus dem Kamerun-Gebiete. Bihang till Kungliga Svenska Vetenskapsakademiens Handlingar, 24, 1 - 31, pls. 1 - 4.
Balss, H. (1916) Crustacea II: Decapoda Macrura und Anomura (ausser Fam. Paguridae). In: Michaelsen, W. (Ed.), Beitrage der Meeresfauna Westafrikas. L. Friederichsen & Co., Hamburg, pp. 13 - 46.
Banner, D. M. & Banner, A. H. (1982) The alpheid shrimp of Australia. Part III: The remaining alpheids, principally the genus Alpheus and the family Ogyrididae. Records of the Australian Museum, 34, 1 - 357. https: // doi. org / 10.3853 / j. 0067 - 1975.34.1982.434
Christoffersen M. L. (1998) Malacostraca. Eucarida. Caridea. Crangonoidea and Alpheoidea (Except Glyphocrangonidae and Crangonidae). In: Young, P. S. (Ed.), Catalogue of Crustacea of Brazil. Serie Livros N. 6. Museu Nacional, Rio de Janeiro, pp. 351 - 372.
Christoffersen, M. L. (1980) Taxonomia e distribuicao geografica dos Alpheoidea (Crustacea, Decapoda, Natantia) do Brasil, Uruguay e norte da Argentina, incluindo consideracles sobre a divisao do sul do continente em provincias biogeograficas marinhas. PhD Thesis, University of S o Paulo, S o Paulo, 467 pp.
Christoffersen, M. L. (1984) The western Atlantic snapping shrimps related to Alpheus heterochaelis Say (Crustacea, Caridea) with the description of a new species. Papeis Avulsos de Zoologia, 35, 189 - 208.
Coutiere, H. (1899) Les Alpheidae . Morphologie externe et interne, formes larvaires, bionomie. Annales des Sciences Naturelles, Zoologie et Paleontologie, Serie 8, 9, 1 - 559, pls. 1 - 6. Masson, Paris. https: // doi. org / 10.5962 / bhl. title. 13143
Crosnier, A. & Forest, J. (1965) Note preliminaire sur les Alpheidae recuellis par la Calypso dans l'Atlantique oriental tropical (Crustacea Decapoda Natantia). Bulletin du Museum National d'Histoire Naturelle, Paris, Serie 2, 36, 602 - 610.
Crosnier, A. & Forest, J. (1966) Crustaces Decapodes: Alpheidae. In: Campagnes de la Calypso dans le Golfe de Guinee et aux Iles Principe, S o Tom et Annobon (1956), et Campagne aux Iles du Cap Vert (1959). Part 19. Resultats Scientifiques des Campagnes de la Calypso. 7 (27). Annales de l'Institut Oceanographique de Monaco, 44, 199 - 314. https: // doi. org / 10.5962 / bhl. title. 13143
Cunha, A. M., Terossi, M., Mantelatto, F. L. & Almeida, A. O. de (2017) Morphological and molecular analyses support the amphi-Atlantic distribution and taxonomic status of the snapping shrimp Alpheus intrinsecus Spence Bate, 1888 (Crustacea: Decapoda: Alpheidae). Zootaxa, 4303 (4), 573 - 589. https: // doi. org / 10.11646 / zootaxa. 4303.4.8
Hailstone, S. Jr. (1835) Notices of another species of Pontophilus, and of a crustacean allied to the genus Hippolyte. The Magazine of Natural History and Journal of Zoology, Botany, Mineralogy, Geology, and Meteorology, 8, 270 - 273.
Holthuis L. B. (1951) The caridean Crustacea of tropical West Africa. Atlantide Report, 2, 1 - 187.
Longhurst, A. R. (1958) An ecological survey of the West African marine benthos. Colonial Office, Fisheries Publication, 11, 1 - 102.
Monod, T. (1927) Crustacea IV. Decapoda (excl. Palaemonidae, Atyidae et Potamomdae). In: Monod, T. (Ed.), Contribution a l'etude de la faune du Cameroun. Faune des Colonies Francaises, 1, 593 - 624.
