Stromateoidei
|
publication ID |
https://doi.org/10.1093/zoolinnean/zlab058 |
|
DOI |
https://doi.org/10.5281/zenodo.6771673 |
|
persistent identifier |
https://treatment.plazi.org/id/0E16878B-FFFC-FFCB-FCFB-FECCFEF2F938 |
|
treatment provided by |
Plazi (2022-06-27 06:56:26, last updated 2024-11-27 09:30:39) |
|
scientific name |
Stromateoidei |
| status |
|
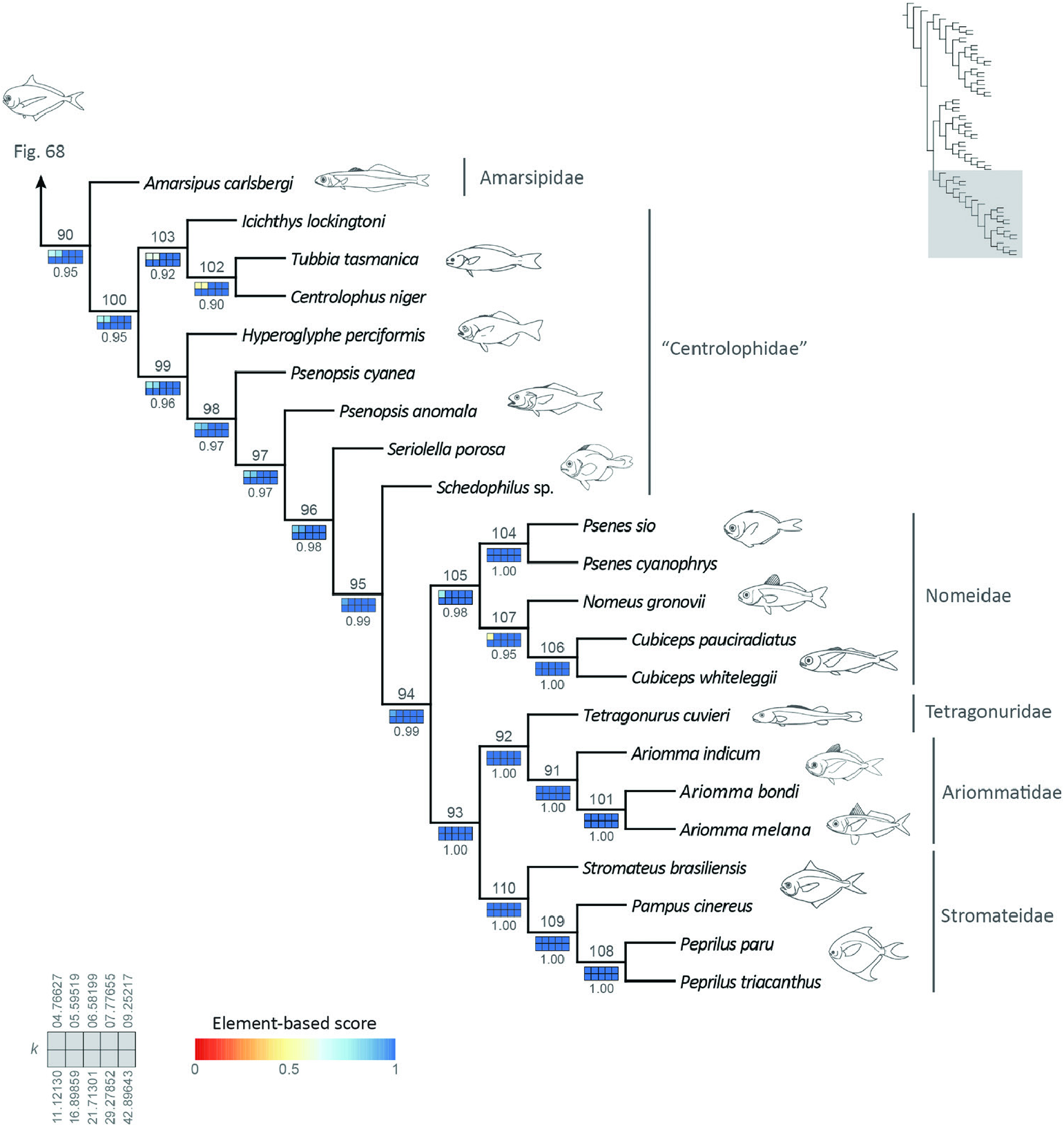
Node 100 = Stromateoidei (new usage for non-Amarsipidae Stromateiformes )
Icichthys lockingtoni , Tubbia tasmanica , Centrolophus niger , Hyperoglyphe perciformis , Psenopsis cyanea , Psenopsis anomala , Seriolella porosa , Schedophilus sp. , Psenes sio , Psenes cyanophrys , Nomeus gronovii , Cubiceps whiteleggii , Cubiceps pauciradiatus , Tetragonurus cuvieri , Ariomma indicum , Ariomma bondi , Ariomma melana , Stromateus brasiliensis , Pampus cinereus , Peprilus triacanthus , Peprilus paru .
Unambiguous synapomorphies: Character 3 (16> 18): number of branched pectoral-fin rays increased to 18; character 41 (0> 1): vomerine teeth present; character 49 (0> 1): fourth upper pharyngeal tooth plate considerably longer than wide; character 108 (0> 1): orbitopectoral branch of the ramus lateralis accessorius (RLA-OP) lateral to the levator operculi; character 117 (0> 1): adductor hyomandibulae insertion advancing onto anterior portion of the endopterygoid; character 124 (0> 1): sphincter oesophagi pars anterior absent; character 129 (1> 0): dorsalis portion of the rectus communis associated with hypobranchial 3; character 130 (0> 1): fibres of the rectus communis disposed between hypobranchial 3 and urohyal; character 