Monod, T. (1928) Additions a ma liste des decapodes marins du Cameroun. Bulletin du Museum d'Histoire Naturelle, 34, 252.
Norman, A. M. (1868) On the British species of Alpheus, Typton, and Axius, and on Alpheus Edwardsii of Audouin. Annals and Magazine of Natural History, Series 4, 2, 173 - 178. https: // doi. org / 10.1080 / 00222936808695776
Pachelle, P. P. G., Anker, A., Mendes, C. B. & Bezerra, L. E. A. (2016) Decapod crustaceans from the state of Ceara, northeastern Brazil: an updated checklist of marine and estiarine species, with 23 new records. Zootaxa, 4131 (1), 1 - 63. https: // doi. org / 10.11646 / zootaxa. 4131.1.1
Powell, C. B. (1979) Three alpheid shrimps of a new genus from West African fresh and brackish waters: taxonomy and ecologial zonation (Crustacea Decapoda Natantia). Revue de Zoologie Africaine, 93, 116 - 150.
Rathbun, M. J. (1900) The decapod crustaceans of West Africa. Proceedings of the United States National Museum, 22, 271 - 316. https: // doi. org / 10.5479 / si. 00963801.22 - 1199.271
Rochebrune, A. T. de (1883) Diagnoses d'arthropodes nouveaux propres la Senegambie. Bulletin de la Societe Philomathique de Paris, Serie 7, 7, 167 - 182.
Rossignol, M. 1962. Catalogue des crustaces decapodes brachyoures, anomoures et macroures littoraux en collection au Centre d'Oceanographie de Pointe-Noire. Cahiers de l'ORSTOM, Oceanographie, 2, 111 - 138.
Schmitt, W. L. (1926) The macruran, anomuran and stomatopod crustaceans collected by the American Museum Congo Expedition, 1909 - 1915. Bulletin of the American Museum of Natural History, 53, 1 - 67, pls. 1 - 9.
Soledade, G. O. & Almeida, A. O. de (2013) Snapping shrimps of the genus Alpheus Fabricius, 1798 from Brazil (Caridea: Alpheidae): updated checklist and key for identification. Nauplius, 21, 89 - 122. https: // doi. org / 10.1590 / S 0104 - 64972013000100010
Soledade, G. O., Oliveira, M. V. & Almeida, A. O. de (2017) A specimen of the snapping shrimp Alpheus pontederiae de Rochebrune, 1883 with symmetric chelipeds. Spixiana, 40, 181 - 184.
Spence Bate, C. (1888) Report on the Crustacea Macrura collected by the Challenger during the years 1873 - 76. In: Report on the Scientific Results of the Voyage of H. M. S. Challenger during the years 1873 - 76. Vol. 24. Eyre & Spottiswoode, London, pp. i - xc + 1 - 942, pls. 1 - 157.
Vilela, H. (1949) Crustaceos decapodes e estomatopodes da Guine Portuguesa. Anais da Junta de Investigacles Coloniais, Lisboa, 4, 47 - 70.