136 (0> 1): medial contact between antimeres of pharyngoclavicularis internus present; character 146 (0> 1): anterior border of epaxialis anterolateral section reaching or trespassing the vertical line through the middle of the orbit; character 157 (0> 1): pharyngeal sac present; character 194 (0> 1): interradial membrane of the dorsal fin scaled; character 197 (0> 1) interradial membrane of the anal fin scaled.
Support: Relative Bremer = 64%.
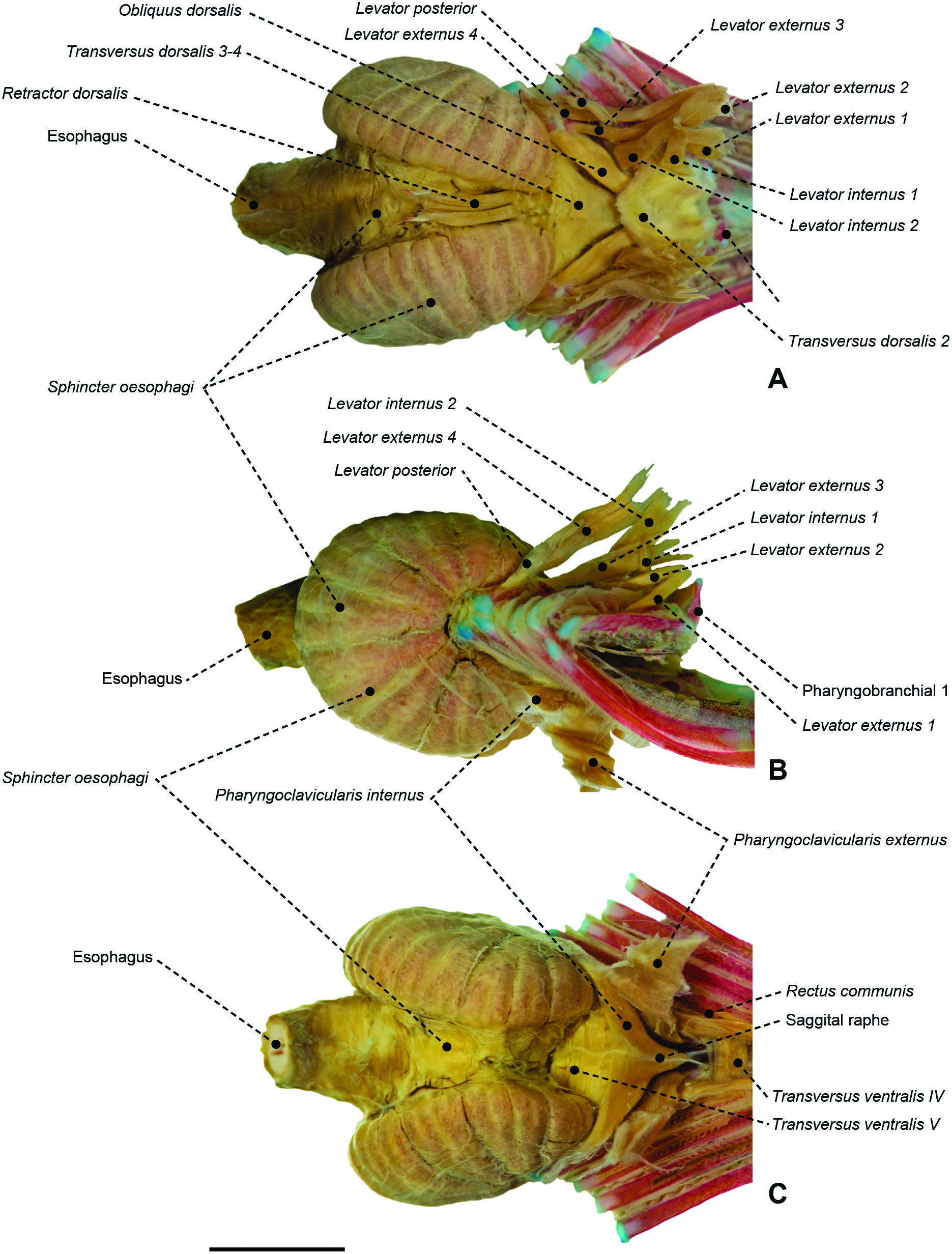
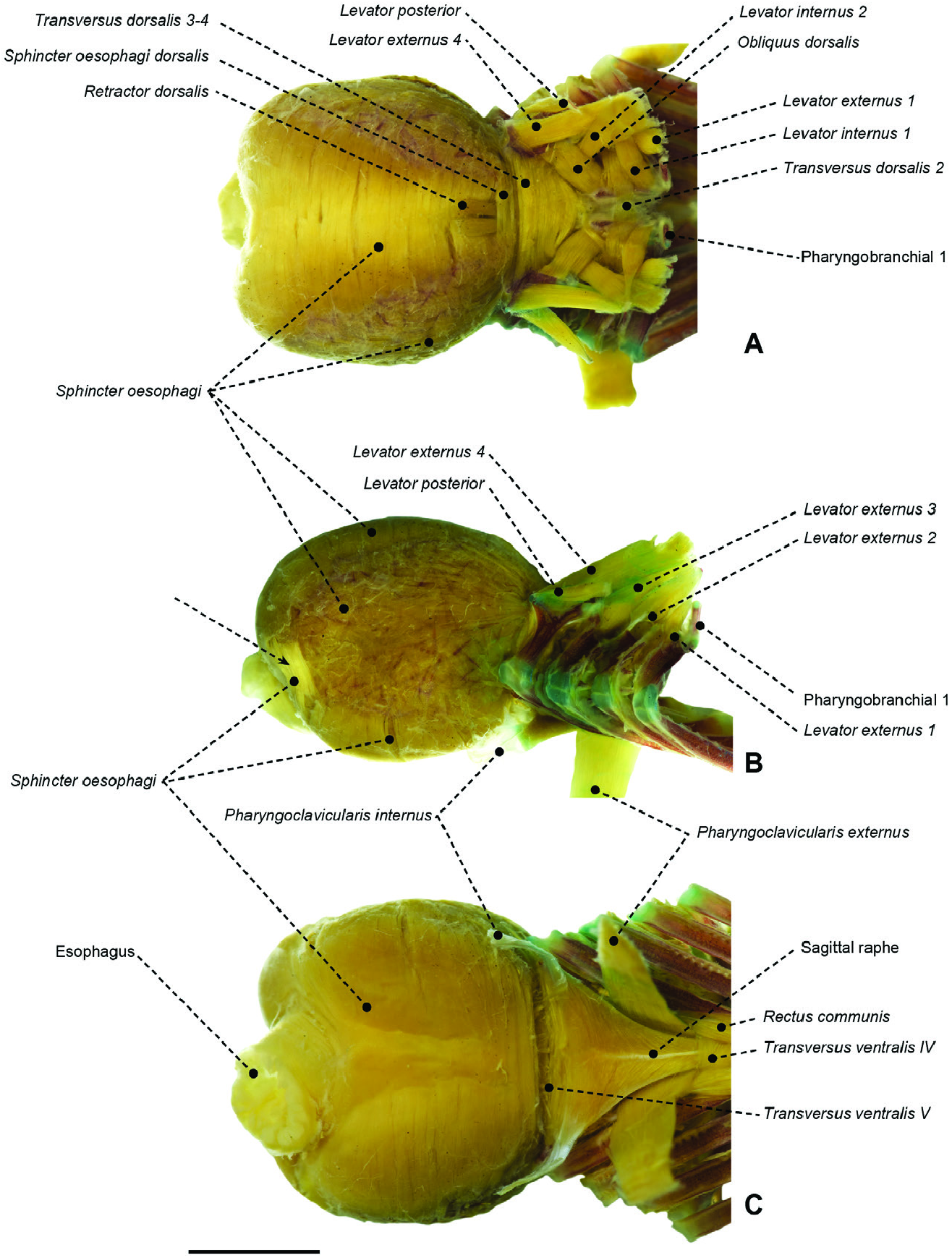
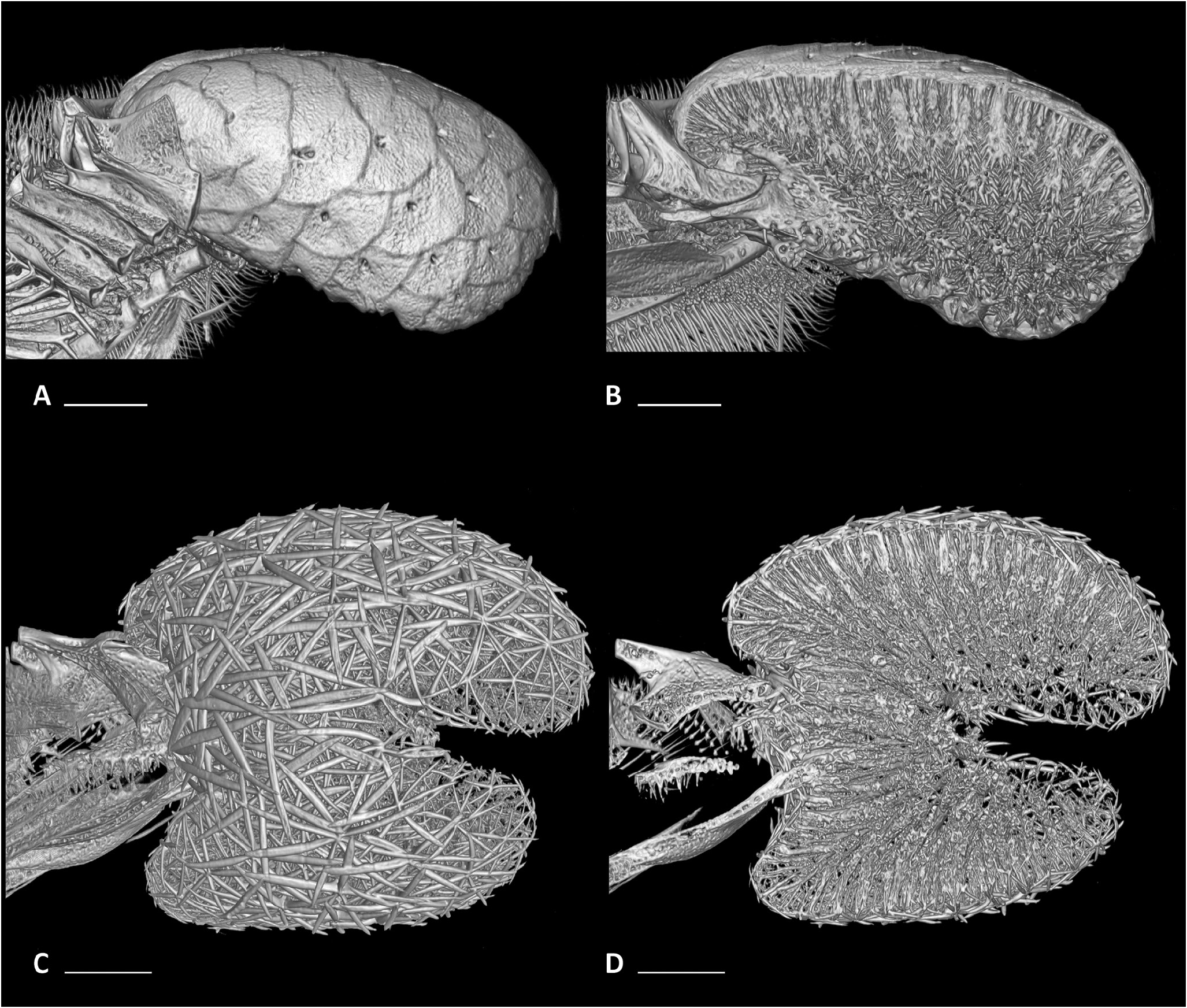
Remarks: The suborder Stromateoidei is herein proposed to encompass all non-amarsipid Stromateiformes , namely the families Centrolophidae , Nomeidae , Tetragonuridae , Ariommatidae and Stromateidae . The monophyly of this clade is supported by 13 morphological synapomorphies and obtained under all searching parameters used in this study. Among the characters supporting node 100, the presence of the pharyngeal sac is obviously the most remarkable synapomorphy of Stromateoidei , because this complex organ is, to our knowledge, unparalleled among vertebrates ( Figs 36–41 View Figure 36 View Figure 37 View Figure 38 View Figure 39 View Figure 40 View Figure 41 , 52–58 View Figure 52 View Figure 53 View Figure 54 View Figure 55 View Figure 56 View Figure 57 View Figure 58 ). Accordingly, a natural assemblage comprising the pharyngeal sacbearing stromateiform fishes has long been recognized by morphological studies (e.g. Regan, 1902; Gilchrist, 1922; Bühler, 1930; Barnard 1948; Isokawa et al., 1965; Haedrich, 1967; Horn, 1984; Datovo et al., 2014).
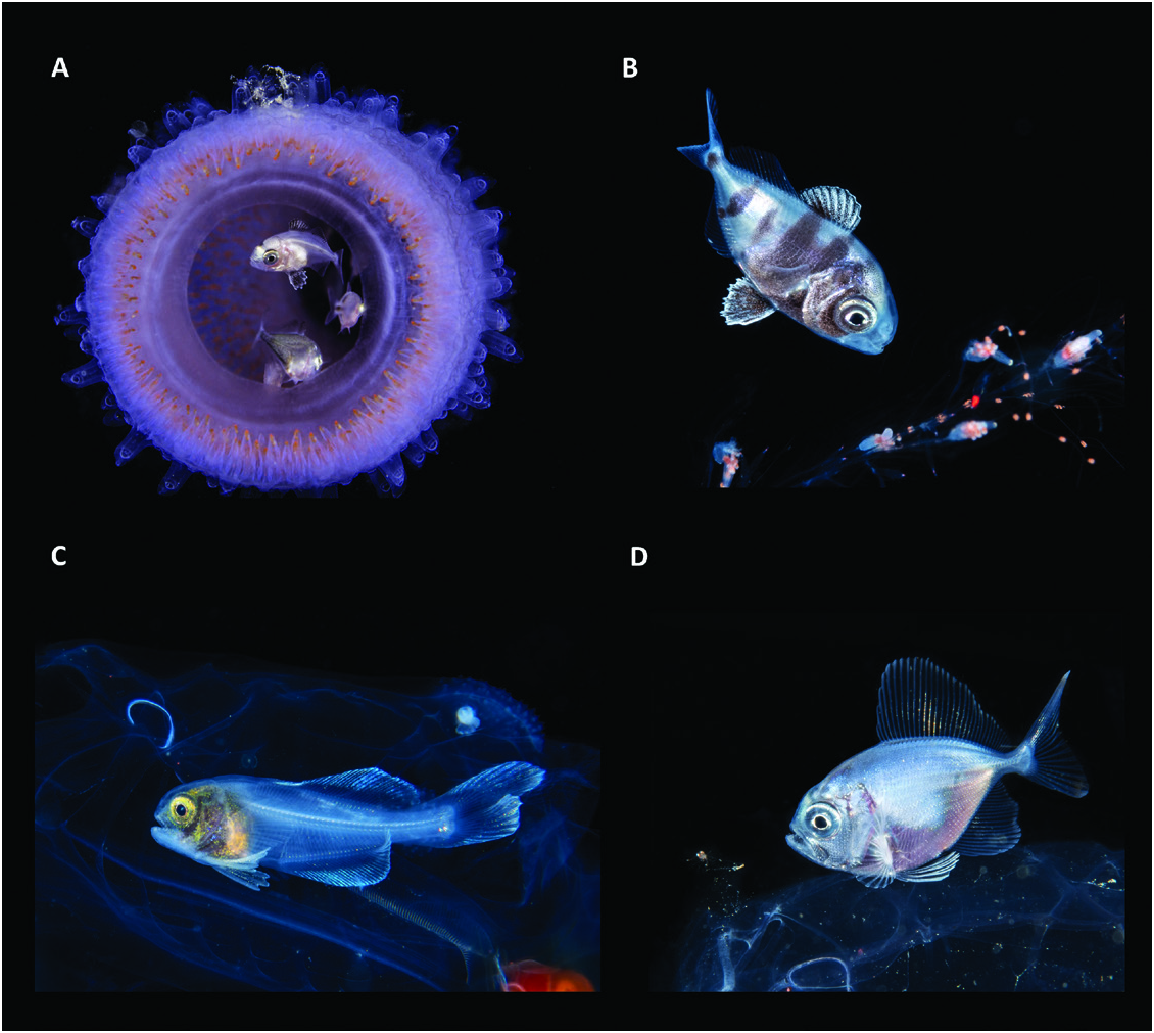
Our study aside, a Stromateoidei clade has been recovered within an explicit phylogenetic context only by Horn (1984), wherein it was supported by two synapomorphies: the presence of the pharyngeal sac (our character 53) and the juvenile association with floating objects (our character 206). Of these two characters, only the presence of a pharyngeal sac is corroborated as a synapomorphy for the Stromateoidei . The association between juvenile fishes and gelatinous organisms is broader than reported by Horn (1984), and it has been reported for juvenile amarsipids ( Janssen & Harbison, 1981; Harbison, 1993) and for some other non-stromateiform percomorphs ( Fig. 65 View Figure 65 ; Supporting Information, Supplementary File S1: Table S3). A character reconstruction of this behaviour (character 206) indicates that it could either be optimized as a synapomorphy for Stromateiformes (node 90; DelTran, i.e. delayed transformation), with parallel acquisitions in bramids, caristiids, carangids and icosteids) or be a synapomorphy for a larger clade (node 76; AccTran, i.e. accelerated transformation), with reversals occurring within carangiforms, scombriforms and other taxa lacking juvenile association with pelagic gelatinous invertebrates.
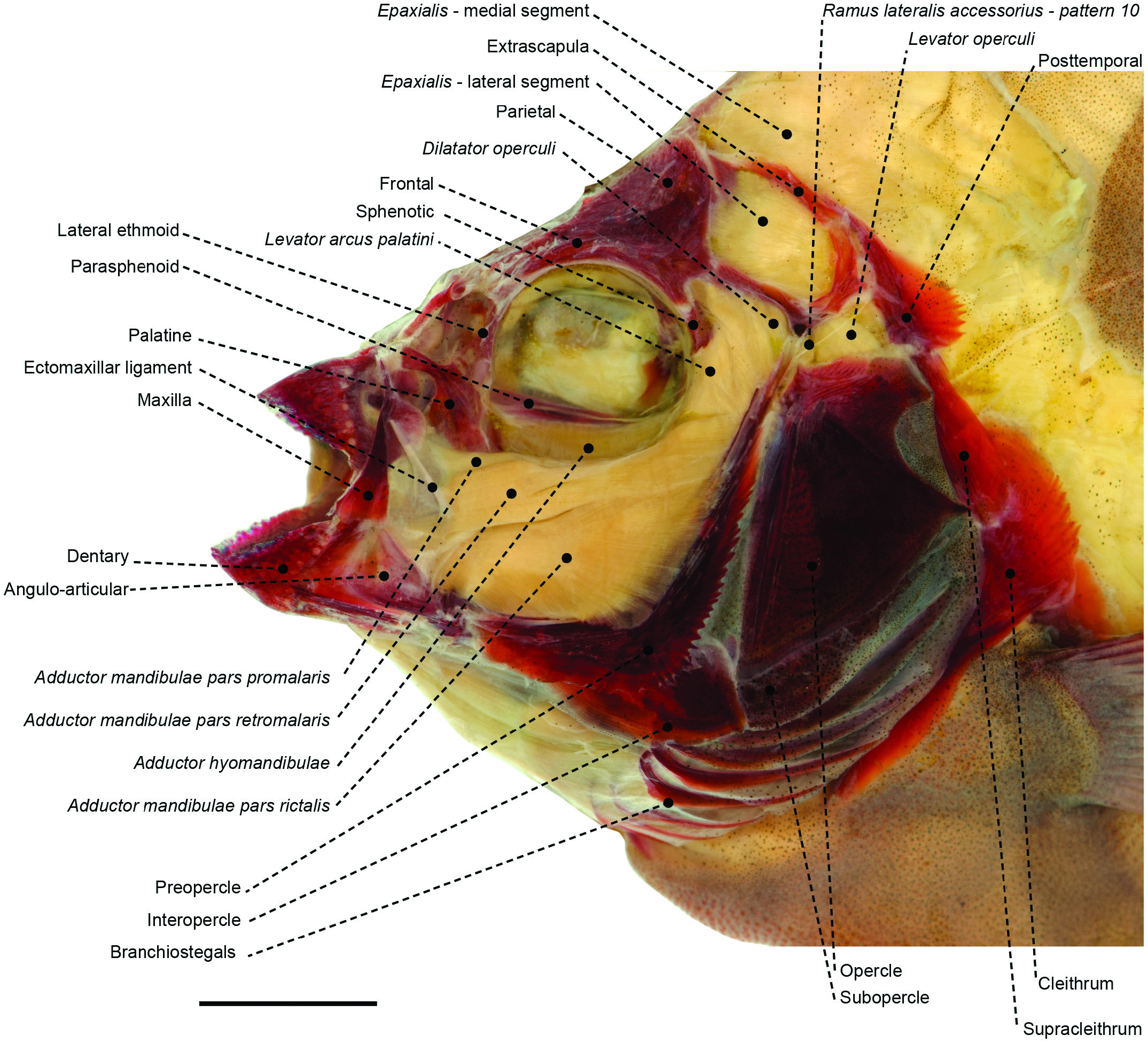
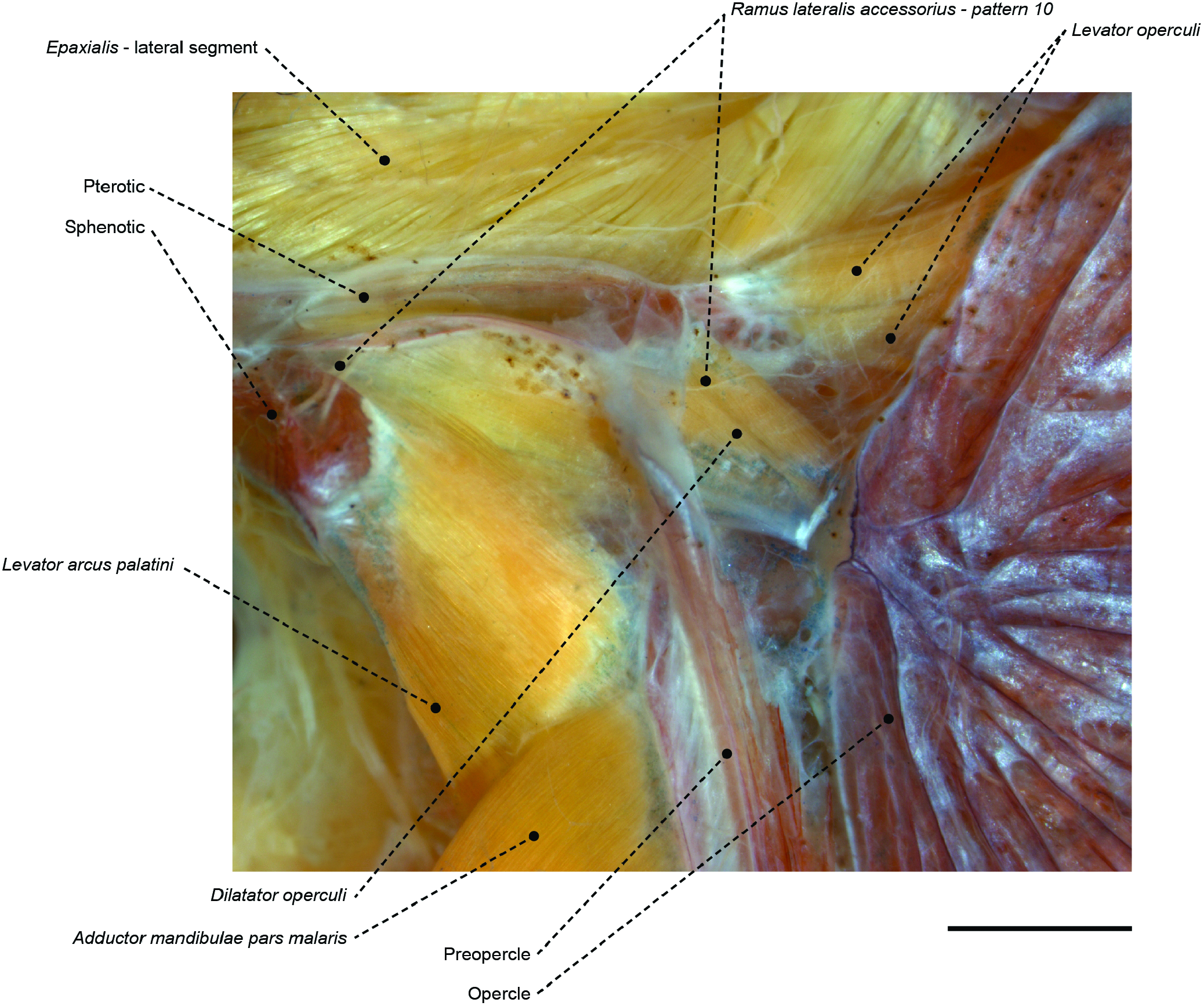
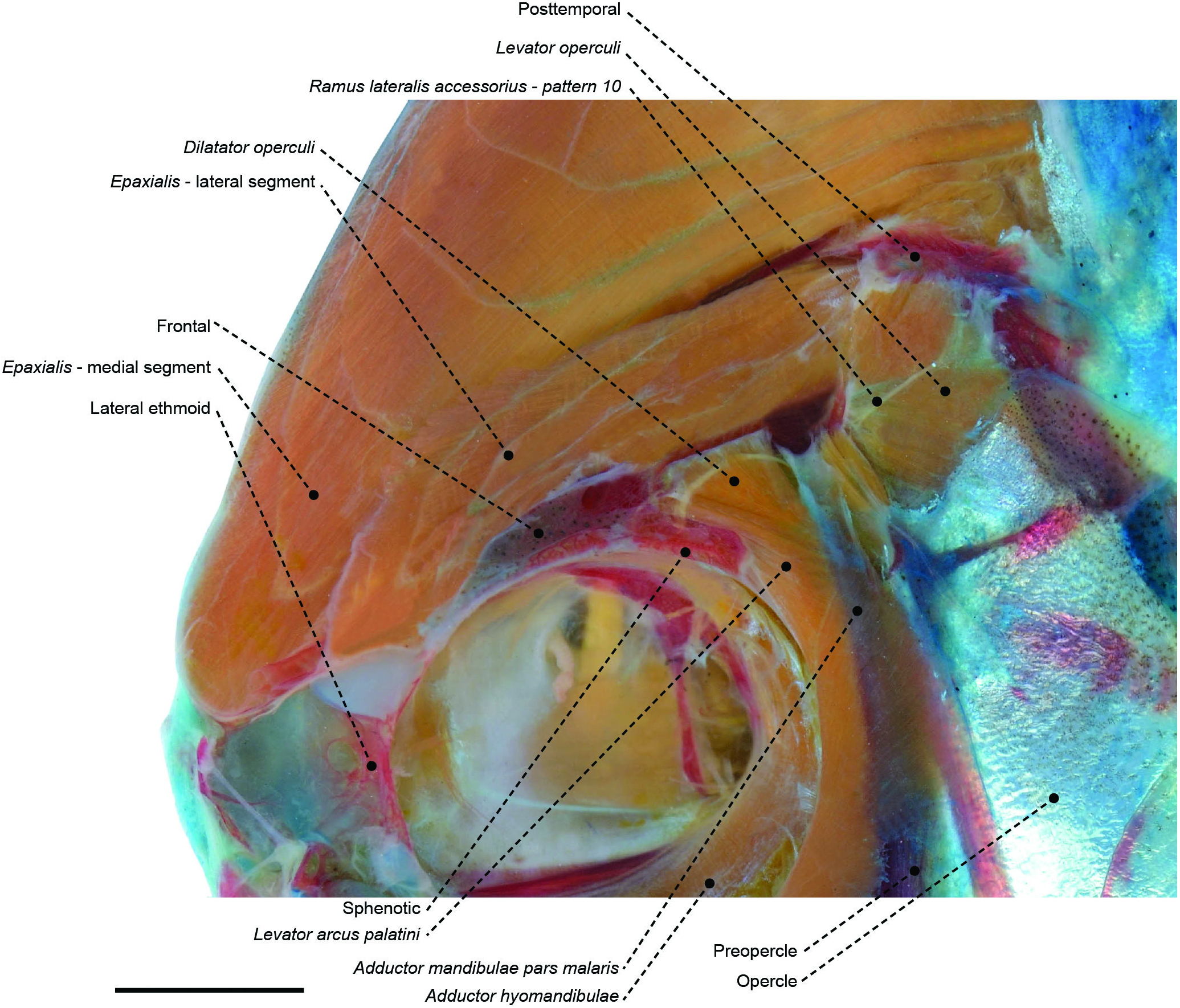
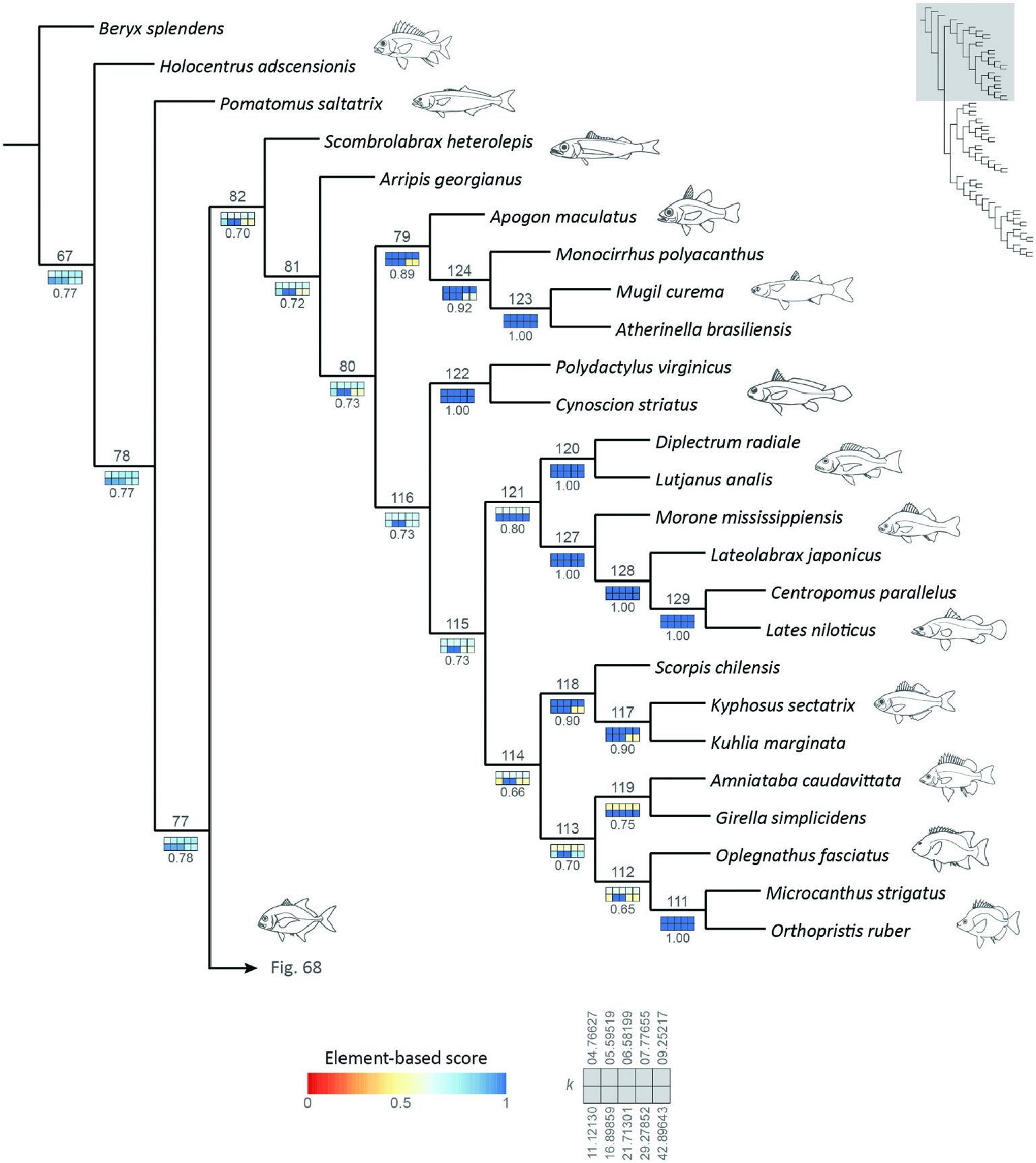
Another synapomorphy for Stromateoidei is the shared pattern 10 of the RLA, in which its orbitopectoral branch overlies the levator arcus palatini, dilatator operculi and levator operculi muscles ( Figs 27 View Figure 27 , 50 View Figure 50 , 51 View Figure 51 ; see character 108). The sharing of this innervation pattern has previously been posited as evidence for a possible relationship between stromateoids and the Kyphosidae s.l. (including Scorpididae , Girellidae and Microcanthidae ), Oplegnathidae , Kuhliidae , Arripidae , Terapontidae ( Freihofer, 1963; Johnson & Fritzsche, 1989; Springer & Johnson, 2004) and Dichistiidae ( Leis & Lingen, 1997) . Yet, our analysis contrasts with these hypotheses by not supporting the monophyly of an RLA-10-pattern fish assemblage. Our topology indicates that this branching pattern has arisen at least four times independently, in: (1) Pomatomidae ( Fig. 67 View Figure 67 , optimized as an autapomorphy); (2) Arripidae ( Fig. 67 View Figure 67 , also optimized as an autapomorphy); (3) a clade encompassing Kyphosidae s.l., Kuhliidae , Terapontidae , Oplegnathidae and Haemulidae , with reversals in Scorpis chilensis and Orthopristis ruber ( Fig. 67 View Figure 67 : node 114, AccTran); and (4) Stromateoidei ( Fig. 69 View Figure 69 : node 100, with reversal in Tubbia , Psenes and Tetragonurus ).
Barnard KH. 1948. Further notes on South African marine fishes. Annals of the South African Museum 36: 341 - 406.
Buhler H. 1930. Die Verdauungsorgane der Stromateidae (Teleost.). Zoomorphology 19: 59 - 115.
Datovo A, de Pinna MCC, Johnson GD. 2014. The infrabranchial musculature and its bearing on the phylogeny of percomorph fishes (Osteichthyes: Teleostei). PLoS One 9: e 110129.
Freihofer WC. 1963. Patterns of ramus lateralis accessorius and their systematic significance in teleostean fishes. Stanford Ichthyological Bulletin 8: 80 - 189.
Gilchrist JDF. 1922. On the oesophagal teeth of the Stromateidae. Annals and Magazine of Natural History 9: 249 - 255.
Haedrich RL. 1967. The stromateoid fishes: systematics and a classification. Bulletin of the Museum of Comparative Zoology 135: 310 - 139.
Harbison GR. 1993. The potential of fishes for the control of gelatinous zooplankton. International Council for the Exploration of the Sea (ICES) 74: 1 - 10.
Horn MH. 1984. Stromateoidei: development and relationships. In: Moser HG, Richards WJ, Cohen DM, Fahay MP, Kendall AW Jr, Richardson SL, eds. Ontogeny and systematics of fishes. Based on an International Symposium dedicated to the memory of Elbert Halvor Ahlstrom. New York: American Society of Ichthyologists and Herpetologists, 620 - 636.