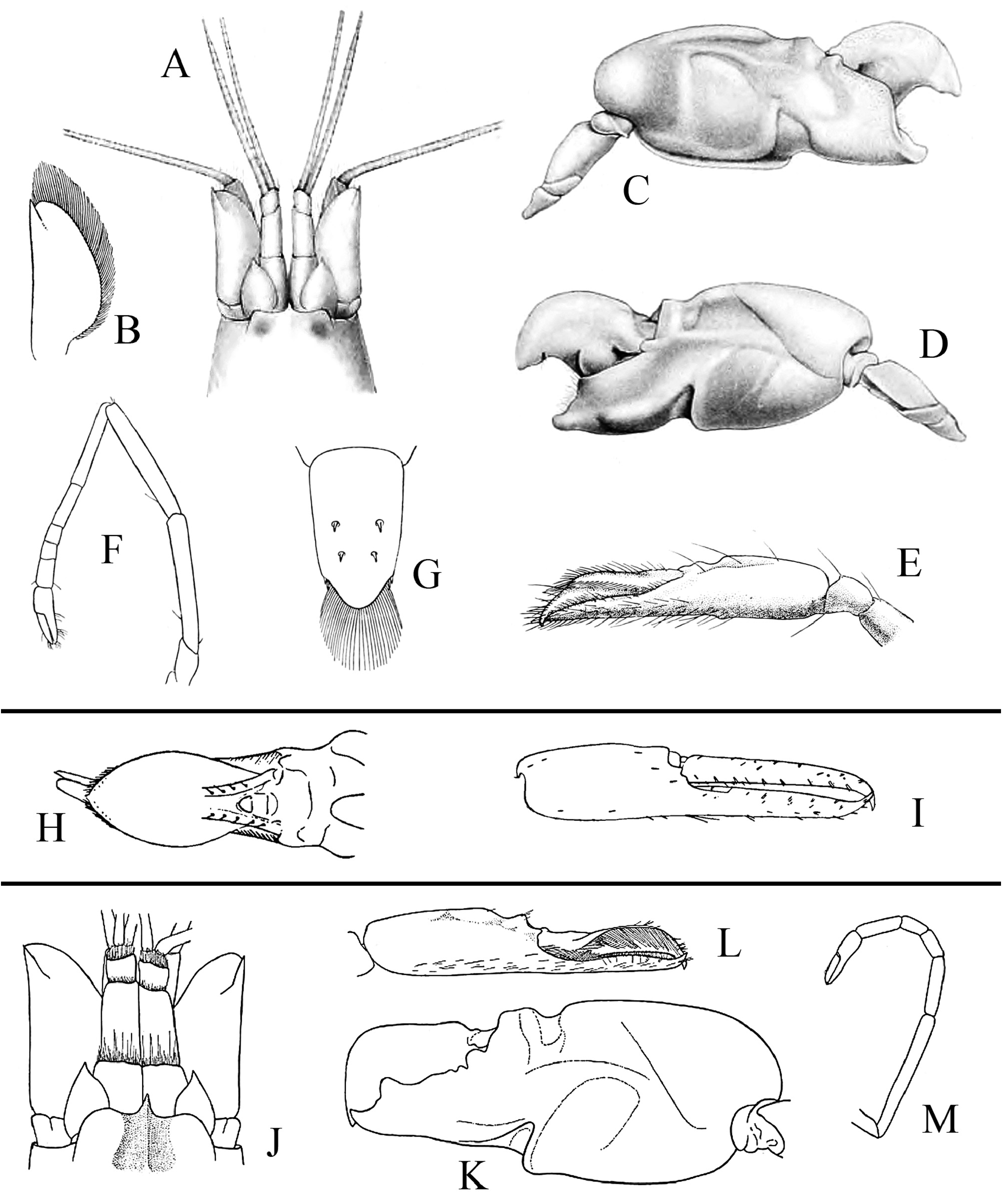
FIGURE 3. Three species of Alpheus described by Kemp (1915), De Man (1920) and Schmitt (1926). Alpheus paludicola Kemp, 1915: syntype, male (cl indet., tl 22.0 mm) from Chilika Lake, India [A–G]; A—frontal region, dorsal view; B—scaphocerite of left antenna, dorsal view; C—(left) major cheliped, mesial view; D—same, lateral view; E—minor (right) cheliped, distal portion, mesial view; F—second pereiopod, lateral view; G—telson, dorsal view (after Kemp 1915). Alpheus eurydactylus De Man, 1920: syntypes, male (cl 13.8 mm) [H] and ovigerous female (cl 15.0 mm) [I] from Java, Indonesia (see text for lectotype / paralectotype designation); H—male minor (right) chela, distal portion of palm and fingers, dorsal view; I—female (right) minor chela, lateral view (after De Man 1924). Alpheus langi (Schmitt, 1926), originally described as Crangon langi, presenly junior synonym of Alpheus pontederiae de Rochebrune, 1883: holotype, male (cl 11.0 mm, tl 34.0 mm) from Congo [J–M]: J—frontal region, dorsal view; K—major (left) chela, lateral view; L—minor (right) chela, lateral view; M—second pereiopod, lateral view (after Schmitt 1926). Not to scale.
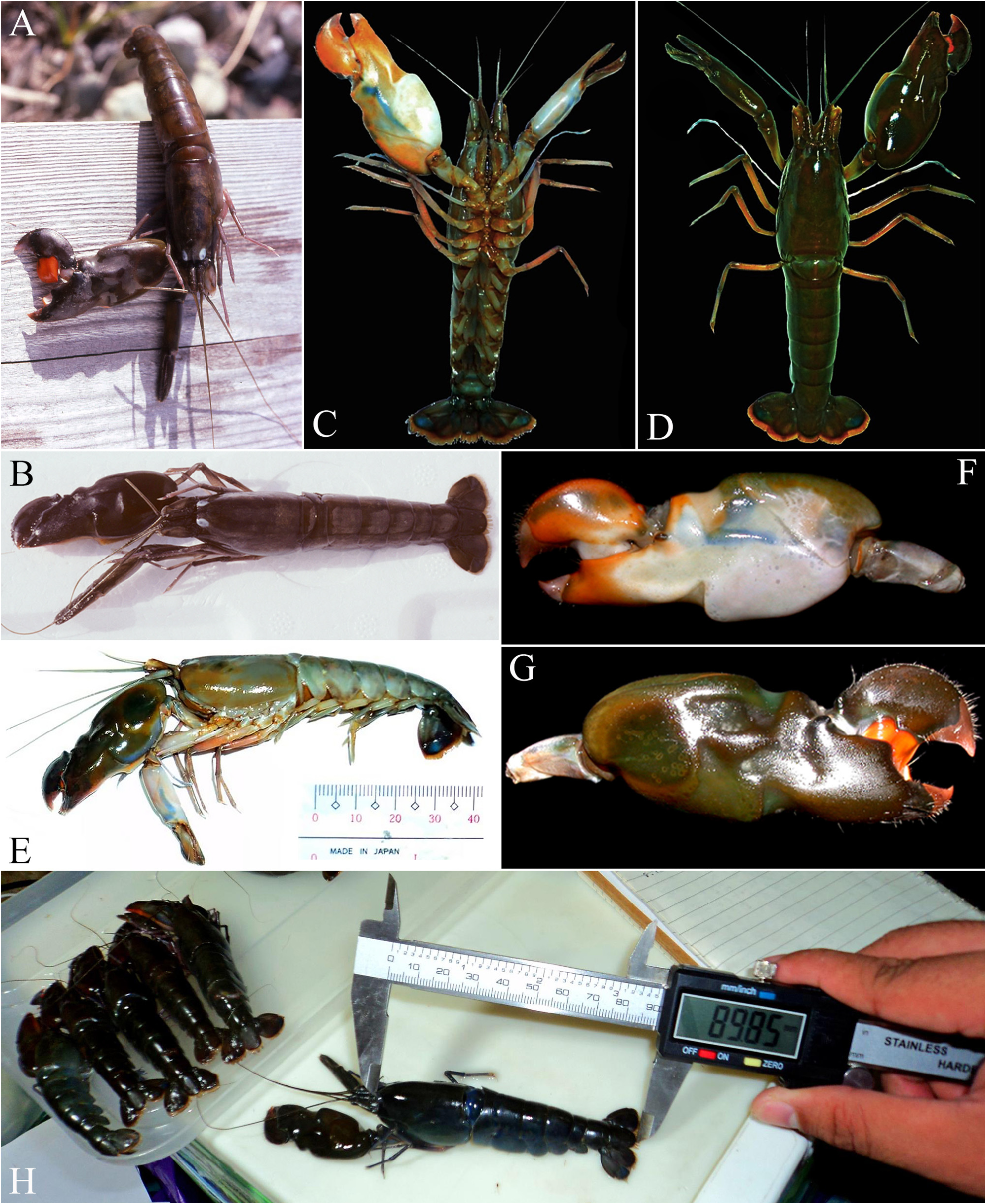
FIGURE 16. Alpheus eurydactylus De Man, 1920: male (cl indet.) from Can Gio, Vietnam (ZMMU) [A]; female (cl indet.) from the same locality (ZMMU) [B, C]; male (cl indet.) from near Bangkok, Thailand (deposition unknown) [D]: A—habitus, dorsal view; B, D—same, lateral view; C—major cheliped, mesial view. Photographs courtesy of Ivan N. Marin (A–C) and Sukkrit Nimitkul (D).