Isokawa S, Kubota K, Kosaikai T, Satomura I, Tsubouchi M, Sera A. 1965. Some contributions to study of esophageal sacs and teeth of fishes. Journal of Nihon University School of Dentistry 7: 103 - 111.
Janssen J, Harbison GR. 1981. Fish in salps: the association of squaretails (Tetragonurus spp.) with pelagic tunicates. Journal of the Marine Biological Association of the United Kingdom 61: 917 - 927.
Johnson GD, Fritzsche RA. 1989. Graus nigra, an omnivorous girellid, with a comparative osteology and comments on relationships of the Girellidae (Pisces: Perciformes). Proceedings of the Academy of Natural Sciences of Philadelphia 141: 1 - 27.
Leis JM, Van der Lingen CD. 1997. Larval development and relationships of the perciform family Dichistiidae (= Coracinidae), the galjoen fishes. Bulletin of Marine Sciences 60: 100 - 116.
Regan CT. 1902. A revision of the fishes of the family Stromateidae. Annals and Magazine of Natural History 10: 115 - 131.
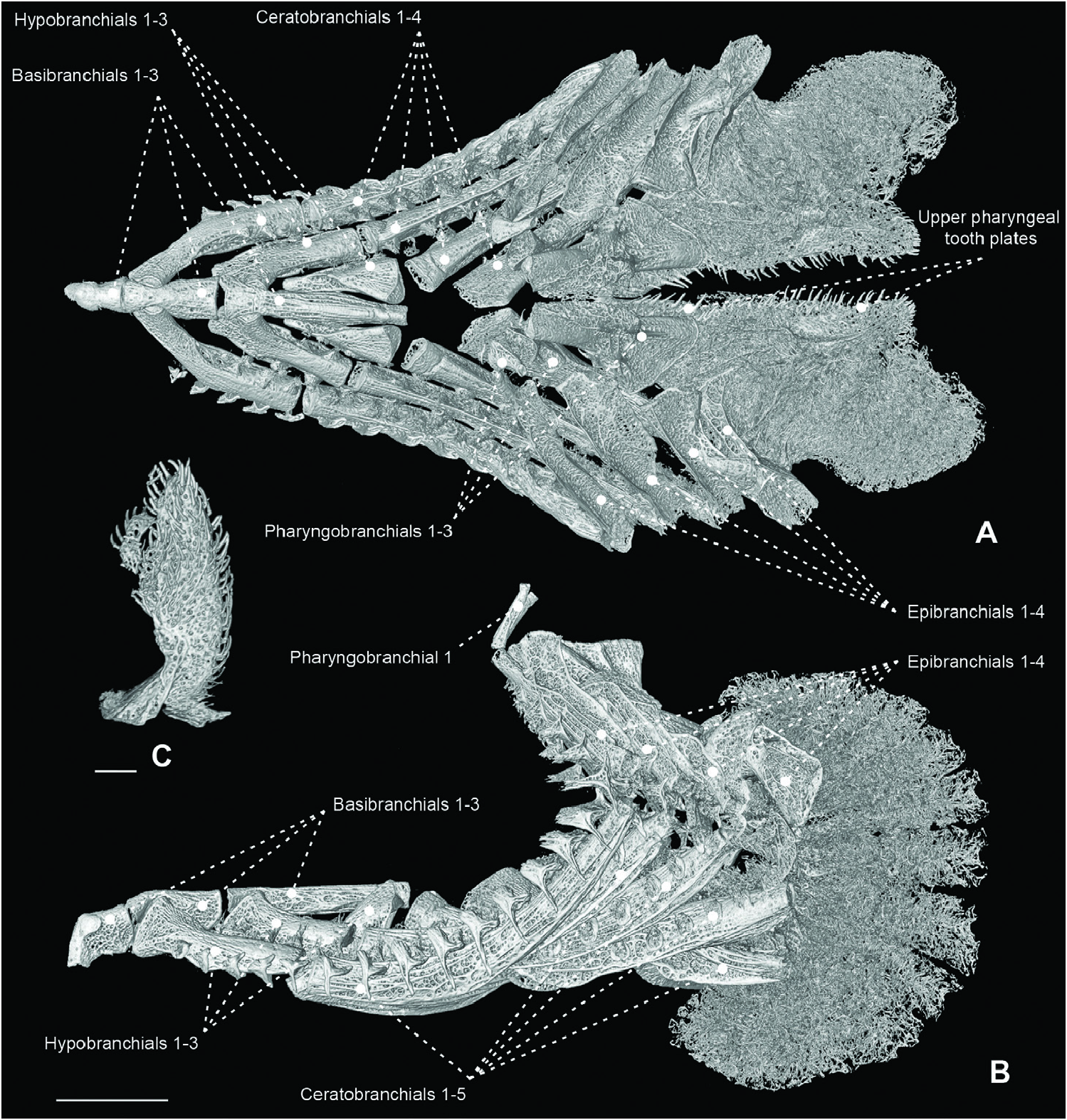
Springer VG, Johnson GD. 2004. Study of the dorsal gillarch musculature of Teleostome fishes, with special reference to the Actinopterygii. Bulletin of the Biological Society of Washington 11: 1 - 205.
Figure 27. Superficial cranial musculoskeletal system of the oplegnathid Oplegnathus fasciatus (MZUSP 28867) in left lateral view. Eye and infraorbital series removed. Scale bar: 4 mm.
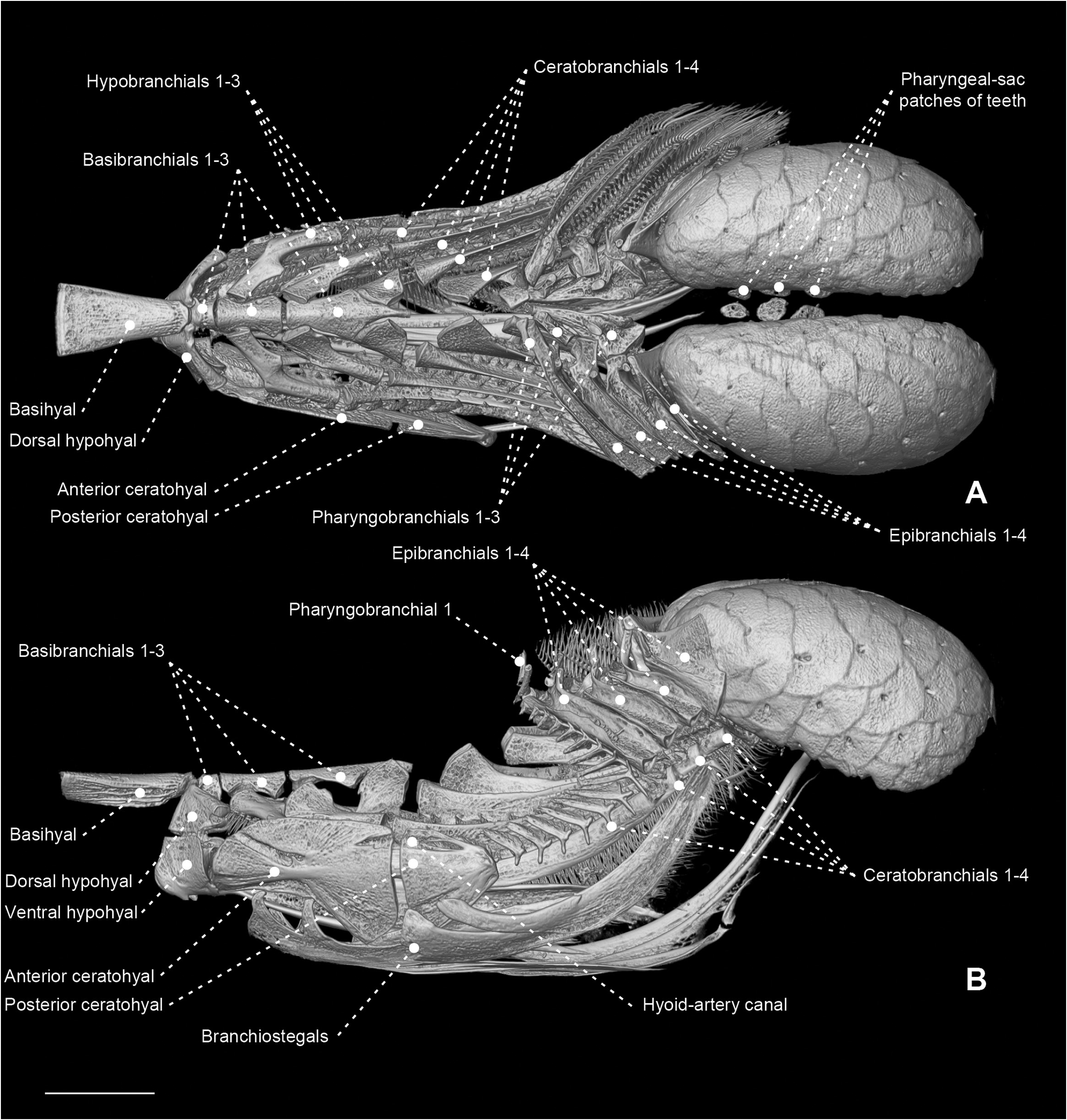
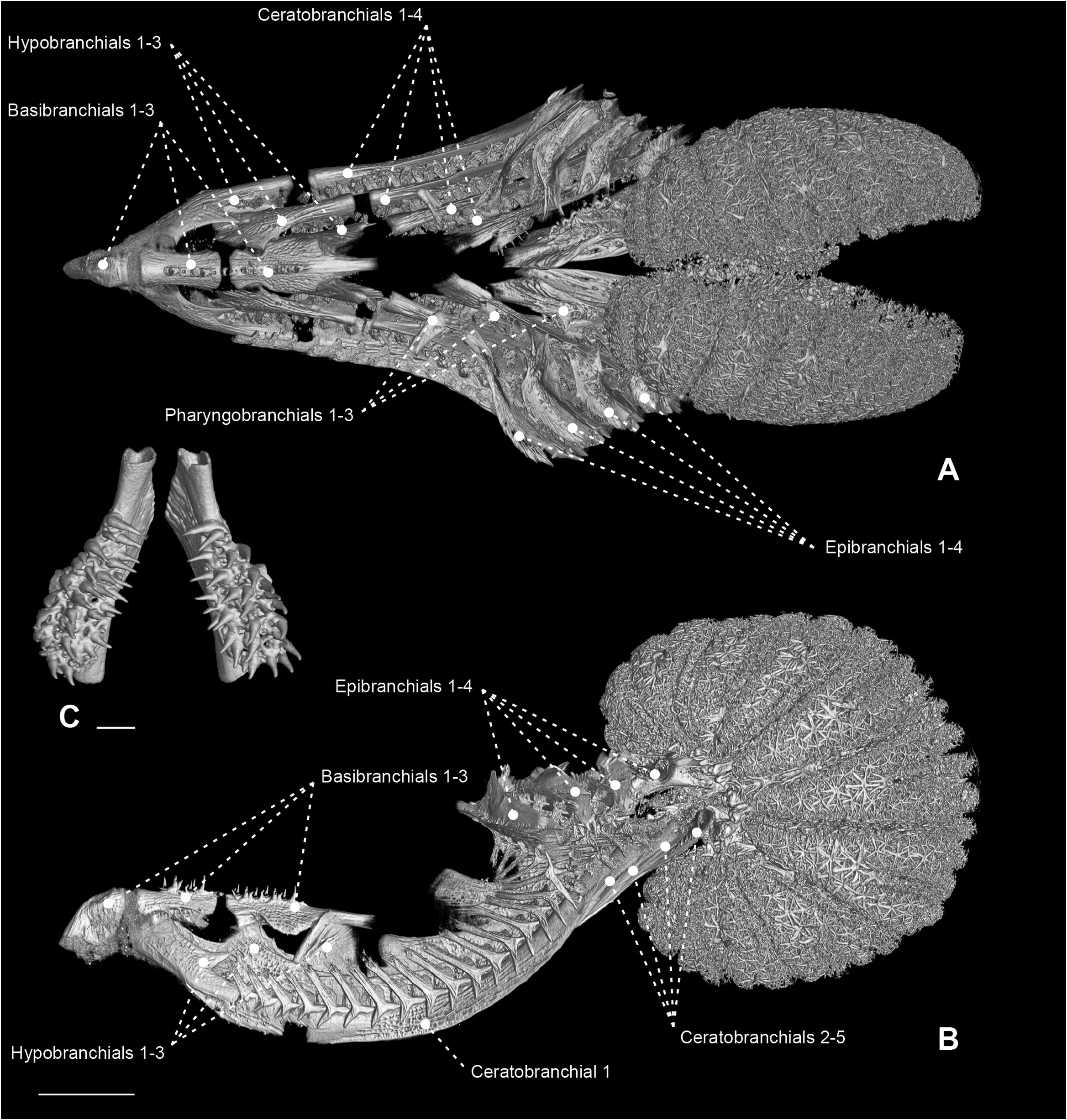
Figure 36. Micro-computed tomography scan of the branchial and pharyngeal-sac skeleton of the ariommatid Ariomma bondi (MZUSP 86717) in dorsal (A) and left lateral (B) view. Scale bar: 4 mm.