FIGURE 32. Alpheus takla sp. nov.: female (cl 23.0 mm) from unknown locality in the Philippines, fisheries import (OUMNH. ZC. 2019.06.68) [A, B]; male (cl indet.) from Bohol, Philippines (not deposited) [C–G]; several specimens from Bohol being measured, largest female at tl 89.85 mm (material not deposited/deposition unknown) [H]; A, B, D, H—habitus, dorsal view (specimen in A, B somewhat discoloured); C—same, ventral view; E—same, lateral view; F—major (left) cheliped, lateral view; G—same, mesial view. Photographs courtesy of Keiichi Nomura (A, B) and Manaklaay Ko (M. Gabe) (C–H).
FIGURE 35. Alpheus takla sp. nov. in its mangrove habitat near Cairns, Queensland, Australia: A—male shrimp extracted from its burrow [not deposited]; B—burrows of A. takla sp. nov. (larger holes) and possibly another, smaller species of Alpheus (smaller holes) at low tide; C—burrows of A. takla sp. nov. at low tide; D—shrimp inside its burrow, either pushing away or pulling into the burrow a fallen mangrove leaf; E—partly flooded burrow funnel with one of the shrimps at the entrance and a small goby; F—general view of the mangrove. Photographs courtesy of Andrew Mitchell.
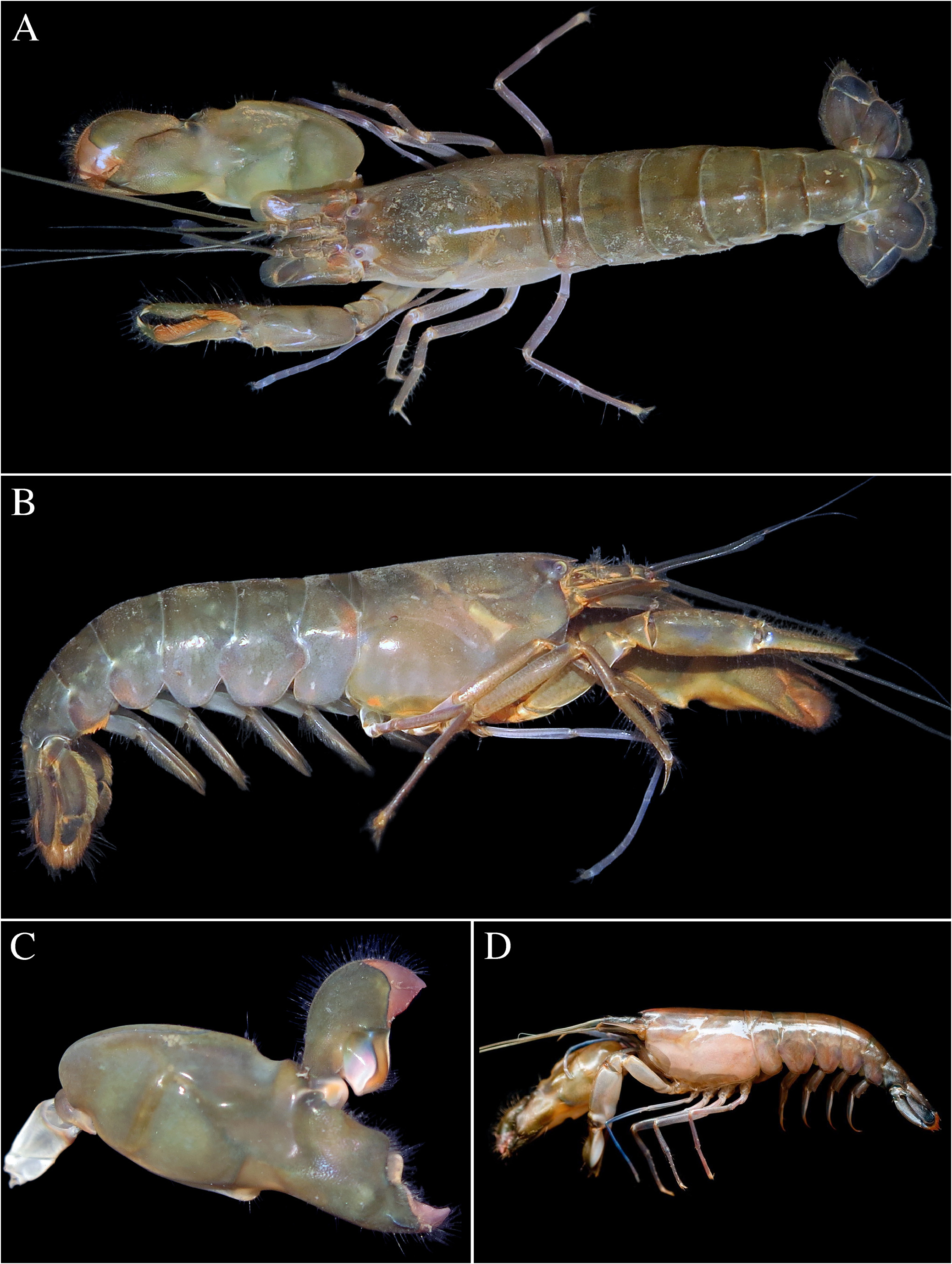