Figure 37. Gill arches of Peprilus sp. (SML 34163). A, dorsal gill arches from left side in dorsal view. B, dorsal gill arches from left side in dorsal view.C, ventral view. Gill rakers omitted. Photographs taken by Miguel Montalvo. Scale bars: 0.5 mm.
Figure 38. Micro-computed tomography scan of the branchial and pharyngeal-sac skeleton of the centrolophid Tubbia tasmanica (CSIRO H 6979-03) in dorsal (A) and left lateral (B) view. Scale bar: 7 mm (in B). C, magnification of enlarged raker associated with epi-ceratobranchial 4 joint. Scale bar: 2 mm (in C).
Figure 39. Micro-computed tomography scan of the branchial and pharyngeal-sac skeleton of the centrolophid Tubbia tasmanica (CSIRO H 6979-03), highlighting an enlarged raker associated with epi-ceratobranchial 4 joint (orange). A, dorsal view, with dorsal gill-arch elements removed. B, frontal view, slightly turned to right. C, parasagittal view, with gillarch elements of the left side removed. Scale bar: 7 mm.
Figure 40. A, B, micro-computed tomography scan of the branchial and pharyngeal-sac skeleton of the nomeid Psenes cyanophrys (MZUSP 106392) in dorsal (A) and left lateral (B) view. Scale bar: 4 mm (in B). C, magnification of third pharyngobranchial and associated pharyngeal tooth plate in ventral view. Scale bar: 1 mm (in C).
Figure 41. A, B, branchial and pharyngeal-sac musculoskeletal system of the tetragonurid Tetragonurus cuvieri (MZUSP 123241) in dorsal (A) and left lateral (B) view. C, a laterally cut pharyngeal sac, showing internal papillary lining and posteriorly elongate upper pharyngeal tooth plates. Scale bar: 5 mm.
Figure 50. The temporal region of the centrolophid Icichthys lockingtoni (OS 16732) in left lateral view, showing associated muscles, bones and the ramus lateralis accessorius pattern 10. Scale bar: 2 mm.
Figure 51. The temporal region of the stromateid Peprilus paru (MZUSP 67608) in left lateral view, showing associated muscles and bones. Eye and infraorbital series removed. In pattern 10, the ramus lateralis accessorius emerges from the sphenotic and extends caudally superficial to the levator arcus palatini, dilatator operculi and levator operculi. Scale bar: 4 mm.
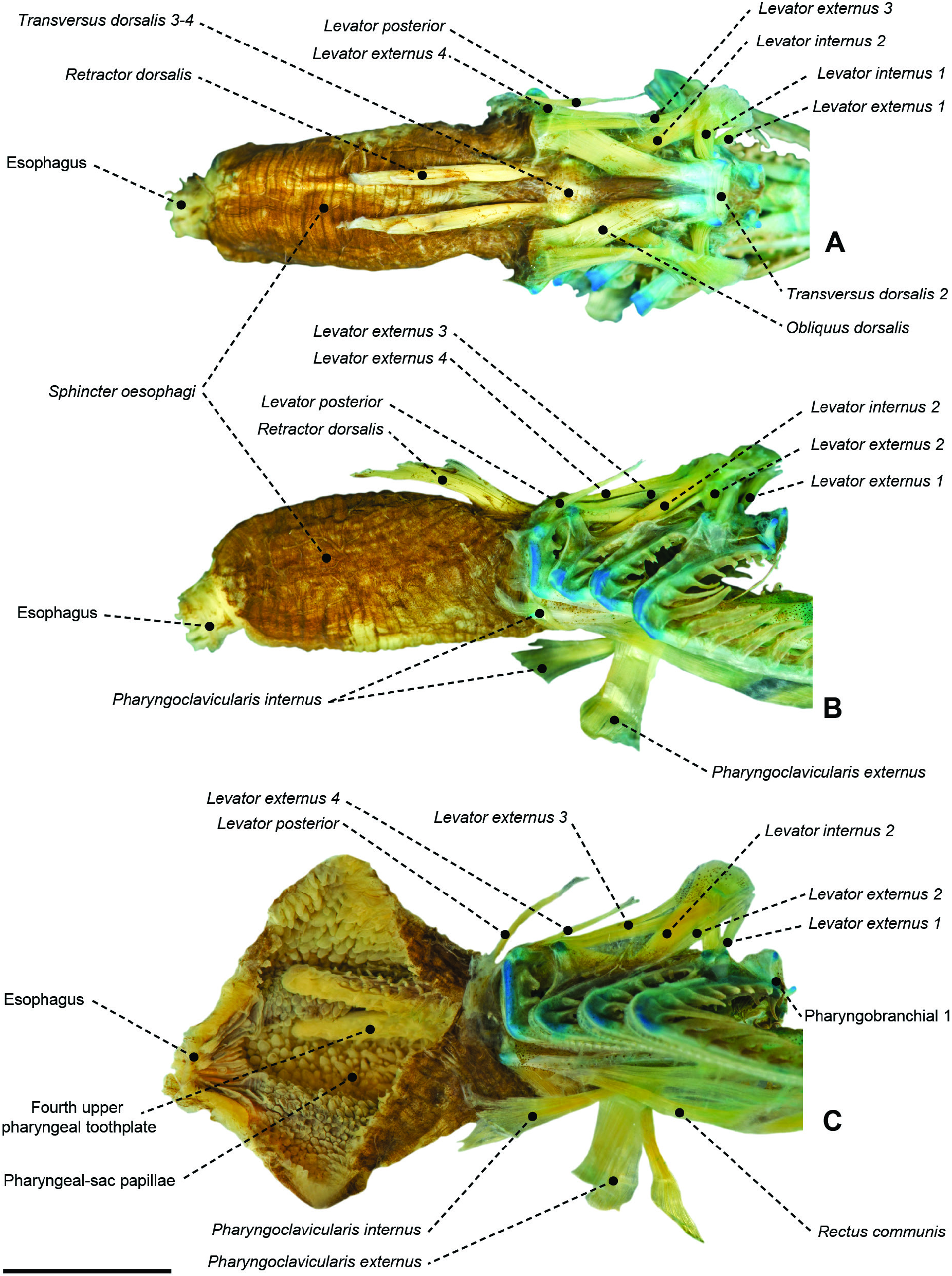
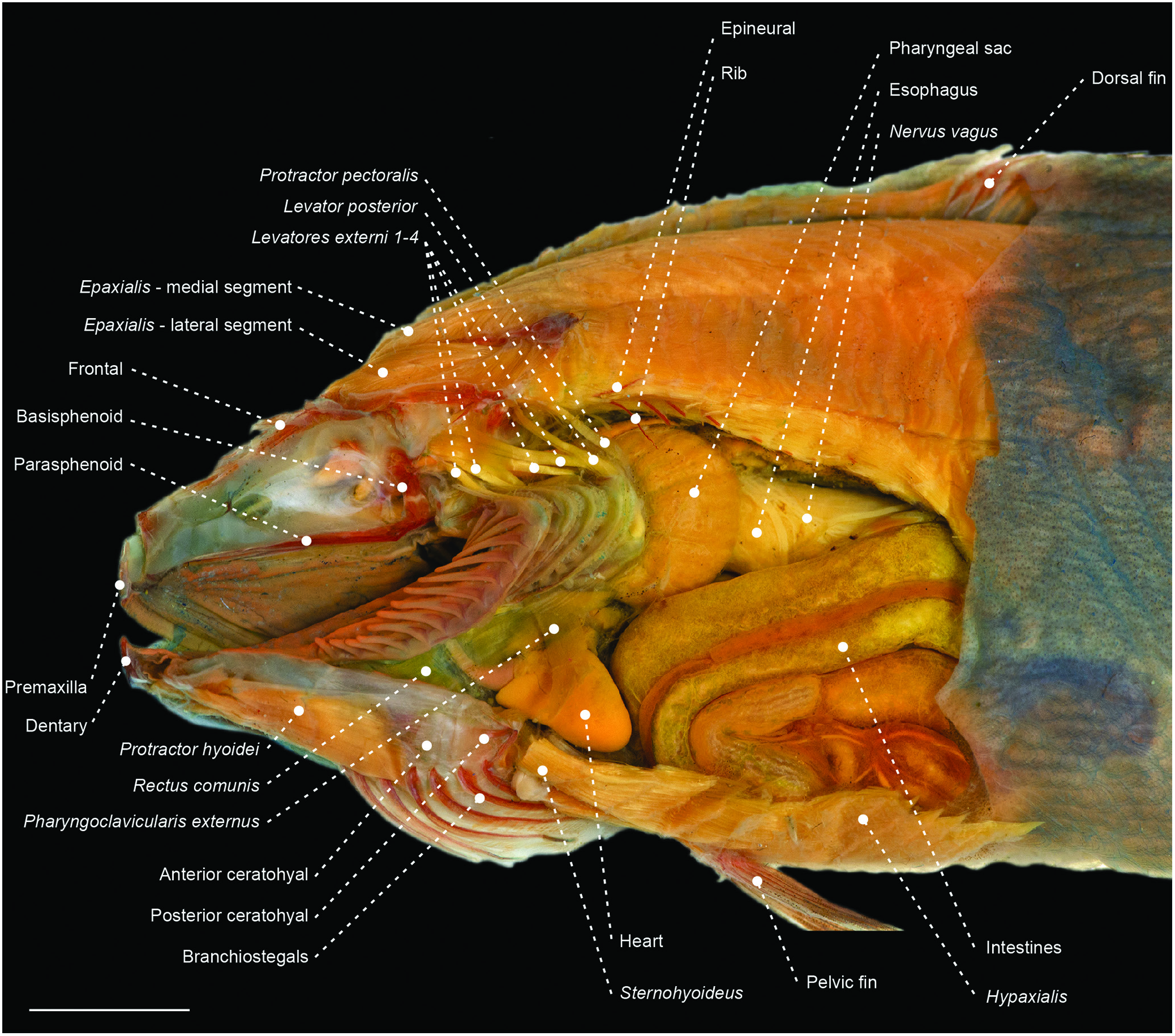
Figure 52. Left lateral view of the cranial and abdominal anatomy of the centrolophid Icichthys lockingtoni (OS 16732), depicting gill arches, pharyngeal sac and anterior portion of the abdominal cavity. Orbital, adductor mandibulae, opercular, hyopalatal and pectoral girdle complexes removed. Scale bar: 6 mm.
Figure 53. A, cranial and abdominal anatomy of the tetragonurid Tetragonurus cuvieri (MZUSP 123241). Gill arches, pharyngeal sac and anterior portion of the abdominal cavity (orbital, adductor mandibulae, opercular, hyopalatal and pectoral girdle complexes removed). Scale bar: 10 mm (in A). B, higher magnification of the gill arch muscles and bones. Scale bar: 2 mm (in B). C, higher magnification pharyngeal sac and anteriormost abdominal organs. Scale bar: 2 mm (in C).
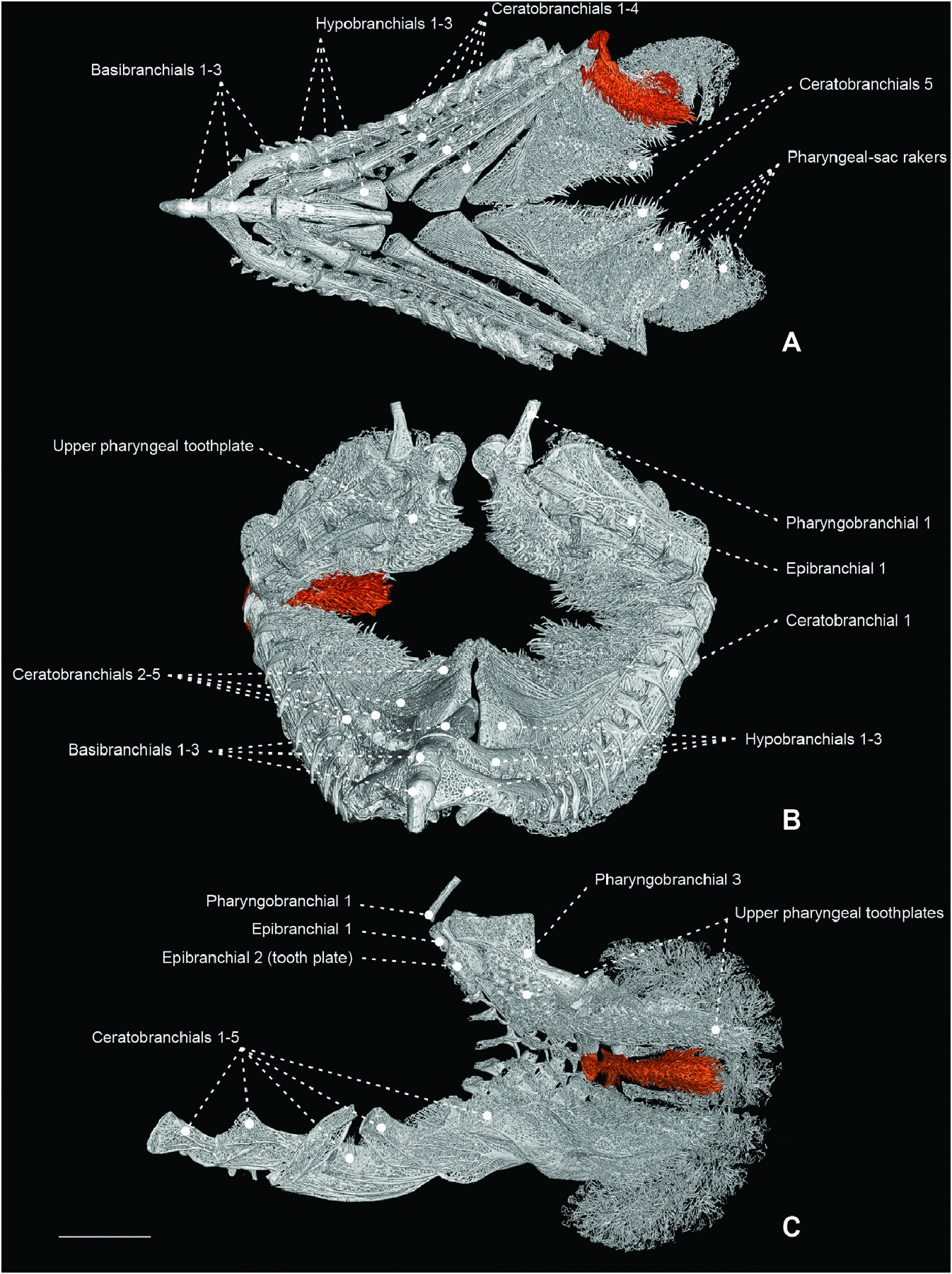
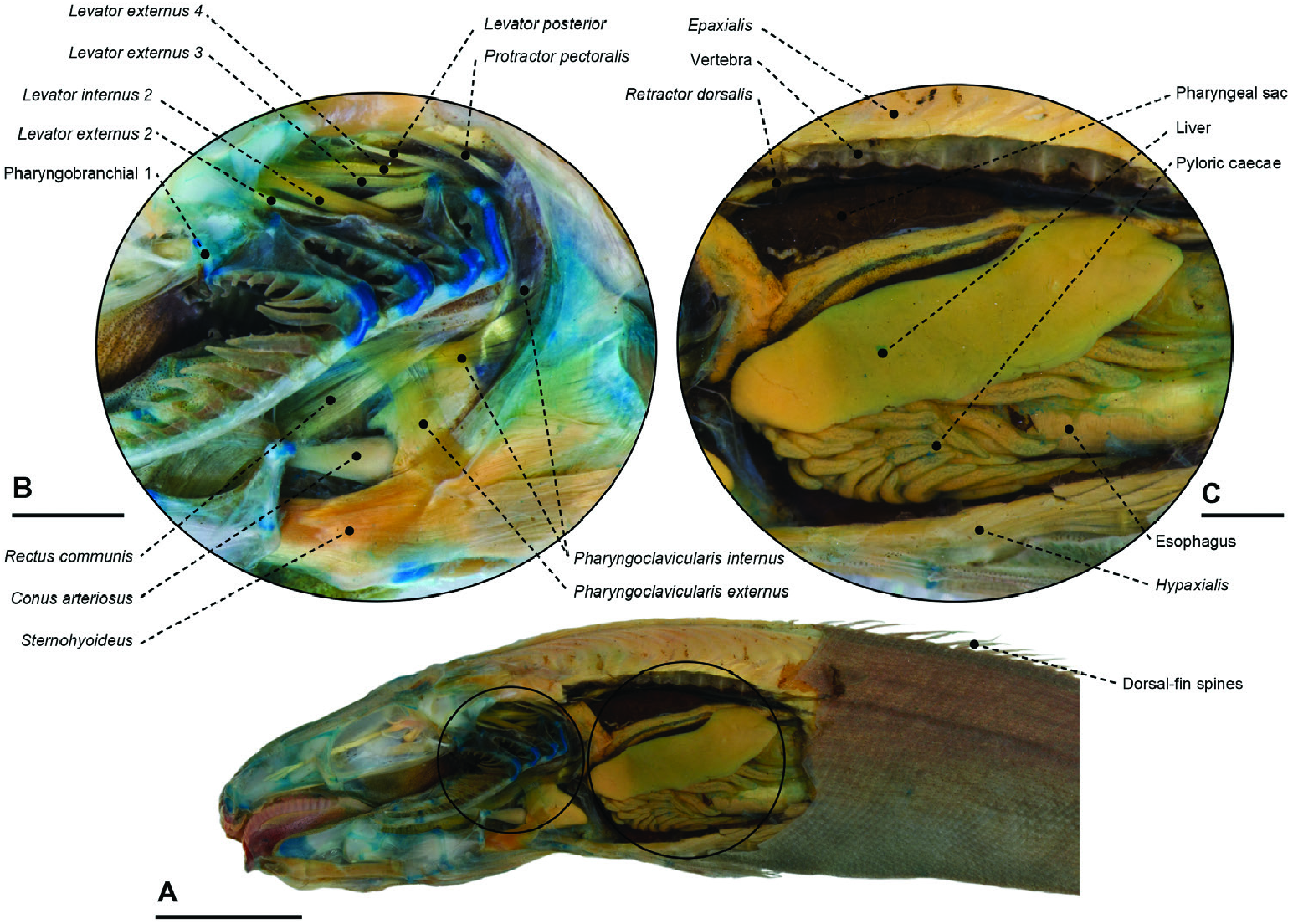
Figure 54. Dorsal view of branchial and pharyngeal-sac musculoskeletal system of the ariommatid Ariomma melana (MZUSP 123246; A) and the centrolophid Hyperoglyphe perciformis (MZUSP 119733; B). Scale bars: 5 mm. Arrow points to the dorsal expansion of the pharyngeal sac.