FIGURE 47. Alpheus pontederiae de Rochebrune, 1883: female (cl indet.) from Orinoco Delta, Venezuela (MZUSP) [A–E]; male (cl indet.) from the same locality (MZUSP) [F–H]; A—frontal region, dorsal view; B, F—major (left) cheliped, mesial view; C—same, lateral view; D—same, chela fingers opened, mesial view; E—minor (right) cheliped, mesial view; G—minor (right) cheliped, lateral view; H, same, chela, dorsal view.
FIGURE 48. Alpheus pontederiae de Rochebrune, 1883: male (cl indet.) from near Bragança, Pará, Brazil (MPEG) [A–C]; male (cl ~6.0 mm) from S„o Tomé, S„o Tomé and Príncipe (OUMNH.ZC. 2011.06.8) [D] and its habitat near Porto Alegre, S„o Tomé [E]; A, D—habitus, dorsal view; B—same, lateral view; C—frontal region and chelipeds, dorsal view; E—mangrove channel near Porto Alegre, S„o Tomé, collection locality of the specimen in D. Photographs by the author.
FIGURE 52. Distributional maps for species of Alpheus treated in this study:A—A. takla sp. nov.; B—A. mangalis sp. nov. and A. cf. mangalis sp. nov.; C—A. songkla Banner & Banner, 1966 and A. cf. songkla (see text); D—A. paludicola Kemp, 1915, A. nipa Banner & Banner, 1985 and A. bunburius Banner & Banner, 1982; E—A. pontederiae de Rochebrune, 1883; F—A. firmus Kim & Abele, 1988 and A. cf. firmus (see text).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Alpheus pontederiae de Rochebrune, 1883
| Anker, Arthur 2023 |
Alpheus pontederiae
| Rossignol, M. 1962: 131 |
| Longhurst, A. R. 1958: 31 |
| Holthuis L. B. 1951: 85 |
| Coutiere, H. 1899: 37 |
Alpheus Pontederiae de Rochebrune 1883: 174
| Rochebrune, A. T. de 1883: 174 |