Figure 55. Branchial and pharyngeal-sac musculoskeletal system of the centrolophid Icichthys lockingtoni (OS 16732) in dorsal (A), lateral (B) and ventral (C) view. Scale bar: 5 mm.
Figure 56. Branchial and pharyngeal-sac musculoskeletal system of the nomeid Cubiceps whiteleggii (MZUSP 67590) in dorsal (A), lateral (B) and ventral (C) view. Scale bar: 6 mm.
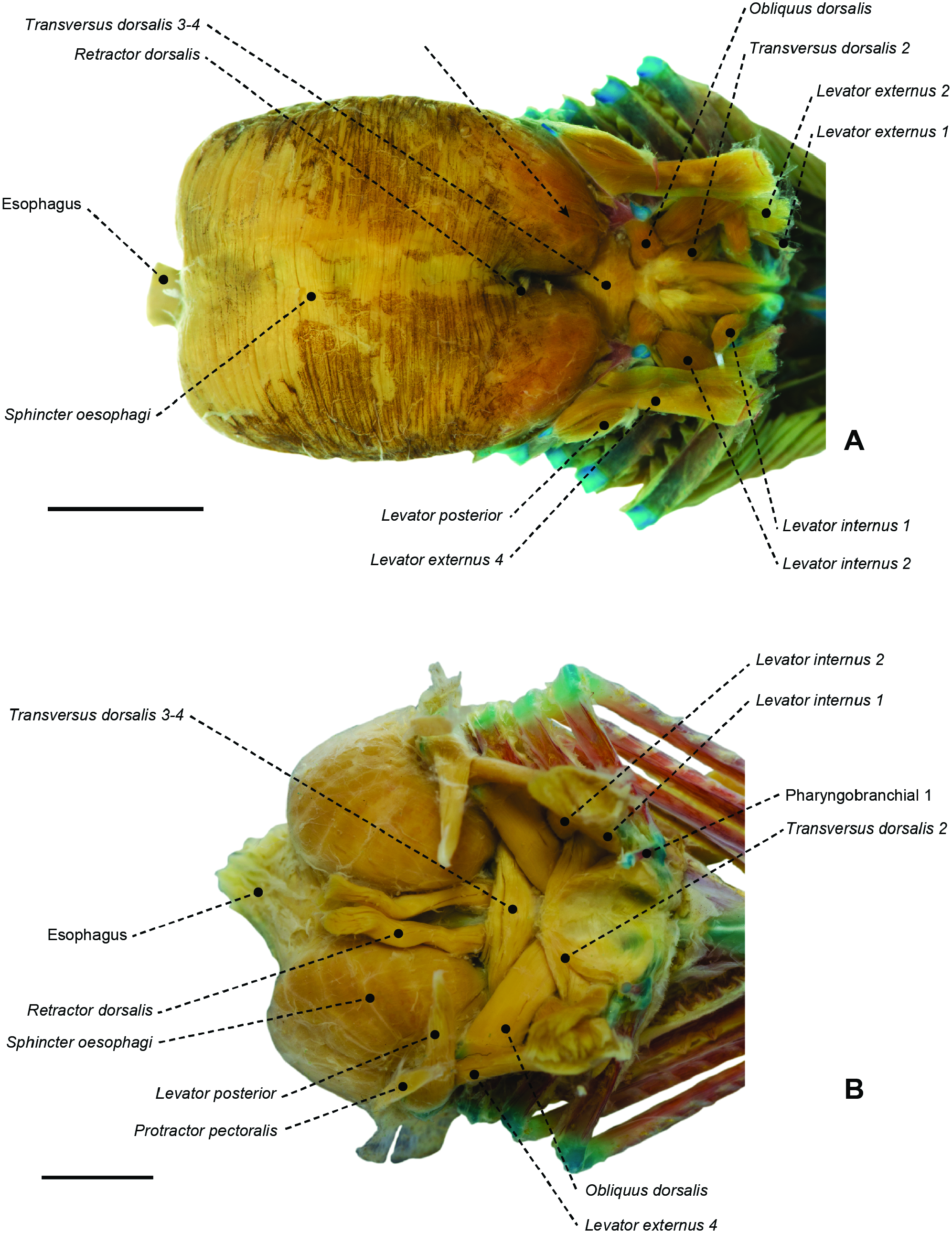
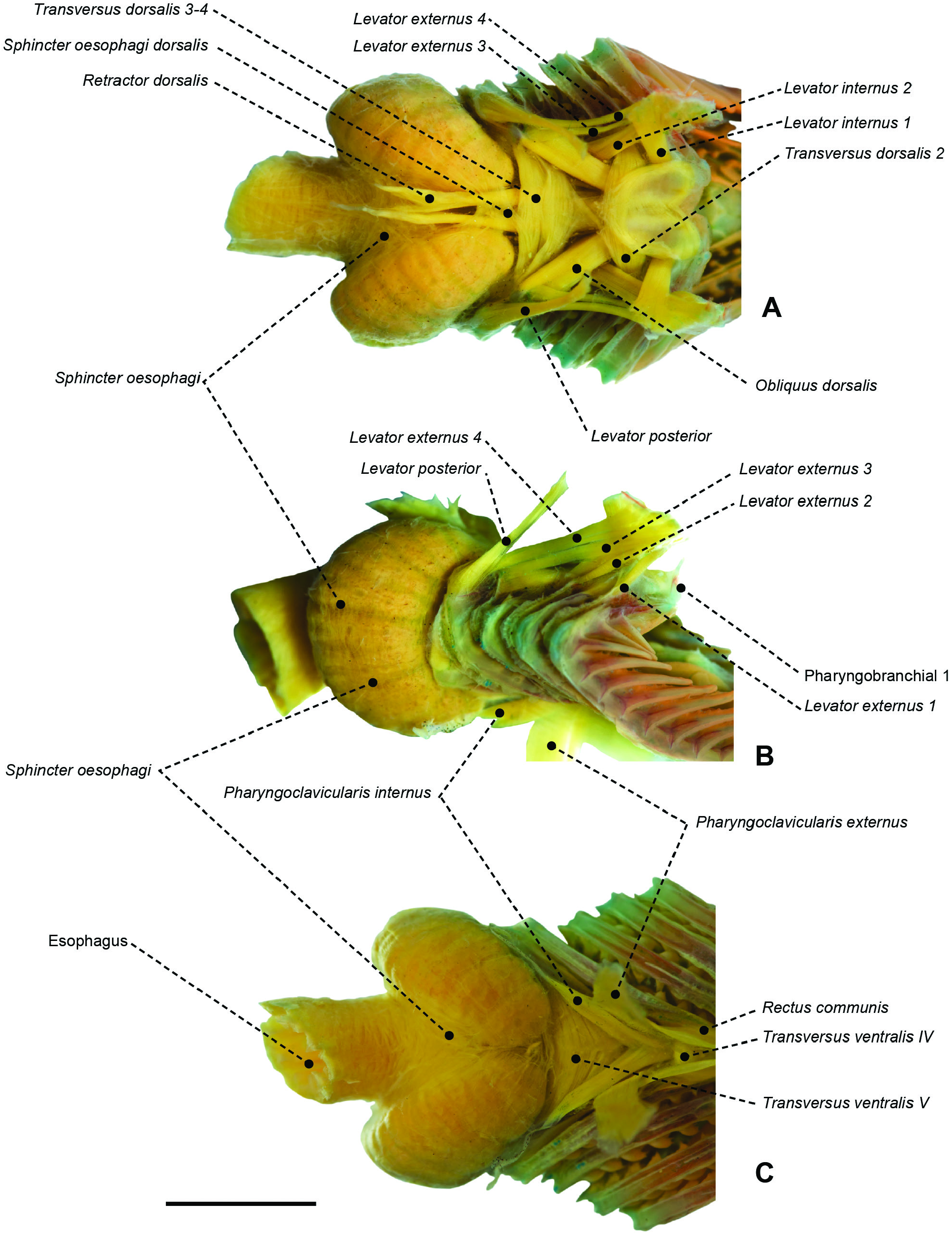
Figure 57. Branchial and pharyngeal-sac musculoskeletal system of the stromateid Stromateus brasiliensis (MZUSP 51279) in dorsal (A), lateral (B) and ventral (C) view. Arrow indicates posterolateral set of sphincter oesophagi fibres. Scale bar: 5 mm.
Figure 58. Micro-computed tomography scans of pharyngeal-sac skeletons in left lateral view, whole (A, C) and sagittal (B, D) view. A, B, the ariommatid Ariomma bondi (MZUSP 86717). C, D, the stromateid Peprilus triacanthus (MZUSP 123240). Scale bar: 2 mm.
Figure 65. Examples of the association between juvenile fishes and pelagic, gelatinous invertebrates. A, B, the nomeid Nomeus gronovii sheltering inside a pyrosome (A) and swimming around the tentacles of a pelagic siphonophore (B). C, the amarsipid Amarsipus carlsbergi hiding in the abdominal cavity of a pelagic salp. D, a bramid Brama sp. resting on the top of a jellyfish. Images from Suzan Meldonian (A) and Linda Ianniello (B–D).
Figure 67. Reference tree obtained under implied-weights parsimony (k = 13.56554), with sensitivity analysis of alternative weighting schemes, highlighting the interrelationships of node 82. Squares representing each value of k are coloured according to their respective element-based scores. Numbers below sensitivity plots indicate the average element-based comparison score for the clade. Node numbers referenced in the text displayed above each clade.
Figure 69. Reference tree obtained under implied-weights parsimony (k = 13.56554), with sensitivity analysis of alternative weighting schemes, highlighting the interrelationships of Stromateiformes (node 90). Squares representing each value of k are coloured according to their respective element-based scores. Numbers below sensitivity plots indicate the average element-based comparison score for the clade. Node numbers referenced in the text displayed above each clade.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Order |